MAPK Cascades and Transcriptional Factors: Regulation of Heavy Metal Tolerance in Plants
Abstract
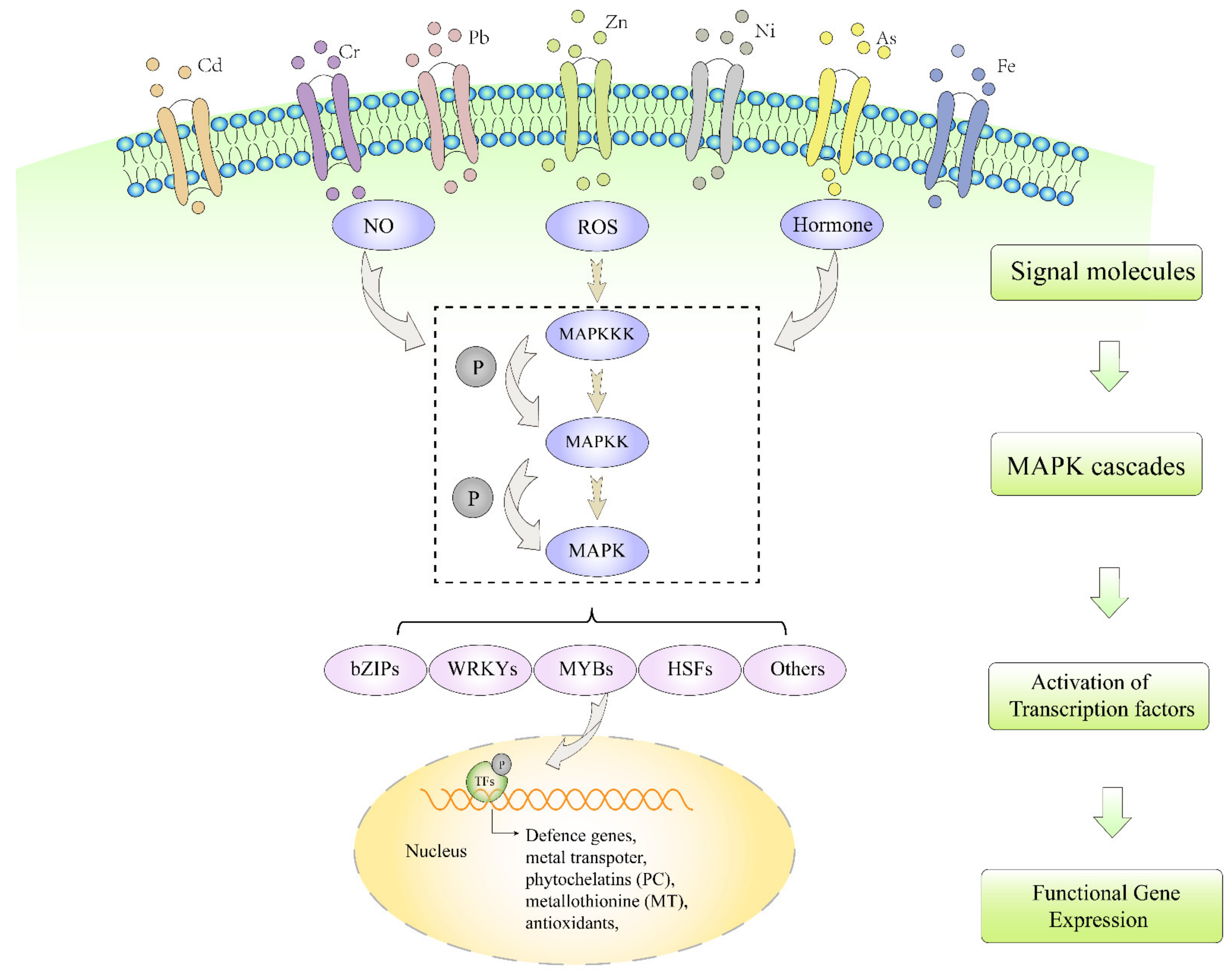
:1. Introduction
2. MAPK Was Directly Activated under Heavy Metal Stress
3. Different Signal Molecules Activate MAPK Pathway under Heavy Metal Stresses
3.1. ROS
3.2. NO
3.3. Plant Hormones
4. Transcriptional Factors Regulate Heavy Metal Tolerance
4.1. bZIP
4.2. MYB
4.3. WRKY
4.4. HSF
4.5. Other TFs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HM | heavy metal |
| MAPK | mitogen-activated protein kinases |
| MAPKKK | MAPKK kinase |
| MAPKK | MAPK kinase |
| ROS | active oxygen |
| H2O2 | hydrogen peroxide |
| SA | salicylic acid |
| ABA | abscisic acid |
| IAA | auxin |
| ET | ethylene |
| bZIP | basic leucine zipper |
| HSF | heat shock transcription factor |
| MYB | myeloblastosis protein |
| ERF | ethylene-responsive transcription factor |
| bHLH | Basic helix–loop–helix |
| WRKY | WRKYGQK domain |
| TFs | transcriptional factors |
| H2S | hydrogen sulfide |
References
- Kosakivska, I.V.; Babenko, L.M.; Romanenko, K.O.; Korotka, I.Y.; Potters, G. Molecular mechanisms of plant adaptive responses to heavy metals stress. Cell Biol. Int. 2021, 45, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Khalili, N.; Razi, S.; Keshavarz-Fathi, M.; Khalili, N.; Rezaei, N. Effects of lead and cadmium on the immune system and cancer progression. J. Environ. Health Sci. 2020, 18, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Abbas, Z.; Seleiman, M.F.; Rizwan, M.; Kalderis, D. Glycine betaine accumulation, significance and interests for heavy metal tolerance in plants. Plants 2020, 9, 896. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N. The mechanism of heavy metal elements in various biological process and its deteriorate effects on the productivity of different crop plants. Int. J. Waste Resour. 2021, 11, 403. [Google Scholar]
- Husen, A. The Harsh Environment and Resilient Plants: An Overview. In Harsh Environ. Plant Resil; Husen, A., Ed.; Springer: Cham, Switzerland, 2021; pp. 1–23. [Google Scholar]
- Haak, D.C.; Fukao, T.; Grene, R.; Hua, Z.; Ivanov, R.; Perrella, G.; Li, S. Multilevel regulation of abiotic stress responses in plants. Front. Plant Sci. 2017, 8, 1564. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Raina, S.K.; Sultan, S.M. Arabidopsis MAPK signaling pathways and their cross talks in abiotic stress response. J. Plant Biochem. Biot. 2020, 29, 1–15. [Google Scholar] [CrossRef]
- Ding, Y.F.; Ding, L.H.; Xia, Y.J.; Wang, F.J.; Zhu, C. Emerging Roles of microRNAs in plant heavy metal tolerance and homeostasis. J. Agric. Food Chem. 2020, 68, 1958–1965. [Google Scholar] [CrossRef]
- Mondal, S.; Pramanik, K.; Ghosh, S.K.; Pal, P.; Ghosh, P.K.; Ghosh, A.; Maiti, T.K. Molecular insight into arsenic uptake, transport, phytotoxicity, and defense responses in plants: A critical review. Planta 2022, 255, 87. [Google Scholar] [CrossRef]
- Hao, S.H.; Wang, Y.R.; Yan, Y.X.; Liu, Y.H.; Wang, J.Y.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 6, 132. [Google Scholar] [CrossRef]
- Kaur, R.; Das, S.; Bansal, S.; Singh, G.; Sardar, S.; Dhar, H.; Ram, H. Heavy metal stress in rice: Uptake, transport, signaling, and tolerance mechanisms. Physiol. Plant 2021, 173, 430–448. [Google Scholar] [CrossRef]
- Sharma, D.; Verma, N.; Pandey, C.; Verma, D.; Bhagat, P.K.; Noryang, S.; Tayyeba, S.; Banerjee, G.; Sinha, A.K. MAP Kinase as Regulators for Stress Responses in Plants: An Overview. In Protein Kinases and Stress Signaling in Plants; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Komis, G.; Šamajová, O.; Ovečka, M.; Šamaj, J. Cell and developmental biology of plant mitogen-activated protein kinases. Annu. Rev. Plant Biol. 2018, 69, 237–265. [Google Scholar] [CrossRef]
- Sharma, S.S.; Kumar, V.; Dietz, K.J. Emerging trends in metalloid-dependent signaling in plants. Trends Plant Sci. 2021, 26, 452–471. [Google Scholar] [CrossRef]
- Andreasson, E.; Ellis, B. Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci. 2010, 15, 106–113. [Google Scholar] [CrossRef]
- Wang, K.; Shao, Z.; Guo, F.; Wang, K.; Zhang, Z. The mitogen-activated protein kinase kinase TaMKK5 mediates immunity via the TaMKK5–TaMPK3–TaERF3 module. Plant Physiol. 2021, 187, 2323–2337. [Google Scholar] [CrossRef]
- Xue, W.X.; Jiang, Y.; Shang, X.S.; Zou, J.H. Characterisation of early responses in lead accumulation and localization of Salix babylonica L. roots. BMC Plant Biol. 2020, 20, 296. [Google Scholar] [CrossRef]
- Jonak, C.; Ökrész, L.; Bögre, L.; Hirt, H. Complexity, cross talk and integration of plant MAP kinase signalling. Curr. Opin. Plant Biol. 2002, 5, 415–424. [Google Scholar] [CrossRef]
- Zhang, M.M.; Zhang, S.Q. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef]
- Bigeard, J.; Hirt, H. Nuclear signaling of plant MAPKs. Front. Plant Sci. 2018, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Wang, C.; Wang, H.; Li, L.; Wang, C. The function of MAPK cascades in response to various stresses in horticultural plants. Front. Plant Sci. 2020, 11, 952. [Google Scholar] [CrossRef]
- Xu, Z.G.; Dong, M.; Peng, X.Y.; Ku, W.Z.; Zhao, Y.L.; Yang, G.Y. New insight into the molecular basis of cadmium stress responses of wild paper mulberry plant by transcriptome analysis. Ecotox. Environ. Safe 2019, 171, 301–312. [Google Scholar] [CrossRef]
- Pandey, C.; Gopal, B.; Krishna, S.A. Differential expression of mitogen activated protein kinase (MAPK) and stress-related genes in rice overexpressing MPK3 and MPK6 under abiotic stress. Int. J. Plant Environ. 2020, 6, 264–269. [Google Scholar]
- Muhammad, T.; Zhang, J.; Ma, Y.; Li, Y.; Zhang, F.; Zhang, Y.; Liang, Y. Overexpression of a mitogen-activated protein kinase SlMAPK3 positively regulates tomato tolerance to cadmium and drought stress. Molecules 2019, 24, 556. [Google Scholar] [CrossRef] [Green Version]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.M.; Ausubel, F.; Sheen, J. MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Sinha, A.K. ROS mediated MAPK signaling in abiotic and biotic stress-striking similarities and differences. Front. Plant Sci. 2015, 6, 769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonak, C.; Nakagami, H.; Hirt, H. Heavy metal stress. activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol. 2004, 136, 3276–3283. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.M.; Kim, K.E.; Kim, K.C.; Xuan, C.N.; Han, H.J.; Mi, S.J.; Kim, H.S.; Sun, H.K.; Park, H.C.; Yun, D.J. Cadmium activates Arabidopsis MPK3 and MPK6 via accumulation of reactive oxygen species. Phytochemistry 2010, 71, 614–618. [Google Scholar] [CrossRef]
- Liu, Y.K.; Liu, L.X.; Qi, J.H.; Dang, P.Y.; Xia, T.S. Cadmium activates ZmMPK3-1 and ZmMPK6-1 via induction of reactive oxygen species in maize roots. Biochem. Bioph. Res. Commun. 2019, 516, 747–752. [Google Scholar] [CrossRef]
- Capone, R.; Tiwari, B.S.; Levine, A. Rapid transmission of oxidative and nitrosative stress signals from roots to shoots in Arabidopsis. Plant Physiol. Biochem. 2004, 42, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Bot, P.; Mun, B.G.; Imran, Q.M.; Hussain, A.; Lee, S.U.; Loake, G.; Yun, B.W. Differential expression of AtWAKL10 in response to nitric oxide suggests a putative role in biotic and abiotic stress responses. PeerJ 2019, 7, e7383. [Google Scholar] [CrossRef] [PubMed]
- Chmielowska-Bąk, J.; Gzyl, J.; Rucińska-Sobkowiak, R.; Arasimowicz-Jelonek, M.; Deckert, J. The new insights into cadmium sensing. Front. Plant Sci. 2014, 5, 245. [Google Scholar]
- Ye, Y.; Li, Z.; Xing, D. Nitric oxide promotes MPK6-mediated caspase-3-like activation in cadmium-induced Arabidopsis thaliana programmed cell death. Plant Cell Environ. 2013, 36, 1–15. [Google Scholar] [CrossRef]
- Jin, C.W.; Mao, Q.Q.; Luo, B.F.; Lin, X.Y.; Du, S.T. Mutation of mpk6 enhances cadmium tolerance in Arabidopsis plants by alleviating oxidative stress. Plant Soil. 2013, 371, 387–396. [Google Scholar] [CrossRef]
- Rao, K.P.; Vani, G.; Kumar, K.; Wankhede, D.P.; Misra, M.; Gupta, M.; Sinha, A.K. Arsenic stress activates MAP kinase in rice roots and leaves. Arch. Biochem. Biophys. 2011, 506, 73–82. [Google Scholar] [CrossRef]
- Khan, S.; Iqbal, N.; Deeba, F.; Jabeen, R. Mitogen activated protein kinase: Function and responses to different stresses in plants. Pak. J. Biochem. Biotechnol. 2020, 1, 1. [Google Scholar] [CrossRef]
- Sanja, M.; Tatjana, P.B.; Roland, G.; Vazquez, K.R.; Thomas, R. Imposed glutathione-mediated redox switch modulates the tobacco wound-induced protein kinase and salicylic acid-induced protein kinase activation state and impacts on defence against Pseudomonas Syringae. J. Exp. Bot. 2015, 66, 1935–1950. [Google Scholar]
- Zhao, F.Y.; Wang, K.; Zhang, S.Y.; Ren, J.; Liu, T.; Wang, X. Crosstalk between ABA, auxin, MAPK signaling, and the cell cycle in cadmium-stressed rice seedlings. Acta Physiol. Plant 2014, 36, 1879–1892. [Google Scholar] [CrossRef]
- Opdenakker, K.; Remans, T.; Keunen, E.; Vangronsveld, J.; Cuypers, A. Exposure of Arabidopsis thaliana to Cd or Cu excess leads to oxidative stress mediated alterations in MAPKinase transcript levels. Environ. Exp. Bot. 2012, 83, 53–61. [Google Scholar] [CrossRef]
- Chmielowska-Bak, J.; Lefèvre, I.; Lutts, S.; Deckert, J. Short term signaling responses in roots of young soybean seedlings exposed to cadmium stress. J. Plant Physiol. 2013, 170, 1585–1594. [Google Scholar] [CrossRef]
- Torres, M.; Forman, H.J. Redox signaling and the MAP kinase pathways. BioFactors 2003, 17, 287–296. [Google Scholar] [CrossRef]
- Nazir, F.; Fariduddin, Q.; Khan, T.A. Hydrogen peroxide as a signaling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 2020, 252, 126486. [Google Scholar] [CrossRef]
- Colcombet, J.; Hirt, H. Arabidopsis MAPKs, a complex signaling network involved in multiple biological processes. Biochem. J. 2008, 413, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Li, G.J.; Meng, X.Z.; Wang, R.G.; Mao, G.H.; Han, L.; Liu, Y.D.; Zhang, S.Q. Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genet. 2012, 8, e1002767. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.H.; Meng, X.Z.; Liu, Y.D.; Zheng, Z.Y.; Chen, Z.X.; Zhang, S.Q. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 2011, 23, 1639–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.X.; Xu, L.; Wang, Y.; Tang, M.J.; Liu, L.W. Genome-and transcriptome-wide characterization of bZIP gene family identifies potential members involved in abiotic stress response and anthocyanin biosynthesis in radish (Raphanus sativus L.). Int. J. Mol. Sci. 2019, 20, 6334. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.X.; Hou, Z.N.; He, Q.L.; Zhang, X.M.; Yan, K.J.; Han, R.L.; Liang, Z.S. Genome-wide characterization and expression analysis of bZIP gene family under abiotic stress in Glycyrrhiza uralensis. Front. Genet. 2021, 12, 754237. [Google Scholar] [CrossRef]
- Pan, C.Y.; Ye, H.F.; Zhou, W.Y.; Wan, S.; Li, M.J.; Lu, M.; Li, S.F.; Zhu, X.D.; Wang, Y.X.; Rao, Y.C. QTL mapping of candidate genes involved in Cd accumulation in rice grain. Chin. Bull. Bot. 2021, 56, 8. [Google Scholar]
- Wolfgang, D.L.; Snoek, B.L.; Berend, S.; Christoph, W. The Arabidopsis bZIP transcription factor family—An update. Curr. Opin. Plant Biol. 2018, 45, 36–49. [Google Scholar]
- Fang, H.H.; Liu, Z.Q.; Long, Y.P.; Liang, Y.L.; Jin, Z.P.; Pei, Y.X. The Ca2+/calmodulin2-binding transcription factor TGA3 elevates LCD expression and H2S production to bolster Cr6+ tolerance in Arabidopsis. Plant J. 2017, 91, 1038–1050. [Google Scholar] [CrossRef] [Green Version]
- Farinati, S.; Dalcorso, G.; Varotto, S.; Furini, A. The Brassica juncea BjCdR15, an ortholog of Arabidopsis TGA3, is a regulator of cadmium uptake, transport and accumulation in shoots and confers cadmium tolerance in transgenic plants. New Phytol. 2010, 185, 964–978. [Google Scholar] [CrossRef]
- Huang, C.J.; Zhou, J.H.; Jie, Y.C.; Xing, H.C.; Zhong, Y.L.; Yu, W.L.; She, W.; Ma, Y.S.; Liu, Z.H.; Zhang, Y. A ramie bZIP transcription factor BnbZIP2 is involved in drought, salt, and heavy metal stress response. DNA Cell Biol. 2016, 35, 776–786. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, R.L.; Ju, Q.; Li, W.Q.; Tran, L.S.P.; Xu, J. The R2R3-MYB transcription factor MYB49 regulates cadmium accumulation. Plant Physiol. 2019, 180, 01380–2018. [Google Scholar] [CrossRef]
- Lilay, G.H.; Persson, D.P.; Castro, P.H.; Liao, F.; Assuno, A. ArabidopsisbZIP19 and bZIP23 act as zinc sensors to control plant zinc status. Nat. Plants 2021, 7, 137–143. [Google Scholar] [CrossRef]
- Lilay, G.H.; Castro, P.H.; Campilho, A.; Assunção, A.G. The Arabidopsis bZIP19 and bZIP23 activity requires zinc deficiency-insight on regulation from complementation lines. Front. Plant Sci. 2018, 9, 1955. [Google Scholar] [CrossRef]
- Hu, S.B.; Yu, Y.; Chen, Q.H.; Mu, G.M.; Shen, Z.G.; Zheng, L.Q. OsMYB45 plays an important role in rice resistance to cadmium stress. Plant Sci. 2017, 264, 1–8. [Google Scholar] [CrossRef]
- Sapara, K.K.; Khedia, J.; Agarwal, P.; Gangapur, D.R.; Agarwal, P.K. SbMYB15 transcription factor mitigates cadmium and nickel stress in transgenic tobacco by limiting uptake and modulating antioxidative defence system. Funct. Plant Biol. 2019, 46, 702–714. [Google Scholar] [CrossRef]
- Agarwal, P.; Mitra, M.; Banerjee, S.; Roy, S. MYB4 transcription factor, a member of R2R3-subfamily of MYB domain protein, regulates cadmium tolerance via enhanced protection against oxidative damage and increases expression of PCS1 and MT1C in Arabidopsis. Plant Sci. 2020, 297, 110501. [Google Scholar] [CrossRef]
- Xu, Z.G.; Ge, Y.; Zhang, W.; Zhao, Y.L.; Yang, G.Y. The walnut JrVHAG1 gene is involved in cadmium stress response through ABA-signal pathway and MYB transcription regulation. BMC Plant Biol. 2018, 18, 19. [Google Scholar] [CrossRef] [Green Version]
- Van de Mortel, J.E.; Schat, H.; Moerland, P.D.; Van Themaat, E.V.L.; Van Der Ent, S.J.O.E.R.D.; Blankestijn, H.; Ghandilyan, A.; Tsiatsiani, S.; Aarts, M.G. Expression differences for genes involved in lignin, glutathione and sulphate metabolism in response to cadmium in Arabidopsis thaliana and the related Zn/Cd-hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 2010, 31, 301–324. [Google Scholar] [CrossRef]
- Wang, F.Z.; Chen, M.X.; Yu, L.J.; Xie, L.J.; Yuan, L.B.; Qi, H.; Xiao, M.; Guo, W.; Zhe, C.; Yi, K. OsARM1, an R2R3 MYB transcription factor, is involved in regulation of the response to Arsenic stress in rice. Front. Plant Sci. 2017, 8, 1868. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, H.Y.; Chen, Y.F. The transcription factor MYB40 is a central regulator in arsenic resistance in Arabidopsis. Plant Commun. 2021, 2, 100234. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wu, X.M.; Ling, H.Q.; Yang, W.C. Transgenic expression of DwMYB2 impairs iron transport from root to shoot in Arabidopsis thaliana. Cell Res. 2006, 16, 830–840. [Google Scholar] [CrossRef] [Green Version]
- Karanja, B.K.; Fan, L.; Xu, L.; Wang, Y.; Zhu, X.; Tang, M.; Wang, R.; Zhang, F.; Muleke, E.M.; Liu, L. Genome-wide characterization of the WRKY gene family in radish (Raphanus sativus L.) reveals its critical functions under different abiotic stresses. Plant Cell Rep. 2017, 36, 1757–1773. [Google Scholar] [CrossRef]
- Han, Y.Y.; Fan, T.T.; Zhu, X.Y.; Wu, X.; Ouyang, J.; Jiang, L.; Cao, S.Q. WRKY12 represses GSH1 expression to negatively regulate cadmium tolerance in Arabidopsis. Plant Mol. Biol. 2019, 99, 149–159. [Google Scholar] [CrossRef]
- Sheng, Y.B.; Yan, X.X.; Huang, Y.Y.; Han, Y.; Zhang, C.; Ren, Y.B.; Fan, T.T.; Xiao, F.M.; Liu, Y.S.; Cao, S.Q. The WRKY transcription factor, WRKY13, activates PDR8 expression to positively regulate cadmium tolerance in Arabidopsis. Plant Cell Environ. 2019, 42, 891–903. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, W.; Ji, T.T.; Ye, L.; Lu, Y.T.; Yuan, T.T. WRKY13 enhances cadmium tolerance by promoting D-CYSTEINE DESULFHYDRASE and hydrogen sulfide production. Plant Physiol. 2020, 183, 345–357. [Google Scholar] [CrossRef]
- Cai, Z.D.; Xian, P.Q.; Wang, H.; Lin, R.B.; Lian, T.X.; Cheng, Y.B.; Ma, Q.B.; Nian, H. Transcription factor GmWRKY142 confers cadmium resistance by up-regulating the cadmium tolerance 1-like genes. Front. Plant Sci. 2020, 11, 724. [Google Scholar] [CrossRef]
- Li, C.X.; Yan, J.Y.; Ren, J.Y.; Sun, L.; Xu, C.; Li, G.X.; Ding, Z.J.; Zheng, S.J. A WRKY transcription factor confers aluminum tolerance via regulation of cell wall modifying genes. J. Integr. Plant Biol. 2020, 62, 1176–1192. [Google Scholar] [CrossRef]
- Castrillo, G.; Sanchez-Bermejo, E.; de Lorenzo, L.; Crevillen, P.; Fraile-Escanciano, A.; Tc, M.; Mouriz, A.; Catarecha, P.; Sobrino-Plata, J.; Olsson, S. WRKY6 transcription factor restricts arsenate uptake and transposon activation in Arabidopsis. Plant Cell 2013, 25, 2944–2957. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.S.; Jiang, J.; Han, X.J.; Zhang, Y.X.; Zhuo, R.Y. Identification, expression analysis of the Hsf family, and characterization of class A4 in Sedum alfredii Hance under cadmium stress. Int. J. Mol. Sci. 2018, 19, 1216. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.S.; Yu, M.; Li, H.; Wang, Y.; Lu, Z.C.; Zhang, Y.X.; Liu, M.Y.; Qiao, G.R.; Wu, L.H.; Han, X.J. SaHsfA4c from Sedum alfredii Hance enhances cadmium tolerance by regulating ROS-scavenger activities and heat shock proteins expression. Front. Plant Sci 2020, 11, 142. [Google Scholar] [CrossRef] [Green Version]
- Shim, D.; Hwang, J.U.; Lee, J.; Lee, S.; Choi, Y.; An, G.; Martinoia, E.; Lee, Y. Orthologs of the class A4 heat shock transcription factor HsfA4a confer cadmium tolerance in wheat and rice. Plant Cell 2009, 21, 4031–4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gang, S.; Yuan, S.; Wen, X.; Xie, Z.; Lou, L.; Hu, B.; Cai, Q.; Xu, B. Transcriptome analysis of Cd-treated switchgrass root revealed novel transcripts and the importance of HSF/HSP network in switchgrass Cd tolerance. Plant Cell Rep. 2018, 37, 1485–1497. [Google Scholar]
- Cai, S.Y.; Zhang, Y.; Xu, Y.P.; Qi, Z.Y.; Li, M.Q.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal. Res. 2017, 62, e12387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Yang, J.L.; Li, W.L.; Chen, Y.X.; Lu, H.; Zhao, S.C.; Li, D.D.; Wei, M.; Li, C.H. PuHSFA4a enhances tolerance to excess Zn by regulating ROS production and root development in Populus. Plant Physiol. 2019, 180, 1495. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; He, F.; Zhu, B.; Ren, M.J.; Tang, H. NAC transcription factors from Aegilops markgrafii reduce cadmium concentration in transgenic wheat. Plant Soil 2020, 449, 39–50. [Google Scholar] [CrossRef]
- Lou, H.Q.; Fan, W.; Jin, J.F.; Xu, J.M.; Chen, W.W.; Yang, J.L.; Zheng, S.J. A NAC-type transcription factor confers aluminium resistance by regulating cell wall-associated receptor kinase 1 and cell wall pectin. Plant Cell Environ. 2020, 43, 463–478. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.B.; Yan, X.X.; Liu, Y.L.; Wang, R.; Fan, T.T.; Ren, Y.B.; Tang, X.F.; Xiao, F.M.; Liu, Y.S. Zinc-finger transcription factor ZAT6 positively regulates cadmium tolerance through the Glutathione-Dependent pathway in Arabidopsis. Plant Physiol. 2016, 171, 707–719. [Google Scholar] [CrossRef]
- Yao, X.; Cai, Y.R.; Yu, D.Q.; Liang, G. bHLH104 confers tolerance to cadmium stress in Arabidopsis thaliana. J. Integr. Plant Biol. 2018, 60, 69–80. [Google Scholar] [CrossRef]
- Wu, H.L.; Chen, C.L.; Du, J.; Liu, H.F.; Cui, Y.; Zhang, Y.; He, Y.J.; Wang, Y.Q.; Chu, C.C.; Feng, Z.Y. Co-overexpression FIT with AtbHLH38 or AtbHLH39 in Arabidopsis-enhanced cadmium tolerance via increased cadmium sequestration in roots and improved iron homeostasis of shoots. Plant Physiol. 2012, 158, 790–800. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.T.; Yang, W.M.; Lu, W.; Wang, Y.; Qi, X.T. Transcription factors PvERF15 and PvMTF-1 form a cadmium stress transcriptional pathway. Plant Physiol. 2017, 173, 1565. [Google Scholar] [CrossRef] [Green Version]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Li, N.N.; Wang, J.C.; Song, W.Y. Arsenic uptake and translocation in plants. Plant Cell Physiol. 2016, 57, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.; Ghafoor, A.; Du, X. Insights into drought stress signaling in plants and the molecular genetic basis of cotton drought tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, M.; Wang, P.; Cox, K.J.; Duan, L.; Dever, J.K.; Shan, L.; Li, Z.; He, P. Regulation of cotton (Gossypium hirsutum) drought responses by mitogen-activated protein (MAP) kinase cascade-mediated phosphorylation of GhWRKY59. New Phytol. 2017, 215, 1462–1475. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Liu, G.D.; Sarkhosh, A.; Fernandez-Zapata, J.C.; Martinez, N.J.; Garcia-Sanchez, F. Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef]
- Neeta, L.; Mohan, B.S.; Prem, L.B. Biological parts for engineering abiotic stress tolerance in plants. BioDesign Res. 2022, 2022, 41. [Google Scholar]
| Signals | Heavy Metal | Plant | MAPK | Reference |
|---|---|---|---|---|
| ROS | - | Arabidopsis thaliana | MEKK1-MKK4/5-MPK3/6 | [25] |
| - | Arabidopsis thaliana | MEKK1-MKK2-MPK4/6 | [26] | |
| Cu, Cd | Medicago sativa | SIMK, MMK2, MMK3 and SAMK | [27] | |
| Cu | Arabidopsis thaliana | MAPK | [28] | |
| Cd | Zea mays | ZmMPK3-1/ZmMPK6-1 | [29] | |
| Cd | Arabidopsis thaliana | MPK3 and MPK6 | [30] | |
| NO | - | Arabidopsis thaliana | AtWAKL10 | [31] |
| - | MAPK | [32] | ||
| Cd | Arabidopsis thaliana | MAPK | [33] | |
| Cd | Arabidopsis thaliana | MPK6 | [34] | |
| Ar | Oryza sativa | MAPK/MPK | [35] | |
| Hormone | ||||
| Arabidopsis thaliana | AtMPK3/AtMPK6 | [36] | ||
| SA | - | Nicotiana tabacum | SIPK | [37] |
| ABA/IAA | Cd | Oryza sativa | MAPK | [38] |
| ET | Cd | Glycine max | MAPK/MAPKK2 | [32] |
| Family | Genes | Heavy Metals | Function | Number of Phosphorylation Sites | Reference |
|---|---|---|---|---|---|
| bZIP | RsbZIP010 | Cd, Cr and Pb | RsbZIP010 exhibited downregulated expression under Cd, Cr and Pb stresses. | [46] | |
| GubZIP | Cd | GubZIPs were expressed specifically in different tissues under cadmium stress | [47] | ||
| LOC_Os02g52780/OsbZIP23 | Cd | LOC_Os02g52780 related to the tolerance of rice to Cd stress and affected Cd accumulation in rice grains. | 39 | [48] | |
| BjCdR15 | Cr | TGA3 elevates LCD expression and H2S production to bolster Cr6+ tolerance in Arabidopsis. | 35 | [49,50,51] | |
| BnbZIP2 BnbZIP3 | Cd | Over expression of BnbZIP2 exhibited more sensitivity to drought and heavy metal Cd stress. | 0/44 | [52] | |
| ABI5 | Cd | ABI5 interacts with MYB49 and prevented its binding to the downstream genes, resulting in inactivation of IRT1 and reduced Cd uptake. | 46 | [53] | |
| bZIP19,23 | Zn | Zinc sensors to control plant zinc status. | 25/17 | [54,55] | |
| MYB | OsMYB45 | Cd | Under Cd stress, OsMYB45 is highly expressed. Mutation of OsMYB45 resulted in hypersensitivity to Cd treatment. | 37 | [56] |
| SbMYB15 | Cd, Ni | Overexpression of SbMYB15 conferred. Cadmium and nickel tolerance in transgenic tobacco | 45 | [57] | |
| AtMYB4 | Cd | MYB4 regulates Cd-tolerance via the coordinated activity of improved anti-oxidant defense systems and through the enhanced expression of PCS1 and MT1C under Cd-stress in Arabidopsis. | 40 | [58] | |
| JrMYB2 | Cd | JrMYB2 acts as an upstream regulator of JrVHAG1 to improve CdCl2 stress tolerance stress tolerance. | [59] | ||
| AtMYB72 | Zn, Fe | The Arabidopsis MYB72 knockout mutant was more sensitive to excess Zn or Fe deficiency than wild-type. | 43 | [60] | |
| OsARM1 | As | OsARM1 regulates arsenic absorption and root-to-shoot translocation. | 19 | [61] | |
| AtMYB40 | As | AtMYB40 enhances plant As (V) tolerance and reduces As(V) uptake. | 28 | [62] | |
| DwMYB2 | Fe | The translocation of iron from root to shoot is affected by the DwMYB2. | 39 | [63] | |
| WRKY | RsWRKY | Cd | RsWRKY transcripts were significantly elevated under Cd and Pb treatments. | [64] | |
| AtWRKY12 | Cd | WRKY12 represses GSH1 expression to negatively regulates cadmium tolerance in Arabidopsis. | 31 | [65] | |
| AtWRKY13 | Cd | Activates PDR8 expression to positively regulate cadmium tolerance in Arabidopsis. | 49 | [66] | |
| AtWRKY13 | Cd | WRKY13 activation of DCD during cadmium stress. | 49 | [67] | |
| GmWRKY142 | Cd | GmWRKY142 confers cadmium resistance by upregulating the cadmium tolerance 1-like genes. | 54 | [68] | |
| AtWRKY47 | Al | A WRKY transcription factor confers aluminum tolerance via regulation of cell wall modifying genes. | 63 | [69] | |
| AtWRKY6 | As | WRKY6 transcription factor restricts arsenate uptake and transposon activation in Arabidopsis. | 66 | [70] | |
| HSF | SaHsfA4c | Cd | The expression of SaHsfA4c was induced by cadmium and enhanced Cd tolerance by ROS -scavenger activities and shock proteins expression. | 39 | [71,72] |
| TaHsfA4a OsHsfA4a | Cd | HsfA4a of wheat and rice confers Cd tolerance by upregulating MT gene expression. | 46 | [73] | |
| PvBip1 | Cd | HSF/HSP participates in the reconstruction of protein conformation and improves intracellular homeostasis to increase cadmium tolerance. | 64 | [74] | |
| HSF1A | Cd | HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. | 55 | [75] | |
| PuHSFA4a | Zn | PuHSFA4 activates the antioxidant system and root development–related genes and directly targets PuGSTU17 and PuPLA. | 39 | [76] | |
| Others | AemNAC2 | Cd | Overexpression of AemNAC2 led to reduced cadmium concentration. | 52 | [77] |
| VuNAR1 | Al | VuNAR1 regulates Al resistance by regulating cell wall pectin metabolism via directly binding to the promoter of WAK1 and inducing its expression. | 24 | [78] | |
| ZAT6 | Cd | ZAT6 coordinately activates PC synthesis–related gene expression and directly targets GSH1 to positively regulate Cd accumulation and tolerance in Arabidopsis. | 40 | [79] | |
| AtbHLH104 AtbHLH38 AtbHLH39 | Cd | AtbHLHs positively regulates genes involved in heavy metal absorption and detoxification. | 27/27/35 | [80,81] | |
| HIPP22 | Cd | MYB49 binds to the promoter regions of the HIPP22 and HIPP44, resulting in upregulation Cd accumulation. | 14 | [76] | |
| PvERF15 | Cd | PvERF15 and PvMTF-1 form a cadmium-stress transcriptional pathway. | 44 | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Han, X.; Lu, Z.; Qiu, W.; Yu, M.; Li, H.; He, Z.; Zhuo, R. MAPK Cascades and Transcriptional Factors: Regulation of Heavy Metal Tolerance in Plants. Int. J. Mol. Sci. 2022, 23, 4463. https://doi.org/10.3390/ijms23084463
Li S, Han X, Lu Z, Qiu W, Yu M, Li H, He Z, Zhuo R. MAPK Cascades and Transcriptional Factors: Regulation of Heavy Metal Tolerance in Plants. International Journal of Molecular Sciences. 2022; 23(8):4463. https://doi.org/10.3390/ijms23084463
Chicago/Turabian StyleLi, Shaocui, Xiaojiao Han, Zhuchou Lu, Wenmin Qiu, Miao Yu, Haiying Li, Zhengquan He, and Renying Zhuo. 2022. "MAPK Cascades and Transcriptional Factors: Regulation of Heavy Metal Tolerance in Plants" International Journal of Molecular Sciences 23, no. 8: 4463. https://doi.org/10.3390/ijms23084463
APA StyleLi, S., Han, X., Lu, Z., Qiu, W., Yu, M., Li, H., He, Z., & Zhuo, R. (2022). MAPK Cascades and Transcriptional Factors: Regulation of Heavy Metal Tolerance in Plants. International Journal of Molecular Sciences, 23(8), 4463. https://doi.org/10.3390/ijms23084463






