Probing Interleukin-6 in Stroke Pathology and Neural Stem Cell Transplantation
Abstract
:1. Introduction to Stroke Epidemiology and Treatment
2. Interleukin-6 as an Inflammatory Mediator in Stroke
3. Applications and Benefits of Neural Stem Cells
4. Mechanisms of Stem Cell Therapy for Stroke
An Introduction to Interleukin-6 Signaling in Stem Cells
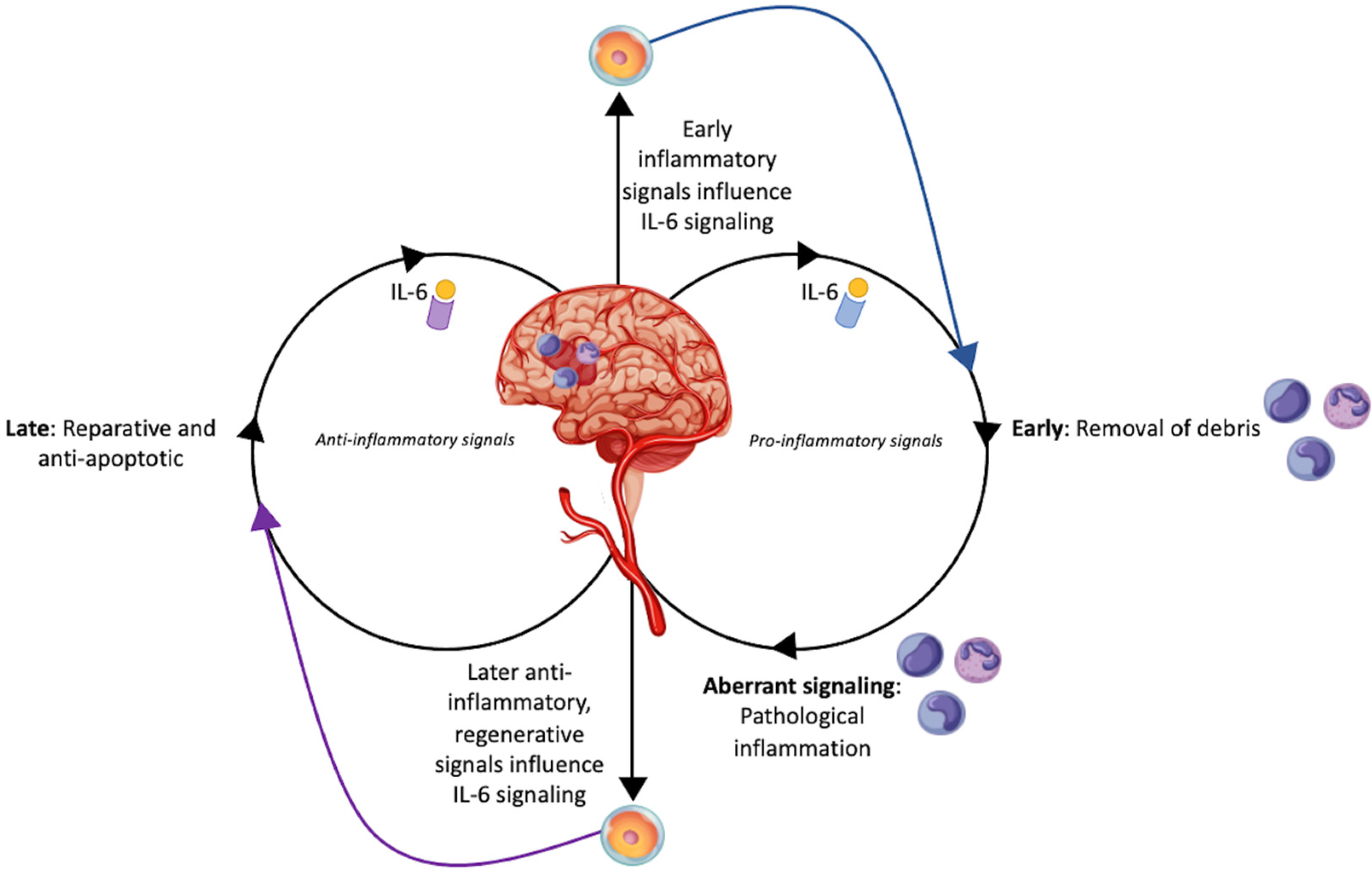
5. Stem Cells and Interleukin-6: The Occasionally Disparate, Occasionally Cooperative Roles in Ischemic Stroke
5.1. Preclinical Studies Describing the Synergistic Role of IL-6 in the Setting of Stroke
5.2. Preclinical Studies Describing the Disparate Role of IL-6 in the Setting of Stroke
| Citation | Sample | Time to Transplantation (Post Induction) | Cell Type | Cell Pretreatment | Route | Dosage | Results |
|---|---|---|---|---|---|---|---|
| Gutiérrez-Fernández et al. (2011) [64] | MCAO rats | 30 min | Bone marrow-derived MSCs | - | Intravenous or intra-arterial | 2 × 106 cells | Intravenous injection produces the highest levels of IL-6. Both routes decrease apoptosis, enhance angiogenesis, and improve neurological function. |
| Sakata et al. (2012) [63] | MCAO mice | 6 h or 7 days | NSCs | IL-6 | Intracerebral | 1 × 105 cells | IL-6 preconditioning increases NSC tolerance for oxidative stress and induces angiogenesis. |
| Huang et al. (2014) [32] | MCAO mice | 24 h | hNSCs | - | Intracerebral | 1 × 106 cells | Within 24 h of hippocampal transplantation, hNSCs decreased expression of inflammatory cytokines (IL-6, IL-1β and TNF-α, monocyte chemotactic protein-1, macrophage inflammatory protein-1α) and adhesion molecules, reduced BBB damage, and improved behavioral function. |
| Eckert et al. (2015) [33] | MCAO rats | 24 h | hiPSC-NSCs | - | Intracerebral | 1 × 106 cells | Downregulation of IL-6, TNF-α, and IL-1β was associated with reduced microglial activation, enhanced BBB restoration, and preserved neurological function. |
| Yang et al. (2017) [66] | MCAO rats | 24 h | Multipotent Adult Progenitor Cells | - | Intravenous | 1.2 × 107 cells | MAPC transplant reduced IL-6 and IL-1β while increasing IL-10 in serum. Stroke recovery was dependent on intact spleen, acting by restoring spleen mass reduction caused by stroke. |
| Chi et al. (2018) [67] | MCAO rats | 0, 12, or 24 h | Adipose-derived MSCs | - | Intravenous | 2 × 106 cells | BBB permeability decreases by downregulating expression of IL-6, IL-1β and TNF-α. ER stress response is reduced, inducing anti-apoptotic factors. Infarct area and neurological function is ameliorated. |
| Cheng et al. (2018) [68] | MCAO rats | 15 min | Bone marrow-derived MSCs | - | Intracerebral | 1 × 105 cells | MSC transplant reduced inflammatory cytokines (IL-6, IL-1β and TNF-α), tight junction protein loss, IgG leakage, and matrix metalloproteinase expression to attenuate infarct volume and neurological function. |
| Gong et al. (2019) [72] | CA rats | 1 h | Adipose-derived MSCs | - | Intravenous | 5 × 106 cells | Stimulation of IL-6 and BDNF expression results in improved neurological deficits reduces hippocampal apoptosis. |
| Kaneko et al. (2020) [65] | MCAO rats | 3 h to 1 week | Bone marrow-derived NCS-01 cells | - | Intra-arterial or intravenous | 7.5 × 106 cells | NCS-01 cells secrete IL-6 and bFGF while contributing to filopodia formation. This results in improved motor function, neurological behavior, and reduction of infarct volume. |
| Salehi et al. (2020) [73] | MCAO rats | Immediate | Epidermal neural crest stem cells or bone marrow-derived MSCs | - | Intra-arterial or intravenous | 2 × 106 cells | Neural crest stem cell transplantation results in greater expression of IL-6, BDNF, nestin, Sox10, doublecortin, β-III tubulin and GFAP while decreasing expression of nerotrophin-3 and IL-10. Both cell types decreased infarct volume and improved functional outcome. |
| Boese et al. (2020) [34] | MCAO mice | 24 h | hNSCs | - | Intravenous | 1 × 106 cells | In delayed tPA treatment, hNSCs decreased expression of proinflammatory factors (including IL-6), decreases matrix metalloprotease expression, increased BDNF expression, and attenuated BBB damage. |
| Sun et al. (2020) [58] | MCAO mice | 1 h | HUMSCs | VX-765 | Intracerebral | 1 × 105 cells | VX-765 pretreatment downregulates pro-inflammatory cytokines, including IL-6), while upregulating anti-inflammatory cytokines to reduce apoptosis and neuroinflammation. |
| Chen et al. (2022) [61] | MCAO rats | 24 h | Bone marrow-derived MSCs | Roxadustat | Intracerebral | 5 × 105 cells | Roxadustat pretreatment decreases IL-6, IL-1β and TNF-α expression, resulting in improved neurological function. |
| Ranjbaran et al. (2022) [74] | 2VO rats | 2 h | Adipose-derived MSCs | - | Intraperitoneal | 1 × 106 cells | Inhibition of IL-6 and TNF-α results in decreased apoptosis in the hippocampus and improved memory deficits. |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donkor, E.S. Stroke in the 21(st) Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef] [Green Version]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Yang, L.; Qian, J.; Yang, B.; He, Q.; Wang, J.; Weng, Q. Challenges and Improvements of Novel Therapies for Ischemic Stroke. Front. Pharmacol. 2021, 12, 721156. [Google Scholar] [CrossRef]
- Anthony, S.; Cabantan, D.; Monsour, M.; Borlongan, C.V. Neuroinflammation, Stem Cells, and Stroke. Stroke 2022, 53, 1460–1472. [Google Scholar] [CrossRef]
- Tian, G.; Ji, Z.; Lin, Z.; Pan, S.; Yin, J. Cerebral autoregulation is heterogeneous in different stroke mechanism of ischemic stroke caused by intracranial atherosclerotic stenosis. Brain Behav. 2021, 11, e01907. [Google Scholar] [CrossRef]
- Zarriello, S.; Tuazon, J.P.; Corey, S.; Schimmel, S.; Rajani, M.; Gorsky, A.; Incontri, D.; Hammock, B.D.; Borlongan, C.V. Humble beginnings with big goals: Small molecule soluble epoxide hydrolase inhibitors for treating CNS disorders. Prog. Neurobiol. 2019, 172, 23–39. [Google Scholar] [CrossRef]
- Prakash, R.; Carmichael, S.T. Blood-brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr. Opin. Neurol. 2015, 28, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Hadanny, A.; Rittblat, M.; Bitterman, M.; May-Raz, I.; Suzin, G.; Boussi-Gross, R.; Zemel, Y.; Bechor, Y.; Catalogna, M.; Efrati, S. Hyperbaric oxygen therapy improves neurocognitive functions of post-stroke patients—A retrospective analysis. Restor. Neurol. Neurosci. 2020, 38, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Rusyniak, D.E.; Kirk, M.A.; May, J.D.; Kao, L.W.; Brizendine, E.J.; Welch, J.L.; Cordell, W.H.; Alonso, R.J. Hyperbaric Oxygen Therapy in Acute Ischemic Stroke. Stroke 2003, 34, 571–574. [Google Scholar] [CrossRef] [Green Version]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta 2016, 1863, 1218–1227. [Google Scholar] [CrossRef]
- Suzuki, S.; Tanaka, K.; Suzuki, N. Ambivalent aspects of interleukin-6 in cerebral ischemia: Inflammatory versus neurotrophic aspects. J. Cereb. Blood Flow Metab. 2009, 29, 464–479. [Google Scholar] [CrossRef]
- Xing, Z.; Gauldie, J.; Cox, G.; Baumann, H.; Jordana, M.; Lei, X.F.; Achong, M.K. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Investig. 1998, 101, 311–320. [Google Scholar] [CrossRef]
- Landreth, G.E. Growth factors. In Basic Neurochemistry; Siegel, G.J., Agranoff, B.W., Albers, R.W., Fisher, S.K., Uhler, M.D., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999; pp. 383–398. [Google Scholar]
- Ziegler, L.; Wallen, H.; Aspberg, S.; de Faire, U.; Gigante, B. IL6 trans-signaling associates with ischemic stroke but not with atrial fibrillation. BMC Neurol. 2021, 21, 306. [Google Scholar] [CrossRef]
- Kaminska, J.; Dymicka-Piekarska, V.; Chrzanowski, R.; Sawicki, K.; Milewska, A.J.; Zinczuk, J.; Tylicka, M.; Jadeszko, M.; Mariak, Z.; Kratz, E.M.; et al. IL-6 Quotient (The Ratio of Cerebrospinal Fluid IL-6 to Serum IL-6) as a Biomarker of an Unruptured Intracranial Aneurysm. J. Inflamm. Res. 2021, 14, 6103–6114. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, S.; Li, Y.; Sun, Y.; Xiong, X.; Hu, X.; Chen, J.; Qiu, S. Interleukins and Ischemic Stroke. Front. Immunol. 2022, 13, 828447. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Malik, R.; Gill, D.; Franceschini, N.; Sudlow, C.L.M.; Dichgans, M.; Invent Consortium; CHARGE Inflammation Working Group. Interleukin-6 Signaling Effects on Ischemic Stroke and Other Cardiovascular Outcomes: A Mendelian Randomization Study. Circ. Genom. Precis. Med. 2020, 13, e002872. [Google Scholar] [CrossRef]
- Hudobenko, J. Ischemic Stroke Damage Is Reduced by Inhibition of IL-6 Signaling with Tocilizumab; The University of Texas MD Anderson Cancer Center: Houston, TX, USA, 2018. [Google Scholar]
- Croci, D.M.; Wanderer, S.; Strange, F.; Gruter, B.E.; Sivanrupan, S.; Andereggen, L.; Casoni, D.; von Gunten, M.; Widmer, H.R.; Di Santo, S.; et al. Tocilizumab Reduces Vasospasms, Neuronal Cell Death, and Microclot Formation in a Rabbit Model of Subarachnoid Hemorrhage. Transl. Stroke Res. 2021, 12, 894–904. [Google Scholar] [CrossRef]
- Gertz, K.; Kronenberg, G.; Kalin, R.E.; Baldinger, T.; Werner, C.; Balkaya, M.; Eom, G.D.; Hellmann-Regen, J.; Krober, J.; Miller, K.R.; et al. Essential role of interleukin-6 in post-stroke angiogenesis. Brain 2012, 135, 1964–1980. [Google Scholar] [CrossRef] [Green Version]
- Loddick, S.A.; Turnbull, A.V.; Rothwell, N.J. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J. Cereb. Blood Flow Metab. 1998, 18, 176–179. [Google Scholar] [CrossRef]
- Matsuda, S.; Wen, T.C.; Morita, F.; Otsuka, H.; Igase, K.; Yoshimura, H.; Sakanaka, M. Interleukin-6 prevents ischemia-induced learning disability and neuronal and synaptic loss in gerbils. Neurosci. Lett. 1996, 204, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Sawamoto, K.; Suzuki, S.; Suzuki, N.; Adachi, K.; Kawase, T.; Mihara, M.; Ohsugi, Y.; Abe, K.; Okano, H. Blockade of interleukin-6 signaling aggravates ischemic cerebral damage in mice: Possible involvement of Stat3 activation in the protection of neurons. J. Neurochem. 2005, 94, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, M.; Preynat-Seauve, O.; De Bruin, K.; Pepper, M.S. Stem cell therapy for neurological disorders. S. Afr. Med. J. 2019, 109, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Yandava, B.D.; Billinghurst, L.L.; Snyder, E.Y. “Global” cell replacement is feasible via neural stem cell transplantation: Evidence from the dysmyelinated shiverer mouse brain. Proc. Natl. Acad. Sci. USA 1999, 96, 7029–7034. [Google Scholar] [CrossRef] [Green Version]
- Doetsch, F.; García-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997, 17, 5046–5061. [Google Scholar] [CrossRef] [Green Version]
- Djavadian, R.L. Serotonin and neurogenesis in the hippocampal dentate gyrus of adult mammals. Acta Neurobiol. Exp. 2004, 64, 189–200. [Google Scholar]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Corti, S.; Nizzardo, M.; Simone, C.; Falcone, M.; Donadoni, C.; Salani, S.; Rizzo, F.; Nardini, M.; Riboldi, G.; Magri, F.; et al. Direct reprogramming of human astrocytes into neural stem cells and neurons. Exp. Cell Res. 2012, 318, 1528–1541. [Google Scholar] [CrossRef] [Green Version]
- Shahbazi, E.; Moradi, S.; Nemati, S.; Satarian, L.; Basiri, M.; Gourabi, H.; Zare Mehrjardi, N.; Günther, P.; Lampert, A.; Händler, K.; et al. Conversion of Human Fibroblasts to Stably Self-Renewing Neural Stem Cells with a Single Zinc-Finger Transcription Factor. Stem Cell Rep. 2016, 6, 539–551. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Wong, S.; Snyder, E.Y.; Hamblin, M.H.; Lee, J.P. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res. Ther. 2014, 5, 129. [Google Scholar] [CrossRef] [Green Version]
- Eckert, A.; Huang, L.; Gonzalez, R.; Kim, H.S.; Hamblin, M.H.; Lee, J.P. Bystander Effect Fuels Human Induced Pluripotent Stem Cell-Derived Neural Stem Cells to Quickly Attenuate Early Stage Neurological Deficits After Stroke. Stem Cells Transl. Med. 2015, 4, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Boese, A.C.; Eckert, A.; Hamblin, M.H.; Lee, J.P. Human neural stem cells improve early stage stroke outcome in delayed tissue plasminogen activator-treated aged stroke brains. Exp. Neurol. 2020, 329, 113275. [Google Scholar] [CrossRef] [PubMed]
- Bacigaluppi, M.; Pluchino, S.; Peruzzotti-Jametti, L.; Kilic, E.; Kilic, U.; Salani, G.; Brambilla, E.; West, M.J.; Comi, G.; Martino, G.; et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain 2009, 132, 2239–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, S.; Lee, S.H.; Kim, S.U.; Yoon, B.W. Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen. Res. 2016, 11, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.L.; Chopp, M.; Roberts, C.; Liu, X.; Wei, M.; Nejad-Davarani, S.P.; Wang, X.; Zhang, Z.G. Stroke increases neural stem cells and angiogenesis in the neurogenic niche of the adult mouse. PLoS ONE 2014, 9, e113972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Li, J.; Liu, Y.; Chen, X.; Lu, H.; Kang, Q.; Li, W.; Gao, M. Human embryonic neural stem cell transplantation increases subventricular zone cell proliferation and promotes peri-infarct angiogenesis after focal cerebral ischemia. Neuropathology 2011, 31, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Goderie, S.K.; Jin, L.; Karanth, N.; Sun, Y.; Abramova, N.; Vincent, P.; Pumiglia, K.; Temple, S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 2004, 304, 1338–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mine, Y.; Tatarishvili, J.; Oki, K.; Monni, E.; Kokaia, Z.; Lindvall, O. Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiol. Dis. 2013, 52, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Kim, M.; Jeong, S.W.; Kim, S.U.; Yoon, B.W. Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci. Lett. 2003, 343, 129–133. [Google Scholar] [CrossRef]
- Huang, L.; Reis, C.; Boling, W.W.; Zhang, J.H. Stem Cell Therapy in Brain Ischemia: The Role of Mitochondrial Transfer. Stem Cells Dev. 2020, 29, 555–561. [Google Scholar] [CrossRef]
- Cho, Y.M.; Kim, J.H.; Kim, M.; Park, S.J.; Koh, S.H.; Ahn, H.S.; Kang, G.H.; Lee, J.-B.; Park, K.S.; Lee, H.K. Mesenchymal Stem Cells Transfer Mitochondria to the Cells with Virtually No Mitochondrial Function but Not with Pathogenic mtDNA Mutations. PLoS ONE 2012, 7, e32778. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, S.; Pugin, J. Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Front. Immunol. 2018, 9, 832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, A.; Fadeel, B. Mitochondria released by cells undergoing TNF-α-induced necroptosis act as danger signals. Cell Death Dis. 2014, 5, e1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahrouf-Yorgov, M.; Augeul, L.; Da Silva, C.C.; Jourdan, M.; Rigolet, M.; Manin, S.; Ferrera, R.; Ovize, M.; Henry, A.; Guguin, A.; et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 2017, 24, 1224–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.; Xiong, G.; Feng, H.; Zhang, Z.; Chen, P.; Yan, B.; Chen, L.; Gandhervin, K.; Ma, C.; Li, C.; et al. Donation of mitochondria by iPSC-derived mesenchymal stem cells protects retinal ganglion cells against mitochondrial complex I defect-induced degeneration. Theranostics 2019, 9, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.H.; Lee, J.P. Neural Stem Cells for Early Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 7703. [Google Scholar] [CrossRef]
- Chang, D.-J.; Lee, N.; Park, I.-H.; Choi, C.; Jeon, I.; Kwon, J.; Oh, S.-H.; Shin, D.A.; Do, J.T.; Lee, D.R.; et al. Therapeutic Potential of Human Induced Pluripotent Stem Cells in Experimental Stroke. Cell Transplant. 2013, 22, 1427–1440. [Google Scholar] [CrossRef]
- Pluchino, S.; Zanotti, L.; Rossi, B.; Brambilla, E.; Ottoboni, L.; Salani, G.; Martinello, M.; Cattalini, A.; Bergami, A.; Furlan, R.; et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 2005, 436, 266–271. [Google Scholar] [CrossRef]
- Einstein, O.; Karussis, D.; Grigoriadis, N.; Mizrachi-Kol, R.; Reinhartz, E.; Abramsky, O.; Ben-Hur, T. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol. Cell. Neurosci. 2003, 24, 1074–1082. [Google Scholar] [CrossRef]
- De Feo, D.; Merlini, A.; Laterza, C.; Martino, G. Neural stem cell transplantation in central nervous system disorders: From cell replacement to neuroprotection. Curr. Opin. Neurol. 2012, 25, 322–333. [Google Scholar] [CrossRef]
- Pluchino, S.; Zanotti, L.; Brambilla, E.; Rovere-Querini, P.; Capobianco, A.; Alfaro-Cervello, C.; Salani, G.; Cossetti, C.; Borsellino, G.; Battistini, L.; et al. Immune regulatory neural stem/precursor cells protect from central nervous system autoimmunity by restraining dendritic cell function. PLoS ONE 2009, 4, e5959. [Google Scholar] [CrossRef]
- Einstein, O.; Fainstein, N.; Vaknin, I.; Mizrachi-Kol, R.; Reihartz, E.; Grigoriadis, N.; Lavon, I.; Baniyash, M.; Lassmann, H.; Ben-Hur, T. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann. Neurol. 2007, 61, 209–218. [Google Scholar] [CrossRef]
- Cao, W.; Yang, Y.; Wang, Z.; Liu, A.; Fang, L.; Wu, F.; Hong, J.; Shi, Y.; Leung, S.; Dong, C.; et al. Leukemia inhibitory factor inhibits T helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity 2011, 35, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Lee, J.Y.; Kaneko, Y.; Tuazon, J.P.; Vale, F.; van Loveren, H.; Borlongan, C.V. Human stem cells transplanted into the rat stroke brain migrate to the spleen via lymphatic and inflammation pathways. Haematologica 2019, 104, 1062–1073. [Google Scholar] [CrossRef]
- Asgari Taei, A.; Dargahi, L.; Khodabakhsh, P.; Kadivar, M.; Farahmandfar, M. Hippocampal neuroprotection mediated by secretome of human mesenchymal stem cells against experimental stroke. CNS Neurosci. Ther. 2022, 28, 1425–1438. [Google Scholar] [CrossRef]
- Sun, Z.; Gu, L.; Wu, K.; Wang, K.; Ru, J.; Yang, S.; Wang, Z.; Zhuge, Q.; Huang, L.; Huang, S. VX-765 enhances autophagy of human umbilical cord mesenchymal stem cells against stroke-induced apoptosis and inflammatory responses via AMPK/mTOR signaling pathway. CNS Neurosci. Ther. 2020, 26, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Neal, E.G.; Acosta, S.A.; Kaneko, Y.; Ji, X.; Borlongan, C.V. Regulatory T-cells within bone marrow-derived stem cells actively confer immunomodulatory and neuroprotective effects against stroke. J. Cereb. Blood Flow Metab. 2019, 39, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Zarriello, S.; Neal, E.G.; Kaneko, Y.; Borlongan, C.V. T-Regulatory Cells Confer Increased Myelination and Stem Cell Activity after Stroke-Induced White Matter Injury. J. Clin. Med. 2019, 8, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Lin, X.; Yao, C.; Bingwa, L.A.; Wang, H.; Lin, Z.; Jin, K.; Zhuge, Q.; Yang, S. Transplantation of Roxadustat-preconditioned bone marrow stromal cells improves neurological function recovery through enhancing grafted cell survival in ischemic stroke rats. CNS Neurosci. Ther. 2022, 28, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Philipp, D.; Suhr, L.; Wahlers, T.; Choi, Y.H.; Paunel-Gorgulu, A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res. Ther. 2018, 9, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakata, H.; Narasimhan, P.; Niizuma, K.; Maier, C.M.; Wakai, T.; Chan, P.H. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain 2012, 135, 3298–3310. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fernández, M.; Rodríguez-Frutos, B.; Alvarez-Grech, J.; Vallejo-Cremades, M.T.; Expósito-Alcaide, M.; Merino, J.; Roda, J.M.; Díez-Tejedor, E. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience 2011, 175, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Lee, J.Y.; Tajiri, N.; Tuazon, J.P.; Lippert, T.; Russo, E.; Yu, S.J.; Bonsack, B.; Corey, S.; Coats, A.B.; et al. Translating intracarotid artery transplantation of bone marrow-derived NCS-01 cells for ischemic stroke: Behavioral and histological readouts and mechanistic insights into stem cell therapy. Stem Cells Transl. Med. 2020, 9, 203–220. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Hamilton, J.A.; Valenzuela, K.S.; Bogaerts, A.; Xi, X.; Aronowski, J.; Mays, R.W.; Savitz, S.I. Multipotent Adult Progenitor Cells Enhance Recovery After Stroke by Modulating the Immune Response from the Spleen. Stem Cells 2017, 35, 1290–1302. [Google Scholar] [CrossRef] [Green Version]
- Chi, L.; Huang, Y.; Mao, Y.; Wu, K.; Zhang, L.; Nan, G. Tail Vein Infusion of Adipose-Derived Mesenchymal Stem Cell Alleviated Inflammatory Response and Improved Blood Brain Barrier Condition by Suppressing Endoplasmic Reticulum Stress in a Middle Cerebral Artery Occlusion Rat Model. Med. Sci. Monit. 2018, 24, 3946–3957. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, L.; Qu, M.; Liang, H.; Li, W.; Li, Y.; Deng, L.; Zhang, Z.; Yang, G.Y. Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice. J. Neuroinflamm. 2018, 15, 135. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Li, T.; Ji, T.; Yi, W.; Yang, Z.; Wang, S.; Yang, Y.; Gu, C. AMPK: Potential Therapeutic Target for Ischemic Stroke. Theranostics 2018, 8, 4535–4551. [Google Scholar] [CrossRef]
- Li, J.; Tao, T.; Xu, J.; Liu, Z.; Zou, Z.; Jin, M. HIF-1α attenuates neuronal apoptosis by upregulating EPO expression following cerebral ischemia-reperfusion injury in a rat MCAO model. Int. J. Mol. Med. 2020, 45, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Hou, J.; Li, Y.; Mou, S.; Wang, Z.; Horch, R.E.; Sun, J.; Yuan, Q. The pro-angiogenic role of hypoxia inducible factor stabilizer FG-4592 and its application in an in vivo tissue engineering chamber model. Sci. Rep. 2019, 9, 6035. [Google Scholar] [CrossRef] [Green Version]
- Gong, B.; Dong, Y.; He, C.; Jiang, W.; Shan, Y.; Zhou, B.Y.; Li, W. Intravenous Transplants of Human Adipose-Derived Stem Cell Protect the Rat Brain From Ischemia-Induced Damage. J. Stroke Cerebrovasc. Dis. 2019, 28, 595–603. [Google Scholar] [CrossRef]
- Salehi, M.S.; Pandamooz, S.; Safari, A.; Jurek, B.; Tamadon, A.; Namavar, M.R.; Dianatpour, M.; Dargahi, L.; Azarpira, N.; Fattahi, S.; et al. Epidermal neural crest stem cell transplantation as a promising therapeutic strategy for ischemic stroke. CNS Neurosci. Ther. 2020, 26, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Ranjbaran, M.; Vali, R.; Yaghoobi, Z.; Sehati, F.; Jashn, V.; Kolur, S.M.; Akhondzadeh, F.; Ashabi, G. Adipose-derived mesenchymal stem cells reduced transient cerebral ischemia injury by modulation of inflammatory factors and AMPK signaling. Behav. Brain Res. 2022, 433, 114001. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lockard, G.M.; Alayli, A.; Monsour, M.; Gordon, J.; Schimmel, S.; Elsayed, B.; Borlongan, C.V. Probing Interleukin-6 in Stroke Pathology and Neural Stem Cell Transplantation. Int. J. Mol. Sci. 2022, 23, 15453. https://doi.org/10.3390/ijms232415453
Lockard GM, Alayli A, Monsour M, Gordon J, Schimmel S, Elsayed B, Borlongan CV. Probing Interleukin-6 in Stroke Pathology and Neural Stem Cell Transplantation. International Journal of Molecular Sciences. 2022; 23(24):15453. https://doi.org/10.3390/ijms232415453
Chicago/Turabian StyleLockard, Gavin Miles, Adam Alayli, Molly Monsour, Jonah Gordon, Samantha Schimmel, Bassel Elsayed, and Cesar V. Borlongan. 2022. "Probing Interleukin-6 in Stroke Pathology and Neural Stem Cell Transplantation" International Journal of Molecular Sciences 23, no. 24: 15453. https://doi.org/10.3390/ijms232415453







