Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions
Abstract
:1. Introduction
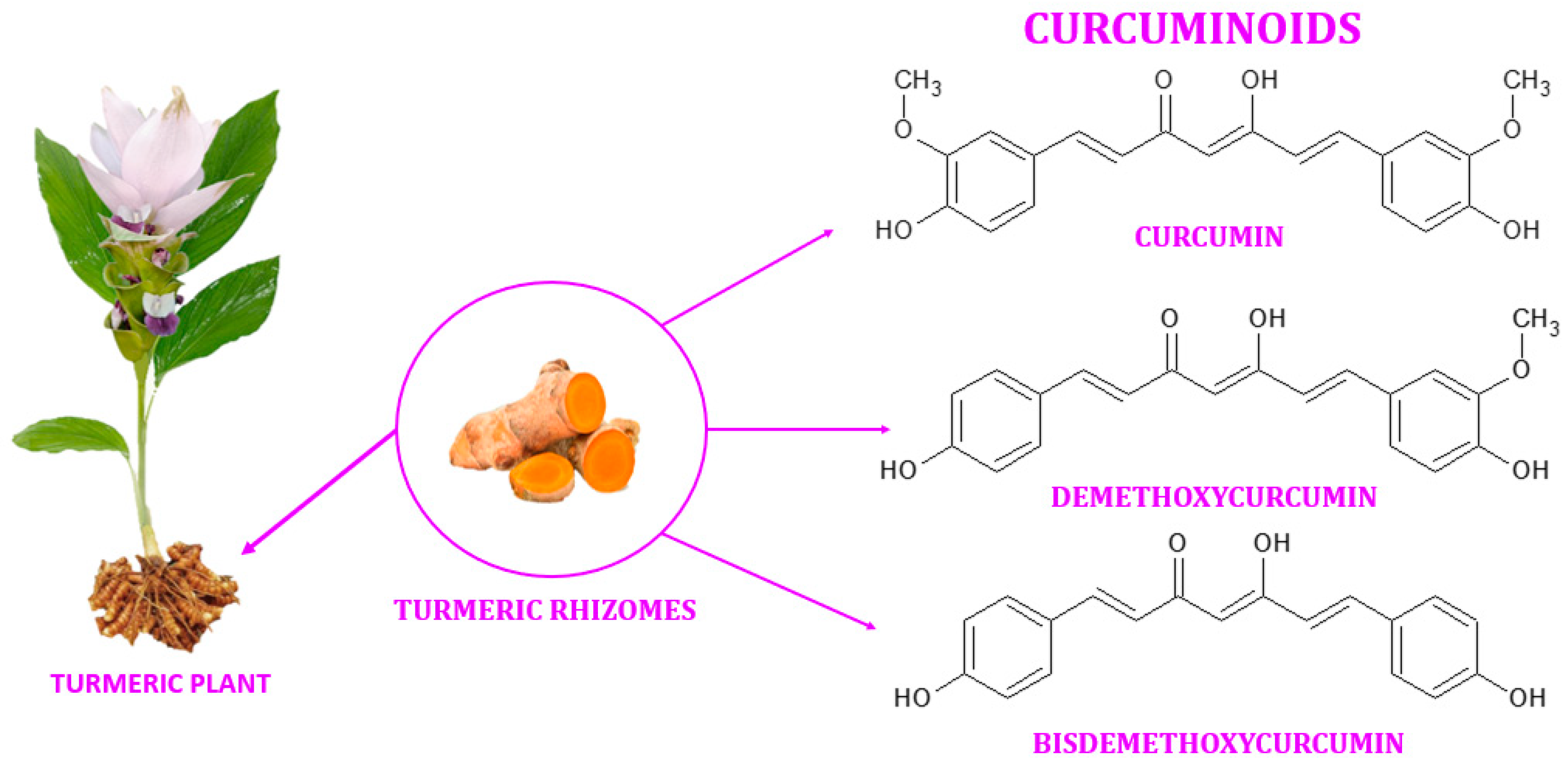
2. Chemistry of Curcumin
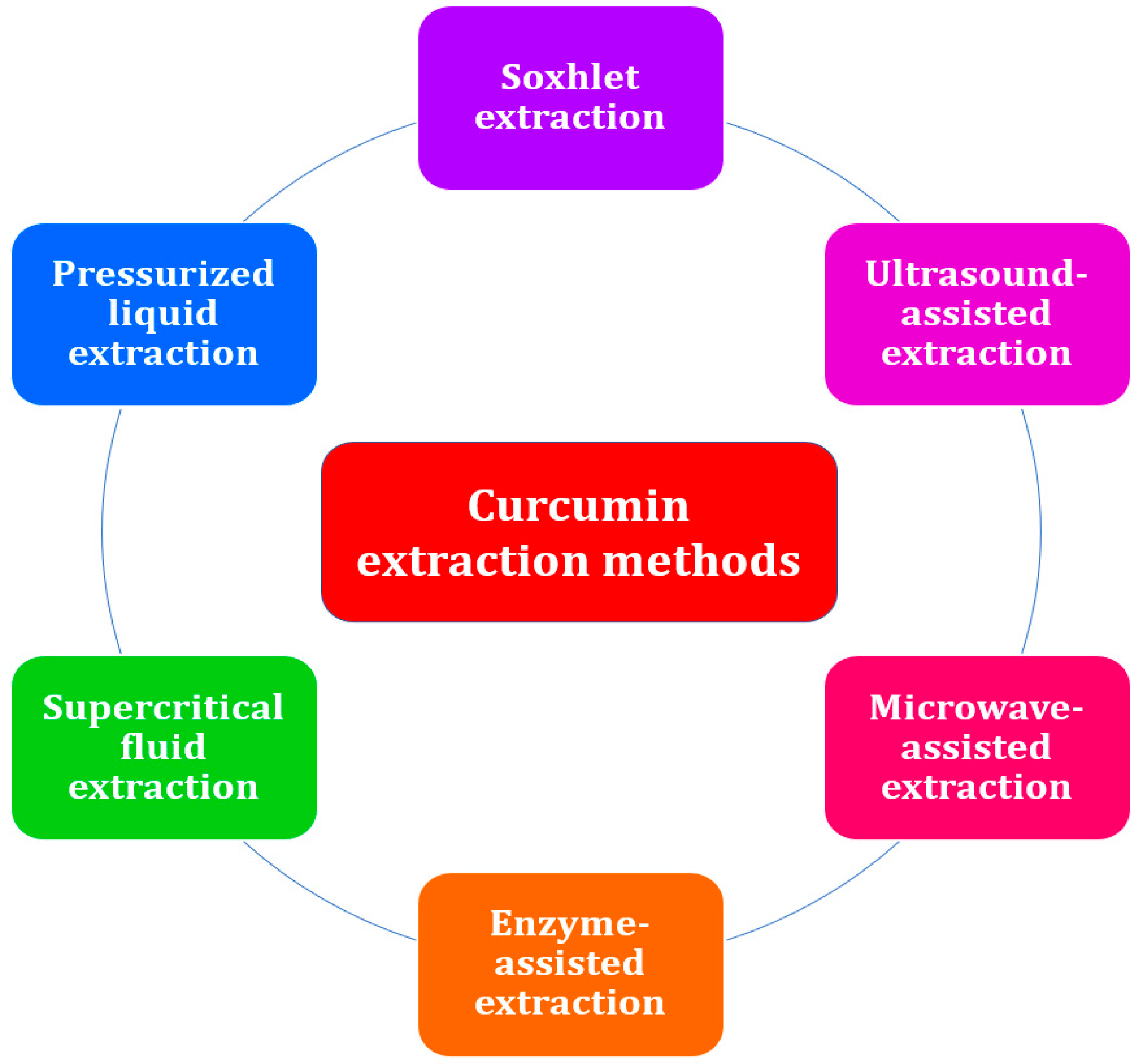
3. Separation of Curcumin from Turmeric
3.1. Solvent Extraction
3.2. Soxhlet Extraction
3.3. Ultrasound-Assisted Extraction
3.4. Microwave-Assisted Extraction
3.5. Enzyme-Assisted Extraction
3.6. Supercritical Fluid Extraction
3.7. Pressurized Liquid Extraction
4. Methods for Identification and Characterization of Curcumin in Plant Extracts
4.1. Spectroscopic Methods
4.2. Chromatographic Methods
4.3. Other Methods
5. Health Benefits of Curcumin
5.1. Antioxidant Properties
5.2. Antibacterial Properties
5.3. Antidepressant Properties
5.4. Diabetes Mellitus
5.5. Anticancer Properties
5.6. Side Effects and Potential Toxicity
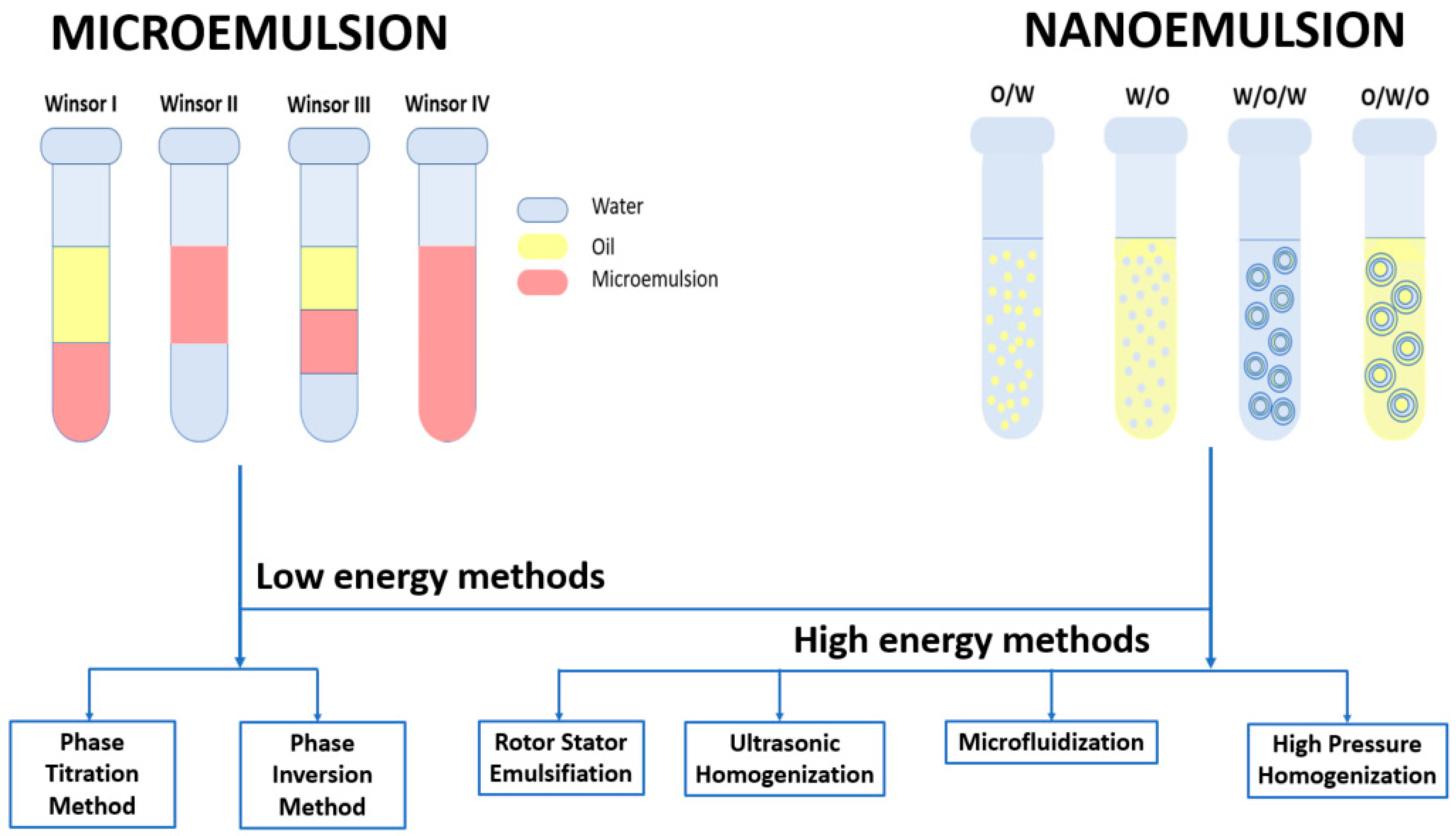
6. Nanoscale Colloidal Systems for Curcumin Delivery
6.1. Preparation of Microemulsions and Nanoemulsions
6.1.1. Phase Titration Method
6.1.2. Phase Inversion Method
6.1.3. Rotor-Stator Emulsification
6.1.4. Ultrasonic Homogenization
6.1.5. Microfluidization
6.1.6. High Pressure Homogenization
6.2. Microemulsions and Nanoemulsions for Curcumin Delivery
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jyotirmayee, B.; Mahalik, G. A review on selected pharmacological activities of Curcuma longa L. Int. J. Food Prop. 2022, 25, 1377–1398. [Google Scholar] [CrossRef]
- Akter, J.; Islam, M.Z.; Takara, K.; Hossain, M.A.; Gima, S.; Hou, D.X. Plant growth inhibitors in turmeric (Curcuma longa) and their effects on Bidens pilosa. Weed Biol. Manage. 2018, 18, 136–145. [Google Scholar] [CrossRef]
- Tavares, K.; Kirk, E.; Motomura-Wages, S.; Calpito, J.; Bingham, J.-P.; Ahmad, A.A.; Flanagan, K.; Uyeda, J.; Kantar, M.B.; Radovich, T.J.K. Genotypic and Environmental Influence on Fresh Rhizome Yield of Turmeric (Curcuma longa L.). Agronomy 2022, 12, 2703. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Sontsa-Donhoung, A.M.; Bahdjolbe, M.; Hawaou; Nwaga, D. Selecting Endophytes for Rhizome Production, Curcumin Content, Biocontrol Potential, and Antioxidant Activities of Turmeric (Curcuma longa). Biomed. Res. Int. 2022, 2022, 8321734. [Google Scholar] [CrossRef]
- Park, J.; Do, S.; Lee, M.; Ha, S.; Lee, K.-G. Preparation of turmeric powder with various extraction and drying methods. Chem. Biol. Technol. Agric. 2022, 9, 39. [Google Scholar] [CrossRef]
- Jiang, T.; Ghosh, R.; Charcosset, C. Extraction, purification and applications of curcumin from plant materials-A comprehensive review. Trends Food Sci. Technol. 2021, 112, 419–430. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Kaushik, M.S.; Mishra, S.K.; Raj, P.; Singh, P.K.; Pandey, K.D. Interaction of turmeric (Curcuma longa L.) with beneficial microbes: A review. 3 Biotech 2017, 7, 357. [Google Scholar] [CrossRef]
- Srivastava, B.B.L.; Ripanda, A.S.; Mwanga, H.M. Ethnomedicinal, Phytochemistry and Antiviral Potential of Turmeric (Curcuma longa). Compounds 2022, 2, 200–221. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocinska, K.; Zielinska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Rathore, M.M.S.; Sharma, P.; Devi, S.; Nagar, J.C.; Khalid, M. Curcumin: A Review for Health Benefits. Int. J. Res. Rev. 2020, 7, 273–290. [Google Scholar]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Urosevic, M.; Nikolic, L.; Gajic, I.; Nikolic, V.; Dinic, A.; Miljkovic, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- El-Menyawy, E.M.; Raslan, M.; Zedan, I.T. Physical characteristics of naturally isolated high-purity curcumin and its application in photosensor appliances. J. Mol. Struct. 2023, 1274, 134445. [Google Scholar] [CrossRef]
- Wahyudi, L.D.; Yu, S.H.; Cho, M.K. The effect of curcumin on the cadmium-induced mitochondrial apoptosis pathway by metallothionein 2A regulation. Life Sci. 2022, 310, 121076. [Google Scholar] [CrossRef]
- Prakhongsil, S.S.P.; Pewlong, W.; Picha, R.; Thamrongsiripak, N. Turmeric Sprout Inhibition and Rhizomes Quality and Post-Harvest Treatment with Gamma Irradiation. Sci. Technol. Asia 2022, 27, 234–241. [Google Scholar] [CrossRef]
- Le-Tan, H.; Fauster, T.; Vladic, J.; Gerhardt, T.; Haas, K.; Jaeger, H. Application of Emerging Cell Disintegration Techniques for the Accelerated Recovery of Curcuminoids from Curcuma longa. Appl. Sci. 2021, 11, 8238. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Smirnova, E.; Karthikeyan, A.; Moniruzzaman, M.; Kalaiselvi, S.; Nam, K.; Goff, G.L.; Min, T. The impact of curcumin on livestock and poultry animal’s performance and management of insect pests. Front. Vet. Sci. 2023, 10, 1048067. [Google Scholar] [CrossRef]
- Lima, M.S.D.; Resende, O.; PlÁCido, G.R.; Silva, J.A.G.E.; CÉLia, J.A.; Caliari, M.; Oliveira, D.E.C.D.; Correia, J.S.; Silva, M.A.P.D. Effects of drying temperature on the bioactive and technological properties of turmeric (Curcuma longa L.) flour. Food Sci. Technol. 2022, 42, e76122. [Google Scholar] [CrossRef]
- Salem, M.A.; El-Shiekh, R.A.; Fernie, A.R.; Alseekh, S.; Zayed, A. Metabolomics-based profiling for quality assessment and revealing the impact of drying of Turmeric (Curcuma longa L.). Sci. Rep. 2022, 12, 10288. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Paucar, A.M.; Machado-Orellana, M.G. Pharmacological Profile, Bioactivities, and Safety of Turmeric Oil. Molecules 2022, 27, 5055. [Google Scholar] [CrossRef] [PubMed]
- Sands, D.C.; Carsky, M.; Donovan, E.; Virgilio, L.L.; Ewart, K.V. Interaction of curcumin with a winter flounder alpha-helical antifreeze protein. Biochem. Biophys. Res. Commun. 2022, 630, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.; Tarafdar, A.; Kumar, D.; Kumar, Y.; Rana, J.S.; Badgujar, P.C. Formulation and characterization of nano-curcumin fortified milk cream powder through microfluidization and spray drying. Food Res. Int. 2022, 160, 111705. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Agrawal, N.; Jaiswal, M. Bioavailability enhancement of curcumin via esterification processes: A review. Eur. J. Med. Chem. Rep. 2022, 6, 100081. [Google Scholar] [CrossRef]
- Hen-Avivi, S.; Savin, O.; Racovita, R.C.; Lee, W.S.; Adamski, N.M.; Malitsky, S.; Almekias-Siegl, E.; Levy, M.; Vautrin, S.; Berges, H.; et al. A Metabolic Gene Cluster in the Wheat W1 and the Barley Cer-cqu Loci Determines beta-Diketone Biosynthesis and Glaucousness. Plant Cell 2016, 28, 1440–1460. [Google Scholar] [CrossRef]
- Racovita, R.C.; Jetter, R. Identification of Polyketides in the Cuticular Waxes of Triticum aestivum cv. Bethlehem. Lipids 2016, 51, 1407–1420. [Google Scholar] [CrossRef]
- Racovita, R.C.; Hen-Avivi, S.; Fernandez-Moreno, J.P.; Granell, A.; Aharoni, A.; Jetter, R. Composition of cuticular waxes coating flag leaf blades and peduncles of Triticum aestivum cv. Bethlehem. Phytochemistry 2016, 130, 182–192. [Google Scholar] [CrossRef]
- Racovita, R.C.; Jetter, R. Identification of In-Chain-Functionalized Compounds and Methyl-Branched Alkanes in Cuticular Waxes of Triticum aestivum cv. Bethlehem. PLoS ONE 2016, 11, e0165827. [Google Scholar] [CrossRef] [PubMed]
- Mague, J.T.; Alworth, W.L.; Payton, F.L. Curcumin and derivatives. Acta Cryst. C 2004, 60, o608–o610. [Google Scholar] [CrossRef] [PubMed]
- Al-Noor, T.; Ali, A.; Al-Sarray, A.; Al-Obaidi, O.; Obeidat, A.; Habash, R. A Short Review: Chemistry of Curcumin and Its Metal Complex Derivatives. J. Univ. Anbar Pure Sci. 2022, 16, 20–26. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, S.; Moulik, S.P. Stability of curcumin in different solvent and solution media: UV-visible and steady-state fluorescence spectral study. J. Photochem. Photobiol. B 2016, 158, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Ojo, O.A.; Adeyemo, T.R.; Rotimi, D.; Batiha, G.E.; Mostafa-Hedeab, G.; Iyobhebhe, M.E.; Elebiyo, T.C.; Atunwa, B.; Ojo, A.B.; Lima, C.M.G.; et al. Anticancer Properties of Curcumin Against Colorectal Cancer: A Review. Front. Oncol. 2022, 12, 881641. [Google Scholar] [CrossRef]
- Nebrisi, E.E. Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 1248. [Google Scholar] [CrossRef]
- Al Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis. Molecules 2022, 27, 604. [Google Scholar] [CrossRef]
- Idris, F.N.; Nadzir, M.M. Comparative Studies on Different Extraction Methods of Centella asiatica and Extracts Bioactive Compounds Effects on Antimicrobial Activities. Antibiotics 2021, 10, 457. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Carrera, C.; Palma, M.; Barroso, C.G. Alternative Extraction Method of Bioactive Compounds from Mulberry (Morus nigra L.) Pulp Using Pressurized-Liquid Extraction. Food Anal. Methods 2018, 11, 2384–2395. [Google Scholar] [CrossRef]
- Piasecka, I.; Wiktor, A.; Górska, A. Alternative Methods of Bioactive Compounds and Oils Extraction from Berry Fruit By-Products—A Review. Appl. Sci. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Egues, I.; Hernandez-Ramos, F.; Rivilla, I.; Labidi, J. Optimization of Ultrasound Assisted Extraction of Bioactive Compounds from Apple Pomace. Molecules 2021, 26, 3783. [Google Scholar] [CrossRef]
- Cavalluzzi, M.M.; Lamonaca, A.; Rotondo, N.P.; Miniero, D.V.; Muraglia, M.; Gabriele, P.; Corbo, F.; De Palma, A.; Budriesi, R.; De Angelis, E.; et al. Microwave-Assisted Extraction of Bioactive Compounds from Lentil Wastes: Antioxidant Activity Evaluation and Metabolomic Characterization. Molecules 2022, 27, 7471. [Google Scholar] [CrossRef] [PubMed]
- Łubek-Nguyen, A.; Ziemichód, W.; Olech, M. Application of Enzyme-Assisted Extraction for the Recovery of Natural Bioactive Compounds for Nutraceutical and Pharmaceutical Applications. Appl. Sci. 2022, 12, 3232. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Carpena, M.; Barciela, P.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A. Pressurized Liquid Extraction for the Recovery of Bioactive Compounds from Seaweeds for Food Industry Application: A Review. Antioxidants 2023, 12, 612. [Google Scholar] [CrossRef]
- Geow, C.H.; Tan, M.C.; Yeap, S.P.; Chin, N.L. A Review on Extraction Techniques and Its Future Applications in Industry. Eur. J. Lipid Sci. Technol. 2021, 123, 2000302. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- López-Olmos, C.; García-Valverde, M.T.; Hidalgo, J.; Ferrerio-Vera, C.; de Medina, V.S. Comprehensive comparison of industrial cannabinoid extraction techniques: Evaluation of the most relevant patents and studies at pilot scale. Front. Nat. Prod. 2022, 1, 1043147. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Bhatti, S.A.; Hussain, M.H.; Mohsin, M.Z.; Mohsin, A.; Zaman, W.Q.; Guo, M.; Iqbal, M.W.; Siddiqui, S.A.; Ibrahim, S.A.; Ur-Rehman, S.; et al. Evaluation of the antimicrobial effects of Capsicum, Nigella sativa, Musa paradisiaca L., and Citrus limetta: A review. Front. Sustain. Food Syst. 2022, 6, 1043823. [Google Scholar] [CrossRef]
- Ali, A.; Riaz, S.; Sameen, A.; Naumovski, N.; Iqbal, M.W.; Rehman, A.; Mehany, T.; Zeng, X.-A.; Manzoor, M.F. The Disposition of Bioactive Compounds from Fruit Waste, Their Extraction, and Analysis Using Novel Technologies: A Review. Processes 2022, 10, 2014. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sable, S.S.; Gaikwad, S.G.; Gogate, P.R. Ultrasound assisted curcumin recovery from Curcuma aromatica: Understanding the effect of different operating parameters. Chem. Eng. Process.-Process Intensif. 2021, 169, 108604. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.; Forster-Carneiro, T.; Vazquez-Espinosa, M.; Gonzalez-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Sahne, F.; Najafpour, G.D.; Moghadamnia, A.A. Extraction of bioactive compound curcumin from turmeric (Curcuma longa L.) via different routes: A comparative study. Pak. J. Biotechnol. 2016, 13, 173–180. [Google Scholar]
- Mehta, N.; Kumar, P.; Verma, A.K.; Umaraw, P.; Khatkar, S.K.; Khatkar, A.B.; Pathak, D.; Kaka, U.; Sazili, A.Q. Ultrasound-Assisted Extraction and the Encapsulation of Bioactive Components for Food Applications. Foods 2022, 11, 2973. [Google Scholar] [CrossRef]
- Singh, K.; Srichairatanakool, S.; Chewonarin, T.; Prommaban, A.; Samakradhamrongthai, R.S.; Brennan, M.A.; Brennan, C.S.; Utama-Ang, N. Impact of Green Extraction on Curcuminoid Content, Antioxidant Activities and Anti-Cancer Efficiency (In Vitro) from Turmeric Rhizomes (Curcuma longa L.). Foods 2022, 11, 3633. [Google Scholar] [CrossRef]
- Lopeda-Correa, M.; Valdes-Duque, B.E.; Osorio-Tobon, J.F. Ultrasound-Assisted Extraction of Phenolic Compounds from Adenaria floribunda Stem: Economic Assessment. Foods 2022, 11, 2904. [Google Scholar] [CrossRef]
- Insuan, W.; Hansupalak, N.; Chahomchuen, T. Extraction of curcumin from turmeric by ultrasonic-assisted extraction, identification, and evaluation of the biological activity. J. Herbmed. Pharmacol. 2022, 11, 188–196. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Pang, C.; Li, X.; Gao, X. The Advances in the Special Microwave Effects of the Heterogeneous Catalytic Reactions. Front. Chem. 2020, 8, 355. [Google Scholar] [CrossRef]
- Le-Tan, H.; Jaeger, H. Impact of Cell Disintegration Techniques on Curcumin Recovery. Food Eng. Rev. 2022, 14, 655–672. [Google Scholar] [CrossRef]
- Fernandez-Marin, R.; Fernandes, S.C.M.; Andres, M.A.; Labidi, J. Microwave-Assisted Extraction of Curcuma longa L. Oil: Optimization, Chemical Structure and Composition, Antioxidant Activity and Comparison with Conventional Soxhlet Extraction. Molecules 2021, 26, 1516. [Google Scholar] [CrossRef]
- Lianza, M.; Marincich, L.; Antognoni, F. The Greening of Anthocyanins: Eco-Friendly Techniques for Their Recovery from Agri-Food By-Products. Antioxidants 2022, 11, 2169. [Google Scholar] [CrossRef]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaq, I.; Al Jbawi, E.; Saewan, S.A. Traditional and innovative approaches for the extraction of bioactive compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Wassie, T.; Niu, K.; Xie, C.; Wang, H.; Xin, W. Extraction Techniques, Biological Activities and Health Benefits of Marine Algae Enteromorpha prolifera Polysaccharide. Front. Nutr. 2021, 8, 747928. [Google Scholar] [CrossRef]
- Kapadia, P.; Newell, A.S.; Cunningham, J.; Roberts, M.R.; Hardy, J.G. Extraction of High-Value Chemicals from Plants for Technical and Medical Applications. Int. J. Mol. Sci. 2022, 23, 334. [Google Scholar] [CrossRef]
- Yixuan, L.; Qaria, M.A.; Sivasamy, S.; Jianzhong, S.; Daochen, Z. Curcumin production and bioavailability: A comprehensive review of curcumin extraction, synthesis, biotransformation and delivery systems. Ind. Crops Prod. 2021, 172, 114050. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Widmann, A.K.; Wahl, M.A.; Kammerer, D.R.; Daniels, R. Supercritical Fluid Extraction with CO2 of Curcuma longa L. in Comparison to Conventional Solvent Extraction. Pharmaceutics 2022, 14, 1943. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Kiamahalleh, M.V.; Najafpour-Darzi, G.; Rahimnejad, M.; Moghadamnia, A.A.; Kiamahalleh, M.V. High performance curcumin subcritical water extraction from turmeric (Curcuma longa L.). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1022, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.L.; Chung, M.S. Pilot-scale subcritical solvent extraction of curcuminoids from Curcuma long L. Food Chem. 2015, 185, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Tobón, J.F.; Carvalho, P.I.N.; Rostagno, M.A.; Petenate, A.J.; Meireles, M.A.A. Extraction of curcuminoids from deflavored turmeric (Curcuma longa L.) using pressurized liquids: Process integration and economic evaluation. J. Supercrit. Fluids 2014, 95, 167–174. [Google Scholar] [CrossRef]
- Dutta, B. Study of secondary metabolite constituents and curcumin contents of six different species of genus Curcuma. J. Med. Plants Stud. 2015, 3, 116–119. [Google Scholar]
- Shirsath, S.R.; Sable, S.S.; Gaikwad, S.G.; Sonawane, S.H.; Saini, D.R.; Gogate, P.R. Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: Effect of different operating parameters. Ultrason. Sonochem. 2017, 38, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Panichayupakaranant, P.; Lateh, L.; Yuenyongsawad, S.; Chen, H. A green method for preparation of curcuminoid-rich Curcuma longa extract and evaluation of its anticancer activity. Pharmacogn. Mag. 2019, 15, 1377–1398. [Google Scholar] [CrossRef]
- Kurmudle, N.; Kagliwal, L.D.; Bankar, S.B.; Singhal, R.S. Enzyme-assisted extraction for enhanced yields of turmeric oleoresin and its constituents. Food Biosci. 2013, 3, 36–41. [Google Scholar] [CrossRef]
- Chandra, A.; Rekhi, H.; Gautam, A.K.; Arya, R.K. Enzyme-assisted turmeric oil extraction from turmeric rhizomes. Chem. Process Eng. 2022, 43, 183–191. [Google Scholar] [CrossRef]
- Rezaeia, G.D.N.M.S.; Mohammadia, A.A. Moghadamniab, S. Kazemib. Formic Acid and Microwave Assisted Extraction of Curcumin from Turmeric (Curcuma longa L.). Int. J. Eng. 2016, 29, 145–151. [Google Scholar] [CrossRef]
- Joshi, N.D.A.A.K.; Cherekar, M.N. Extraction and Purification of Curcumin from Turmeric. Asian J. Plant Sci. Res. 2021, 11, 256–259. [Google Scholar]
- Chang, L.H.; Jong, T.T.; Huang, H.S.; Nien, Y.F.; Chang, C.M.J. Chang. Supercritical carbon dioxide extraction of turmeric oil from Curcuma longa Linn and purification of turmerones. Sep. Purif. Technol. 2006, 47, 119–125. [Google Scholar] [CrossRef]
- Rohman, A. Mini Review: Analysis of Curcuminoids in Food and Pharmaceutical Products. Int. Food Res. J. 2012, 19, 19–27. [Google Scholar]
- Kotra, V.S.R.; Satyabanta, L.; Goswami, T.K. A critical review of analytical methods for determination of curcuminoids in turmeric. J. Food Sci. Technol. 2019, 56, 5153–5166. [Google Scholar] [CrossRef]
- Gad, H.A.; Bouzabata, A. Application of chemometrics in quality control of Turmeric (Curcuma longa) based on Ultra-violet, Fourier transform-infrared and 1H NMR spectroscopy. Food Chem. 2017, 237, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Perera, K.D.C.; Weragoda, G.K.; Haputhanthri, R.; Rodrigo, S.K. Study of concentration dependent curcumin interaction with serum biomolecules using ATR-FTIR spectroscopy combined with Principal Component Analysis (PCA) and Partial Least Square Regression (PLS-R). Vib. Spectrosc. 2021, 116, 103288. [Google Scholar] [CrossRef]
- Kasemsumran, S.; Apiwatanapiwat, W.; Suttiwijitpukdee, N.; Vaithanomsat, P.; Thanapase, W. Evaluation of Fourier Transform-Near Infrared Spectroscopic Measurements for the Quantification of Curcumin in Turmeric Herbal Medicines. J. Near Infrared Spectrosc. 2014, 22, 113–120. [Google Scholar] [CrossRef]
- Rohman, A.; Sudjadi; Devi; Ramadhani, D.; Nugroho, A. Analysis of Curcumin in Curcuma longa and Curcuma xanthorriza Using FTIR Spectroscopy and Chemometrics. Res. J. Med. Plant 2015, 9, 179–186. [Google Scholar] [CrossRef]
- Wulandari, R.; Sudjadi; Martono, S.; Rohman, A. Liquid Chromatography and Fourier Transform Infrared Spectroscopy for quantitative analysis of individual and total curcuminoid in Curcuma longa extract. J. Appl. Pharm. Sci. 2018, 8, 107–113. [Google Scholar] [CrossRef]
- Ali, Z.; Saleem, M.; Atta, B.M.; Khan, S.S.; Hammad, G. Determination of curcuminoid content in turmeric using fluorescence spectroscopy. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2019, 213, 192–198. [Google Scholar] [CrossRef]
- Vitasari, R.A.; Wibowo, F.R.; Marliyana, S.D.; Wartono, M.W. Isolation and identification of curcumin and bisacurone from rhizome extract of temu glenyeh (Curcuma soloensis. Val). IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012063. [Google Scholar] [CrossRef]
- Vidal-Casanella, O.; Nuñez, N.; Sentellas, S.; Núñez, O.; Saurina, J. Characterization of Turmeric and Curry Samples by Liquid Chromatography with Spectroscopic Detection Based on Polyphenolic and Curcuminoid Contents. Separations 2020, 7, 23. [Google Scholar] [CrossRef]
- Núñez, N.; Vidal-Casanella, O.; Sentellas, S.; Saurina, J.; Núñez, O. Characterization, Classification and Authentication of Turmeric and Curry Samples by Targeted LC-HRMS Polyphenolic and Curcuminoid Profiling and Chemometrics. Molecules 2020, 25, 2942. [Google Scholar] [CrossRef] [PubMed]
- Kroon, M.; van Laarhoven, H.W.M.; Swart, E.L.; Kemper, E.M.; van Tellingen, O. A validated HPLC-MS/MS method for simultaneously analyzing curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetra-hydrocurcumin and piperine in human plasma, urine or feces. Heliyon 2023, 9, e15540. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Kiasat, A.R.; Kolahi, M.; Mirzajani, R.; Nejad, S.M.S. Determination of secondary metabolites including curcumin in Rheum ribes L. and surveying of its antioxidant and anticancer activity. J. Saudi Chem. Soc. 2022, 26, 101479. [Google Scholar] [CrossRef]
- Nsofor, W.N.; Nwaoguikpe, R.N.; Ujowundu, F.N.; Keke, C.O.; Uba, M.T.u.; Edom, C.V. Phytochemical, GC-MS, FTIR and Amino acid profile of methanol extract of Tetrapleura tetraptera fruit. J. Drug Deliv. Ther. 2023, 13, 61–69. [Google Scholar] [CrossRef]
- Kushwaha, P.; Shukla, B.; Dwivedi, J.; Saxena, S. Validated high-performance thin-layer chromatographic analysis of curcumin in the methanolic fraction of Curcuma longa L. rhizomes. Future J. Pharm. Sci. 2021, 7, 178. [Google Scholar] [CrossRef]
- Taha, M.N.; Krawinkel, M.B.; Morlock, G.E. High-performance thin-layer chromatography linked with (bio)assays and mass spectrometry—A suited method for discovery and quantification of bioactive components? Exemplarily shown for turmeric and milk thistle extracts. J. Chromatogr. A 2015, 1394, 137–147. [Google Scholar] [CrossRef]
- Anubala, S.; Sekar, R.; Nagaiah, K. Determination of Curcuminoids and Their Degradation Products in Turmeric (Curcuma longa) Rhizome Herbal Products by Non-aqueous Capillary Electrophoresis with Photodiode Array Detection. Food Anal. Methods 2016, 9, 2567–2578. [Google Scholar] [CrossRef]
- Wu, C.; Wang, W.; Quan, F.; Chen, P.; Qian, J.; Zhou, L.; Pu, Q. Sensitive analysis of curcuminoids via micellar electrokinetic chromatography with laser-induced native fluorescence detection and mixed micelles-induced fluorescence synergism. J. Chromatogr. A 2018, 1564, 207–213. [Google Scholar] [CrossRef]
- Ahmed, A.H.M.T.; Naskar, H.; Banerjee, S.; Ghatak, B.; Das, N.; Tudu, B.; Bandyopadhyay, R. Electrochemical sensor based on molecularly imprinted polymer embedded graphite electrode for detecting curcumin. Sens. Actuators A Phys. 2022, 344, 113748. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, C.; Zhang, Y.; Tao, T. Nanomaterials for fluorescent detection of curcumin. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2022, 265, 120359. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, X.; Dong, W.; Zhou, R.; Shuang, S.; Dong, C. Nitrogen and phosphorus dual-doped carbon dots as a label-free sensor for Curcumin determination in real sample and cellular imaging. Talanta 2018, 183, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Wang, X.; Wang, Y.; Yang, H.; Huang, J.; Cai, Z.; Choi, M.M.F. Boron and nitrogen co-doped carbon dots as a sensitive fluorescent probe for the detection of curcumin. Luminescence 2018, 33, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Opustilova, K.; Lapcikova, B.; Lapcik, L.; Gautam, S.; Valenta, T.; Li, P. Physico-Chemical Study of Curcumin and Its Application in O/W/O Multiple Emulsion. Foods 2023, 12, 1394. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Gancarz, M.; Kondracka, A.; Rusinek, R.; Oniszczuk, A. Curcumin and Weight Loss: Does It Work? Int. J. Mol. Sci. 2022, 23, 639. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef]
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin uptake and metabolism. BioFactors 2013, 39, 14–20. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladacenco, O.; Roza, E.; Costachescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Waheed, T.O.; Hahn, O.; Sridharan, K.; Morke, C.; Kamp, G.; Peters, K. Oxidative Stress Response in Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2022, 23, 3435. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Oxidative Stress: What Is It? Can It Be Measured? Where Is It Located? Can It Be Good or Bad? Can It Be Prevented? Can It Be Cured? Antioxidants 2022, 11, 1431. [Google Scholar] [CrossRef]
- Sathyabhama, M.; Dharshini, L.C.P.; Karthikeyan, A.; Kalaiselvi, S.; Min, T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules 2022, 12, 1405. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Druzga, A.; Katarzyna, J.; Skonieczna-Zydecka, K. Antioxidant Potential of Curcumin-A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Fouad, G.I.; Ahmed, K.A. Curcumin Ameliorates Doxorubicin-Induced Cardiotoxicity and Hepatotoxicity Via Suppressing Oxidative Stress and Modulating iNOS, NF-kappaB, and TNF-alpha in Rats. Cardiovasc. Toxicol. 2022, 22, 152–166. [Google Scholar] [CrossRef]
- Hettiarachchi, S.S.; Perera, Y.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, S.; Rajapakse, R.M.G. Comparison of Antibacterial Activity of Nanocurcumin with Bulk Curcumin. ACS Omega 2022, 7, 46494–46500. [Google Scholar] [CrossRef]
- El-Kattan, N.; Emam, A.N.; Mansour, A.S.; Ibrahim, M.A.; El-Razik, A.B.A.; Allam, K.A.M.; Riad, N.Y.; Ibrahim, S.A. Curcumin assisted green synthesis of silver and zinc oxide nanostructures and their antibacterial activity against some clinical pathogenic multi-drug resistant bacteria. RSC Adv. 2022, 12, 18022–18038. [Google Scholar] [CrossRef]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ozarowski, M.; Karpinski, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Trigo-Gutierrez, J.K.; Vega-Chacon, Y.; Soares, A.B.; Mima, E.G.O. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Shokrgozar, H.; Yazdani, J.; Memar, M.Y.; Sharifi, S.; Ghavimi, M.A. Antibacterial Effects of Curcumin Nanocrystals against Porphyromonas gingivalis Isolated from Patients with Implant Failure. Clin. Pr. 2022, 12, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, P.; Rogacheva, E.; Kremleva, A.; Morozkina, S.; Uspenskaya, M.; Kraeva, L. In-Vitro Antibacterial Activity of Curcumin-Loaded Nanofibers Based on Hyaluronic Acid against Multidrug-Resistant ESKAPE Pathogens. Pharmaceutics 2022, 14, 1186. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.; Nowicki, G.J.; Naylor, K.; Piekarski, R.; Slusarska, B. The Prevalence of Depression Symptoms and Their Socioeconomic and Health Predictors in a Local Community with a High Deprivation: A Cross-Sectional Studies. Int. J. Environ. Res. Public Health 2022, 19, 1797. [Google Scholar] [CrossRef]
- Wu, S.X.; Li, J.; Zhou, D.D.; Xiong, R.G.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Li, H.B. Possible Effects and Mechanisms of Dietary Natural Products and Nutrients on Depression and Anxiety: A Narrative Review. Antioxidants 2022, 11, 2132. [Google Scholar] [CrossRef]
- Balakrishnan, V.; Ng, K.S.; Kaur, W.; Govaichelvan, K.; Lee, Z.L. COVID-19 depression and its risk factors in Asia Pacific—A systematic review and meta-analysis. J. Affect. Disord. 2022, 298, 47–56. [Google Scholar] [CrossRef]
- Stephenson, E.; O’Neill, B.; Kalia, S.; Ji, C.; Crampton, N.; Butt, D.A.; Tu, K. Effects of COVID-19 pandemic on anxiety and depression in primary care: A retrospective cohort study. J. Affect. Disord. 2022, 303, 216–222. [Google Scholar] [CrossRef]
- Zalewska, A.; Galczyk, M.; Van Damme-Ostapowicz, K. Level of Depression during the COVID-19 Pandemic in Poland-A Cross-Sectional Study. Healthcare 2022, 10, 1123. [Google Scholar] [CrossRef] [PubMed]
- Aveiro-Robalo, T.R.; Garlisi-Torales, L.D.; Chuman-Sanchez, M.; Pereira-Victorio, C.J.; Huaman-Garcia, M.; Failoc-Rojas, V.E.; Valladares-Garrido, M.J. Prevalence and Associated Factors of Depression, Anxiety, and Stress in University Students in Paraguay during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 2930. [Google Scholar] [CrossRef] [PubMed]
- Riveros, M.E.; Avila, A.; Schruers, K.; Ezquer, F. Antioxidant Biomolecules and Their Potential for the Treatment of Difficult-to-Treat Depression and Conventional Treatment-Resistant Depression. Antioxidants 2022, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Zhang, J. Curcumin in antidepressant treatments: An overview of potential mechanisms, pre-clinical/clinical trials and ongoing challenges. Basic Clin. Pharmacol. Toxicol. 2020, 127, 243–253. [Google Scholar] [CrossRef]
- Lamanna-Rama, N.; Romero-Miguel, D.; Desco, M.; Soto-Montenegro, M.L. An Update on the Exploratory Use of Curcumin in Neuropsychiatric Disorders. Antioxidants 2022, 11, 353. [Google Scholar] [CrossRef]
- Kulkarni, S.; Dhir, A.; Akula, K.K. Potentials of curcumin as an antidepressant. Sci. World J. 2009, 9, 1233–1241. [Google Scholar] [CrossRef]
- Kanchanatawan, B.; Tangwongchai, S.; Sughondhabhirom, A.; Suppapitiporn, S.; Hemrunrojn, S.; Carvalho, A.F.; Maes, M. Add-on Treatment with Curcumin Has Antidepressive Effects in Thai Patients with Major Depression: Results of a Randomized Double-Blind Placebo-Controlled Study. Neurotox. Res. 2018, 33, 621–633. [Google Scholar] [CrossRef]
- Qi, X.J.; Liu, X.Y.; Tang, L.M.; Li, P.F.; Qiu, F.; Yang, A.H. Anti-depressant effect of curcumin-loaded guanidine-chitosan thermo-sensitive hydrogel by nasal delivery. Pharm. Dev. Technol. 2020, 25, 316–325. [Google Scholar] [CrossRef]
- Sena-Junior, A.S.; Aidar, F.J.; Oliveira, E.S.A.M.; Estevam, C.D.S.; de Oliveira Carvalho, C.R.; Lima, F.B.; Dos Santos, J.L.; Marcal, A.C. Whether or Not the Effects of Curcuma longa Supplementation Are Associated with Physical Exercises in T1DM and T2DM: A Systematic Review. Nutrients 2020, 13, 124. [Google Scholar] [CrossRef]
- Hussain, Y.; Khan, H.; Alotaibi, G.; Khan, F.; Alam, W.; Aschner, M.; Jeandet, P.; Saso, L. How Curcumin Targets Inflammatory Mediators in Diabetes: Therapeutic Insights and Possible Solutions. Molecules 2022, 27, 58. [Google Scholar] [CrossRef]
- Rivera-Mancía, S.; Trujillo, J.; Chaverri, J.P. Utility of curcumin for the treatment of diabetes mellitus: Evidence from preclinical and clinical studies. J. Nutr. Intermed. Metab. 2018, 14, 29–41. [Google Scholar] [CrossRef]
- Altobelli, E.; Angeletti, P.M.; Marziliano, C.; Mastrodomenico, M.; Giuliani, A.R.; Petrocelli, R. Potential Therapeutic Effects of Curcumin on Glycemic and Lipid Profile in Uncomplicated Type 2 Diabetes-A Meta-Analysis of Randomized Controlled Trial. Nutrients 2021, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Ghareghomi, S.; Rahban, M.; Moosavi-Movahedi, Z.; Habibi-Rezaei, M.; Saso, L.; Moosavi-Movahedi, A.A. The Potential Role of Curcumin in Modulating the Master Antioxidant Pathway in Diabetic Hypoxia-Induced Complications. Molecules 2021, 26, 7658. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Atkin, S.L.; Nikiforov, N.G.; Sahebkar, A. Therapeutic Role of Curcumin in Diabetes: An Analysis Based on Bioinformatic Findings. Nutrients 2022, 14, 3244. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Pescinini, E.S.L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Dos Santos Bueno, P.C. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef]
- Cho, J.A.; Park, S.H.; Cho, J.; Kim, J.O.; Yoon, J.H.; Park, E. Exercise and Curcumin in Combination Improves Cognitive Function and Attenuates ER Stress in Diabetic Rats. Nutrients 2020, 12, 1309. [Google Scholar] [CrossRef]
- Najafian, M. The Effects of Curcumin on Alpha Amylase in Diabetics Rats. Zahedan J. Res. Med. Sci. 2015, in press. [CrossRef]
- Lu, X.; Wu, F.; Jiang, M.; Sun, X.; Tian, G. Curcumin ameliorates gestational diabetes in mice partly through activating AMPK. Pharm. Biol. 2019, 57, 250–254. [Google Scholar] [CrossRef]
- Yang, Z.J.; Huang, S.Y.; Zhou, D.D.; Xiong, R.G.; Zhao, C.N.; Fang, A.P.; Zhang, Y.J.; Li, H.B.; Zhu, H.L. Effects and Mechanisms of Curcumin for the Prevention and Management of Cancers: An Updated Review. Antioxidants 2022, 11, 1481. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lin, C.H.; Huang, S.P.; Chen, M.; Lee, T.S. Risk Factors for Female Breast Cancer: A Population Cohort Study. Cancers 2022, 14, 788. [Google Scholar] [CrossRef]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Pouresmaeili, F.; Hosseini, S.J.; Farzaneh, F.; Karimpour, A.; Azargashb, E.; Yaghoobi, M.; Kamarehei, M. Evaluation of environmental risk factors for prostate cancer in a population of Iranian patients. Asian Pac. J. Cancer Prev. 2014, 15, 10603–10605. [Google Scholar] [CrossRef] [PubMed]
- Amadou, A.; Praud, D.; Coudon, T.; Deygas, F.; Grassot, L.; Dubuis, M.; Faure, E.; Couvidat, F.; Caudeville, J.; Bessagnet, B.; et al. Long-term exposure to nitrogen dioxide air pollution and breast cancer risk: A nested case-control within the French E3N cohort study. Environ. Pollut. 2023, 317, 120719. [Google Scholar] [CrossRef] [PubMed]
- Okunromade, O.; Yin, J.; Ray, C.; Adhikari, A. Air Quality and Cancer Prevalence Trends across the Sub-Saharan African Regions during 2005–2020. Int. J. Environ. Res. Public Health 2022, 19, 1342. [Google Scholar] [CrossRef] [PubMed]
- Gawelko, J.; Cierpial-Wolan, M.; Bwanakare, S.; Czarnota, M. Association between Air Pollution and Squamous Cell Lung Cancer in South-Eastern Poland. Int. J. Environ. Res. Public Health 2022, 19, 1598. [Google Scholar] [CrossRef] [PubMed]
- Youogo, L.M.K.; Parent, M.E.; Hystad, P.; Villeneuve, P.J. Ambient air pollution and prostate cancer risk in a population-based Canadian case-control study. Env. Epidemiol. 2022, 6, e219. [Google Scholar] [CrossRef]
- Racovita, R.C.; Secuianu, C.; Ciuca, M.D.; Israel-Roming, F. Effects of Smoking Temperature, Smoking Time, and Type of Wood Sawdust on Polycyclic Aromatic Hydrocarbon Accumulation Levels in Directly Smoked Pork Sausages. J. Agric. Food. Chem. 2020, 68, 9530–9536. [Google Scholar] [CrossRef]
- Racovita, R.C.; Secuianu, C.; Israel-Roming, F. Quantification and risk assessment of carcinogenic polycyclic aromatic hydrocarbons in retail smoked fish and smoked cheeses. Food Control 2021, 121, 107586. [Google Scholar] [CrossRef]
- Barcelos, K.A.; Mendonca, C.R.; Noll, M.; Botelho, A.F.; Francischini, C.R.D.; Silva, M.A.M. Antitumor Properties of Curcumin in Breast Cancer Based on Preclinical Studies: A Systematic Review. Cancers 2022, 14, 2165. [Google Scholar] [CrossRef]
- Wang, S.; Gao, X.; Li, J.; Wei, S.; Shao, Y.; Yin, Y.; Zhang, D.; Tang, M. The anticancer effects of curcumin and clinical research progress on its effects on esophageal cancer. Front. Pharmacol. 2022, 13, 1058070. [Google Scholar] [CrossRef]
- Picetti, R.; Deeney, M.; Pastorino, S.; Miller, M.R.; Shah, A.; Leon, D.A.; Dangour, A.D.; Green, R. Nitrate and nitrite contamination in drinking water and cancer risk: A systematic review with meta-analysis. Environ. Res. 2022, 210, 112988. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, M.; Kesharwani, P.; Johnston, T.P.; Sahebkar, A. Anticancer potential of curcumin-cyclodextrin complexes and their pharmacokinetic properties. Int. J. Pharm. 2023, 631, 122474. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Reihani, R.Z.; Doustvandi, M.A.; Amini, M.; Zargari, F.; Baradaran, B.; Yari, A.; Hashemi, M.; Tohidast, M.; Mokhtarzadeh, A. Synergistic anticancer effects of curcumin and crocin on human colorectal cancer cells. Mol. Biol. Rep. 2022, 49, 8741–8752. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P.; Zhou, M.; Wang, K. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and miRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214. [Google Scholar] [CrossRef]
- Li, P.; Pu, S.; Lin, C.; He, L.; Zhao, H.; Yang, C.; Guo, Z.; Xu, S.; Zhou, Z. Curcumin selectively induces colon cancer cell apoptosis and S cell cycle arrest by regulates Rb/E2F/p53 pathway. J. Mol. Struct. 2022, 1263, 133180. [Google Scholar] [CrossRef]
- Farahani, M.K.; Atashi, A.; Asadi, A. Evaluation of Anticancer Effects of Curcumin on Multicellular Breast Cancer Spheroids. Turk. J. Oncol. 2022, 37, 285–290. [Google Scholar] [CrossRef]
- Bolat, Z.B.; Islek, Z.; Sahin, F.; Ucisik, M.H. Delivery of curcumin within emulsome nanoparticles enhances the anti-cancer activity in androgen-dependent prostate cancer cell. Mol. Biol. Rep. 2023, 50, 2531–2543. [Google Scholar] [CrossRef]
- Gad, S.C. LD50/LC50 (Lethal Dosage 50/Lethal Concentration 50). In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 58–60. [Google Scholar]
- Kohli, K.; Ali, J.; Ansari, M.; Raheman, Z. Curcumin: A natural antiinflammatory agent. Indian J. Pharmacol. 2005, 37, 141–147. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Naz, R.K.; Lough, M.L.; Barthelmess, E.K. Curcumin: A Novel Non-Steroidal Contraceptive with Antimicrobial Properties. Front. Biosci. 2016, 8, 113–128. [Google Scholar] [CrossRef]
- Jantawong, C.; Priprem, A.; Intuyod, K.; Pairojkul, C.; Pinlaor, P.; Waraasawapati, S.; Mongkon, I.; Chamgramol, Y.; Pinlaor, S. Curcumin-loaded nanocomplexes: Acute and chronic toxicity studies in mice and hamsters. Toxicol. Rep. 2021, 8, 1346–1357. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer. Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Gronich, N. Spontaneous bleeding and curcumin: Case report. J. Clin. Images Med. Case Rep. 2022, 3, 2141. [Google Scholar] [CrossRef]
- Burgos-Moron, E.; Calderon-Montano, J.M.; Salvador, J.; Robles, A.; Lopez-Lazaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef]
- Jiao, Y.; Wilkinson, J.t.; Di, X.; Wang, W.; Hatcher, H.; Kock, N.D.; D’Agostino, R., Jr.; Knovich, M.A.; Torti, F.M.; Torti, S.V. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 2009, 113, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar] [CrossRef]
- Hassan, S.A.; Bhateja, S.; Arora, G.; Prathyusha, F. Use of Curcumin in Oral Health-A Review. Indian J. Integr. Med. 2020, 2, 20–23. [Google Scholar]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef]
- Xie, T.; Chen, X.; Chen, W.; Huang, S.; Peng, X.; Tian, L.; Wu, X.; Huang, Y. Curcumin is a Potential Adjuvant to Alleviates Diabetic Retinal Injury via Reducing Oxidative Stress and Maintaining Nrf2 Pathway Homeostasis. Front. Pharmacol. 2021, 12, 3696. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, C.; Zhou, M.; Xu, S.; Xie, Y.; Gao, S.; Yang, Y.; Deng, Z.; Zhang, L.; Shu, J.; et al. Role of curcumin in the treatment of acute kidney injury: Research challenges and opportunities. Phytomedicine 2022, 104, 154306. [Google Scholar] [CrossRef]
- Stamenkovska, M.; Hadzi-Petrushev, N.; Nikodinovski, A.; Gagov, H.; Atanasova-Panchevska, N.; Mitrokhin, V.; Kamkin, A.; Mladenov, M. Application of curcumine and its derivatives in the treatment of cardiovascular diseases: A review. Int. J. Food Prop. 2021, 24, 1510–1528. [Google Scholar] [CrossRef]
- Nazemi, M.M.H.; Jafari, E. Antidepressant Activity of Curcumin by Monoamine Oxidase–A Inhibition. Adv. J. Chem. Sect. B 2019, 1, 3–9. [Google Scholar] [CrossRef]
- Al-Saud, N.B.S. Impact of curcumin treatment on diabetic albino rats. Saudi J. Biol. Sci. 2020, 27, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-T.; Ho, Y.-S. Anticancer effect of curcumin on breast cancer and stem cells. Food Sci. Hum. Wellness 2018, 7, 134–137. [Google Scholar] [CrossRef]
- Abbasi, S.; Radi, M. Food grade microemulsion systems: Canola oil/lecithin:n-propanol/water. Food Chem. 2016, 194, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Jalali-Jivan, M.; Abbasi, S.; Fathi-Achachlouei, B. Lutein extraction by microemulsion technique: Evaluation of stability versus thermal processing and environmental stresses. Lwt 2021, 149, 111839. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, J.; Zheng, M.; Zheng, Y.; Xu, X.; Liu, Y.; Wang, X. Co-surfactant free microemulsions: Preparation, characterization and stability evaluation for food application. Food Chem. 2016, 204, 194–200. [Google Scholar] [CrossRef]
- Jalali-Jivan, M.; Abbasi, S. Novel approach for lutein extraction: Food grade microemulsion containing soy lecithin & sunflower oil. Innov. Food Sci. Emerg. Technol. 2020, 66, 102505. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Liu, H.; Hu, L. Nanoemulsion-based delivery approaches for nutraceuticals: Fabrication, application, characterization, biological fate, potential toxicity and future trends. Food Funct. 2021, 12, 1933–1953. [Google Scholar] [CrossRef]
- Amaral, D.M.F.; Bhargava, K. Essential Oil Nanoemulsions and Food Applications. Adv. Food Technol. Nutr. Sci. -Open J. 2015, 1, 84–87. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-Grade Nanoemulsions: Preparation, Stability and Application in Encapsulation of Bioactive Compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef] [PubMed]
- Mariyate, J.; Bera, A. A critical review on selection of microemulsions or nanoemulsions for enhanced oil recovery. J. Mol. Liq. 2022, 353, 118791. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Food-grade microemulsions and nanoemulsions: Role of oil phase composition on formation and stability. Food Hydrocoll. 2012, 29, 326–334. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Nanoemulsions for health, food, and cosmetics: A review. Environ. Chem. Lett. 2021, 19, 3381–3395. [Google Scholar] [CrossRef]
- Buyuktimkin, T. Water titration studies on microemulsions with a nonionic surfactant derived from castor oil and a series of polar oils. J. Drug Deliv. Sci. Technol. 2020, 56, 101521. [Google Scholar] [CrossRef]
- Sousa, R.P.F.d.; Braga, G.S.; Silva, R.R.d.; Leal, G.L.R.; Freitas, J.C.O.; Madera, V.S.; Garnica, A.I.C.; Curbelo, F.D.S. Formulation and Study of an Environmentally Friendly Microemulsion-Based Drilling Fluid (O/W) with Pine Oil. Energies 2021, 14, 7981. [Google Scholar] [CrossRef]
- Ramalho, Í.M.d.M.; Bezerra, G.S.; Ostrosky, E.A.; Ferrari, M.; Oliveira, V.d.S.; Neto, A.d.O.W.; Quintans, J.d.S.S.; Passos, F.R.S.; Heimfarth, L.; Converti, A.; et al. Chrysin-Loaded Microemulsion: Formulation Design, Evaluation and Antihyperalgesic Activity in Mice. Appl. Sci. 2022, 12, 477. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.F.; Wei, Z.F.; Jiang, J.J.; Peng, J.Q.; He, Q.T.; Xu, W.Y.; Liu, H.M. Microemulsion of Cinnamon Essential Oil Formulated with Tea Polyphenols, Gallic Acid, and Tween 80: Antimicrobial Properties, Stability and Mechanism of Action. Microorganisms 2022, 11, 2. [Google Scholar] [CrossRef]
- Fernández-Peña, L.; Gutiérrez-Muro, S.; Guzmán, E.; Lucia, A.; Ortega, F.; Rubio, G.R. Oil-In-Water Microemulsions for Thymol Solubilization. Colloids Interfaces 2019, 3, 64. [Google Scholar] [CrossRef]
- Sole, I.; Pey, C.M.; Maestro, A.; Gonzalez, C.; Porras, M.; Solans, C.; Gutierrez, J.M. Nano-emulsions prepared by the phase inversion composition method: Preparation variables and scale up. J. Colloid Interface Sci. 2010, 344, 417–423. [Google Scholar] [CrossRef]
- Ee, S.L.; Duan, X.; Liew, J.; Nguyen, Q.D. Droplet size and stability of nano-emulsions produced by the temperature phase inversion method. Chem. Eng. J. 2008, 140, 626–631. [Google Scholar] [CrossRef]
- Calligaris, S.; Valoppi, F.; Barba, L.; Pizzale, L.; Anese, M.; Conte, L.; Nicoli, M.C. Development of Transparent Curcumin Loaded Microemulsions by Phase Inversion Temperature (PIT) Method: Effect of Lipid Type and Physical State on Curcumin Stability. Food Biophys. 2016, 12, 45–51. [Google Scholar] [CrossRef]
- Gauthier, G.; Capron, I. Pickering nanoemulsions: An overview of manufacturing processes, formulations, and applications. JCIS Open 2021, 4, 100036. [Google Scholar] [CrossRef]
- Azmi, N.A.N.; Elgharbawy, A.A.M.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for Food, Pharmaceutical and Cosmetics. Processes 2019, 7, 617. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Niknam, S.M.; Kashaninejad, M.; Escudero, I.; Sanz, M.T.; Beltran, S.; Benito, J.M. Preparation of Water-in-Oil Nanoemulsions Loaded with Phenolic-Rich Olive Cake Extract Using Response Surface Methodology Approach. Foods 2022, 11, 279. [Google Scholar] [CrossRef]
- Gazolu-Rusanova, D.; Lesov, I.; Tcholakova, S.; Denkov, N.; Ahtchi, B. Food grade nanoemulsions preparation by rotor-stator homogenization. Food Hydrocoll. 2020, 102, 105579. [Google Scholar] [CrossRef]
- Fuentes, K.; Matamala, C.; Martínez, N.; Zúñiga, R.N.; Troncoso, E. Comparative Study of Physicochemical Properties of Nanoemulsions Fabricated with Natural and Synthetic Surfactants. Processes 2021, 9, 2002. [Google Scholar] [CrossRef]
- Scholz, P.; Keck, C.M. Nanoemulsions produced by rotor-stator high speed stirring. Int. J. Pharm. 2015, 482, 110–117. [Google Scholar] [CrossRef]
- Ahari, H.; Nasiri, M. Ultrasonic Technique for Production of Nanoemulsions for Food Packaging Purposes: A Review Study. Coatings 2021, 11, 847. [Google Scholar] [CrossRef]
- Kobayashi, D.; Hiwatashi, R.; Asakura, Y.; Matsumoto, H.; Shimada, Y.; Otake, K.; Shono, A. Effects of Operational Conditions on Preparation of Oil in Water Emulsion using Ultrasound. Phys. Procedia 2015, 70, 1043–1047. [Google Scholar] [CrossRef]
- Mahadev, M.; Dubey, A.; Shetty, A. Ultrasonically Fabricated Beta-Carotene Nanoemulsion: Optimization, Characterization and Evaluation of Combinatorial Effect with Quercetin on Streptozotocin-Induced Diabetic Rat Model. Pharmaceutics 2023, 15, 574. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiang, D. Stability of oil-in-water emulsions performed by ultrasound power or high-pressure homogenization. PLoS ONE 2019, 14, e0213189. [Google Scholar] [CrossRef]
- Ali, H.S.M.; Ahmed, S.A.; Alqurshi, A.A.; Alalawi, A.M.; Shehata, A.M.; Alahmadi, Y.M. Boosting Tadalafil Bioavailability via Sono-Assisted Nano-Emulsion-Based Oral Jellies: Box-Behnken Optimization and Assessment. Pharmaceutics 2022, 14, 2592. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Lin, Y.; Li, Z. Ultrasonic-assisted preparation of eucalyptus oil nanoemulsion: Process optimization, in vitro digestive stability, and anti-Escherichia coli activity. Ultrason. Sonochem. 2022, 82, 105904. [Google Scholar] [CrossRef]
- Guzmán, C.; Rojas, M.A.; Aragón, M. Optimization of Ultrasound-Assisted Emulsification of Emollient Nanoemulsions of Seed Oil of Passiflora edulis var. edulis. Cosmetics 2020, 8, 1. [Google Scholar] [CrossRef]
- Alam, A.; Ansari, M.J.; Alqarni, M.H.; Salkini, M.A.; Raish, M. Antioxidant, Antibacterial, and Anticancer Activity of Ultrasonic Nanoemulsion of Cinnamomum Cassia L. Essential Oil. Plants 2023, 12, 834. [Google Scholar] [CrossRef]
- Borkar, S.; Yadav, V.; Dhumal, N. Nanoemulsion as Novel Drug Delivery System: Development, Characterization and Application. Asian J. Pharm. Res. Dev. 2022, 10, 120–127. [Google Scholar] [CrossRef]
- Ganesan, P.; Karthivashan, G.; Park, S.Y.; Kim, J.; Choi, D.K. Microfluidization trends in the development of nanodelivery systems and applications in chronic disease treatments. Int. J. Nanomed. 2018, 13, 6109–6121. [Google Scholar] [CrossRef]
- Ozturk, O.K.; Turasan, H. Applications of microfluidization in emulsion-based systems, nanoparticle formation, and beverages. Trends Food Sci. Technol. 2021, 116, 609–625. [Google Scholar] [CrossRef]
- Goh, P.S.; Ng, M.H.; Choo, Y.M.; Amru, N.B.; Chuah, C.H. Production of Nanoemulsions from Palm-Based Tocotrienol Rich Fraction by Microfluidization. Molecules 2015, 20, 19936–19946. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.I.; Kim, T.G.; Lim, D.H.; Kim, S.B.; Park, S.M.; Hur, T.Y.; Ki, K.S.; Kwon, E.G.; Vijayakumar, M.; Kim, Y.J. Preparation of Nanoemulsions of Vitamin A and C by Microfluidization: Efficacy on the Expression Pattern of Milk-Specific Proteins in MAC-T Cells. Molecules 2019, 24, 2566. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qin, D.; Vu, G.; McClements, D.J. Impact of Operating Parameters on the Production of Nanoemulsions Using a High-Pressure Homogenizer with Flow Pattern and Back Pressure Control. Colloids Interfaces 2023, 7, 21. [Google Scholar] [CrossRef]
- Mohite, P.; Rajput, T.; Pandhare, R.; Sangale, A.; Singh, S.; Prajapati, B.G. Nanoemulsion in Management of Colorectal Cancer: Challenges and Future Prospects. Nanomanufacturing 2023, 3, 139–166. [Google Scholar] [CrossRef]
- Hidajat, M.J.; Jo, W.; Kim, H.; Noh, J. Effective Droplet Size Reduction and Excellent Stability of Limonene Nanoemulsion Formed by High-Pressure Homogenizer. Colloids Interfaces 2020, 4, 5. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Chen, K.; Wang, M. Nano-emulsion prepared by high pressure homogenization method as a good carrier for Sichuan pepper essential oil: Preparation, stability, and bioactivity. Lwt 2022, 154, 112779. [Google Scholar] [CrossRef]
- Yakoubi, S.; Kobayashi, I.; Uemura, K.; Nakajima, M.; Isoda, H.; Ksouri, R.; Saidani-Tounsi, M.; Neves, M.A. Essential-Oil-Loaded Nanoemulsion Lipidic-Phase Optimization and Modeling by Response Surface Methodology (RSM): Enhancement of Their Antimicrobial Potential and Bioavailability in Nanoscale Food Delivery System. Foods 2021, 10, 3149. [Google Scholar] [CrossRef] [PubMed]
- Sohan, M.S.R.; Elshamy, S.; Lara-Valderrama, G.; Changwatchai, T.; Khadizatul, K.; Kobayashi, I.; Nakajima, M.; Neves, M.A. Encapsulation of D-Limonene into O/W Nanoemulsions for Enhanced Stability. Polymers 2023, 15, 471. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin-An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Chen, S.; Zhang, J.; Liang, H. Robust stability and antimicrobial activity of d-limonene nanoemulsion by sodium caseinate and high pressure homogenization. J. Food Eng. 2022, 334, 111159. [Google Scholar] [CrossRef]
- Ma, J.; Song, X.; Luo, J.; Zhao, T.; Yu, H.; Peng, B.; Zhao, S. Molecular Dynamics Simulation Insight into Interfacial Stability and Fluidity Properties of Microemulsions. Langmuir 2019, 35, 13636–13645. [Google Scholar] [CrossRef] [PubMed]
- Garavand, F.; Jalai-Jivan, M.; Assadpour, E.; Jafari, S.M. Encapsulation of phenolic compounds within nano/microemulsion systems: A review. Food Chem. 2021, 364, 130376. [Google Scholar] [CrossRef]
- Fanun, M. Microemulsions as delivery systems. Curr. Opin. Colloid Interface Sci. 2012, 17, 306–313. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; Hamdouch, R.; Mazzacuva, F.; Isacchi, B.; Bilia, A.R. Optimization, characterization and in vitro evaluation of curcumin microemulsions. LWT-Food Sci. Technol. 2014, 59, 148–155. [Google Scholar] [CrossRef]
- Shan, M.; Meng, F.; Tang, C.; Zhou, L.; Lu, Z.; Lu, Y. Surfactin effectively improves bioavailability of curcumin by formation of nano-capsulation. Colloids Surf. B. Biointerfaces 2022, 215, 112521. [Google Scholar] [CrossRef]
- Dourado, D.; Oliveira, M.C.d.; Araujo, G.R.S.d.; Amaral-Machado, L.; Porto, D.L.; Aragão, C.F.S.; Alencar, E.d.N.; Egito, E.S.T.d. Low-surfactant microemulsion, a smart strategy intended for curcumin oral delivery. Colloids Surf. Physicochem. Eng. Asp. 2022, 652, 129720. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, H.Y.; Chi, M.H.; Shen, C.M.; Chen, H.W.; Yang, W.J.; Lee, M.H. Preparation of curcumin microemulsions with food-grade soybean oil/lecithin and their cytotoxicity on the HepG2 cell line. Food Chem. 2014, 154, 282–290. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lin, H.-Y.; Chen, H.-C.; Yu, M.-W.; Lee, M.-H. Stability and characterisation of phospholipid-based curcumin-encapsulated microemulsions. Food Chem. 2009, 116, 923–928. [Google Scholar] [CrossRef]
- Hu, L.; Jia, Y.; Niu, F.; Jia, Z.; Yang, X.; Jiao, K. Preparation and enhancement of oral bioavailability of curcumin using microemulsions vehicle. J. Agric. Food. Chem. 2012, 60, 7137–7141. [Google Scholar] [CrossRef]
- Iqbal, R.; Mehmood, Z.; Baig, A.; Khalid, N. Formulation and characterization of food grade O/W nanoemulsions encapsulating quercetin and curcumin: Insights on enhancing solubility characteristics. Food Bioprod. Process. 2020, 123, 304–311. [Google Scholar] [CrossRef]
- Rahdar, A.; Hajinezhad, M.R.; Sargazi, S.; Zaboli, M.; Barani, M.; Baino, F.; Bilal, M.; Sanchooli, E. Biochemical, Ameliorative and Cytotoxic Effects of Newly Synthesized Curcumin Microemulsions: Evidence from In Vitro and In Vivo Studies. Nanomaterials 2021, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Ameta, R.K.; Singh, M. Preparation and characterization of bionanoemulsions for improving and modulating the antioxidant efficacy of natural phenolic antioxidant curcumin. Chem. Biol. Interact. 2014, 222, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Bharmoria, P.; Bisht, M.; Gomes, M.C.; Martins, M.; Neves, M.C.; Mano, J.F.; Bdikin, I.; Coutinho, J.A.P.; Ventura, S.P.M. Protein-olive oil-in-water nanoemulsions as encapsulation materials for curcumin acting as anticancer agent towards MDA-MB-231 cells. Sci. Rep. 2021, 11, 9099. [Google Scholar] [CrossRef] [PubMed]
- Sari, T.P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Q. Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J. Agric. Food. Chem. 2012, 60, 5373–5379. [Google Scholar] [CrossRef]
- Ali, M.; Khan, N.R.; Subhan, Z.; Mehmood, S.; Amin, A.; Rabbani, I.; Rehman, F.U.; Basit, H.M.; Syed, H.K.; Khan, I.U.; et al. Novel Curcumin-Encapsulated alpha-Tocopherol Nanoemulsion System and Its Potential Application for Wound Healing in Diabetic Animals. Biomed. Res. Int. 2022, 2022, 7669255. [Google Scholar] [CrossRef]
- Dokovic, J.B.; Savic, S.M.; Mitrovic, J.R.; Nikolic, I.; Markovic, B.D.; Randjelovic, D.V.; Antic-Stankovic, J.; Bozic, D.; Cekic, N.D.; Stevanovic, V.; et al. Curcumin Loaded PEGylated Nanoemulsions Designed for Maintained Antioxidant Effects and Improved Bioavailability: A Pilot Study on Rats. Int. J. Mol. Sci. 2021, 22, 7991. [Google Scholar] [CrossRef]
- Vaz, G.; Clementino, A.; Mitsou, E.; Ferrari, E.; Buttini, F.; Sissa, C.; Xenakis, A.; Sonvico, F.; Dora, C.L. In Vitro Evaluation of Curcumin- and Quercetin-Loaded Nanoemulsions for Intranasal Administration: Effect of Surface Charge and Viscosity. Pharmaceutics 2022, 14, 194. [Google Scholar] [CrossRef]
- Soliman, W.E.; Shehata, T.M.; Mohamed, M.E.; Younis, N.S.; Elsewedy, H.S. Enhancement of Curcumin Anti-Inflammatory Effect via Formulation into Myrrh Oil-Based Nanoemulgel. Polymers 2021, 13, 577. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, B.H. Preparation of curcuminoid microemulsions from Curcuma longa L. to enhance inhibition effects on growth of colon cancer cells HT-29. RSC Adv. 2018, 8, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, H.; Budiputra, D.K.; Mauludin, R. Curcumin nanoemulsion for transdermal application: Formulation and evaluation. Drug Dev. Ind. Pharm. 2015, 41, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.B.; Zhou, S.Y.; Zhang, Y.Q.; Wang, J.L.; Tian, Y.D.; Jia, Y.Y.; Sun, Y.J. Therapeutic effects of curcumin nanoemulsions on prostate cancer. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.; Mandal, S.D.; Mandal, S.; Faldu, S.; Patel, J. Nasotransmucosal Delivery of Curcumin-Loaded Mucoadhesive Microemulsions for Treating Inflammation-Related CNS Disorders. Turk. J. Pharm. Sci. 2022, 19, 560–571. [Google Scholar] [CrossRef] [PubMed]

| Extraction Method | Extraction Time | Solvent Used | Additional Technical Information | Extraction Yield | Reference |
|---|---|---|---|---|---|
| Soxhlet Extraction | 48 h | Ethanol | 40 °C | 1.25% | [74] |
| UAE | 2 h | Ethanol | 40 °C, 1:30 (w/v), 240 W, 22 kHz | 73.18% | [53] |
| UAE | 1 h | Ethanol | 35 °C, 250 W, 22 kHz | 72% | [75] |
| MAE | 29.99 min | Ethanol | 160 W, 1:20 (w/v) | 10.32% | [62] |
| MAE | 9 min | Ethanol | 900 W | 88% | [76] |
| EAE | 8 h | Water | Enzyme: α-amylase, incubation time: 5 h | 26.04% | [77] |
| PLE | 20 min | Ethanol | 60 °C, 100 bar | 99 ± 5% | [73] |
| EAE | 8 h | Water | Enzyme: cellulase, 40 °C, pH: 4.5–6 | 25–27% | [78] |
| MAE | 2 min | Acetone | 100 W | 82.4% | [79] |
| UAE | 40 min | Ethanol | 1:10 (w/v), 240 W, 42 kHz | 7.67 ± 1.36% | [59] |
| Soxlet extraction | 7 h | Acetone | 56.53 °C | 4.09% | [80] |
| EAE | 1 h | Water | Enzyme: pectinase, pH: 5, 50 °C | 8.36% | [19] |
| MAE | 2 min | Acetone | 300 W | 3.72% | [55] |
| Soxhlet Extraction | 8 h | Acetone | 60 °C | 6.9% | [55] |
| PLE | 5 min | Water/Ethanol 50/50 | 130 °C, 1:20 (w/v) | 15.8% | [72] |
| SFE | 1 h | SC-CO2 | 70 °C, 425 bar | 0.68–0.73% | [69] |
| UAE | 40 min | Ethanol | 240 W; 42 kHz, 1:30 (w/v) | 16.03% | [59] |
| SFE | 2.5 h | SC-CO2 | 47 °C, 260 bar | 71% | [81] |
| Effect of Curcumin | Mechanism of Action | Reference |
|---|---|---|
| Antioxidant | Can scavenge reactive oxidizing substances | [180] |
| Enhances the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) | [116] | |
| Activates antioxidant factors including Nrf2, heme oxygenase (HO-1), and proliferator-activated receptor-gamma coactivator (PGC-1α) | [181] | |
| Prevents LDL (low-density lipoprotein) oxidation | [182] | |
| Antibacterial | Penetrates bacterial cell membranes and makes them permeable for antibiotic absorption. | [119] |
| Causes phototoxicity and inhibits bacterial growth when exposed to blue light. | [15] | |
| Inhibits the growth of Gram-negative and Gram-positive bacteria | [121] | |
| Antidepressant | Can regulate the concentrations of diverse neurotransmitters, including norepinephrine, dopamine, and serotonin. | [136] |
| Has strong interactions with the MAO enzyme | [183] | |
| Antidiabetic | Can increase Akt and GSK-3β phosphorylation | [141] |
| Can lower glucose levels and decrease insulin resistance, dyslipidemia, and malondialdehyde levels | [184] | |
| Can enhance the expression of the GLUT4 gene | ||
| Can reduce the levels of leptin, resistin, interleukin (IL)-6 IL-1β, and tumour necrosis factor-α | [145] | |
| Anticancer | Inhibits the proliferation of human colon cancer SW620 and MC-38 cells | [165] |
| Has antiproliferation effects by inhibiting the binding activity of NF-kB, AP-1, EGR, and β catenin | [185] | |
| Inhibits liver cancer HepG2 cells by inhibiting heat shock protein 70 (HSP70)—toll-like receptor 4 (TLR4) signaling | [149] | |
| Can inhibit the migration of esophageal cancer tumor cells by enhancing the expression of nonmetastatic gene 23 (Nm 23), antimetastatic protein tissue inhibition metalloproteinase (TIMP)-2, and E-cadherin | [160] |
| Type of System | Particle Size (nm) | Components | Effect | Reference |
|---|---|---|---|---|
| O/W ME | 10.07 ± 0.45 | Vitamin E, Tween 20, Ethanol, Water | Improve solubility and stability and oral uptake of curcumin | [236] |
| O/W NE | 23.23 ± 2.86 | IPM, Tween 80: Glycerol, Surfactin | Improve bioavailability, inhibit activity of Caco2 cell | [237] |
| O/W ME | 21.81 ± 0.20 | Miglyol 812 N, Water, PEG 30 castor oil:Span 80 | Improve solubility, stability and modified release of curcumin at gastrointestinal tract pHs | [238] |
| O/W ME | 80.02 ± 3.29 | Soybean Oil, Lecithin:Tween 80, Water | Enhance anti-Hep G2 activities | [239] |
| O/W ME | 71.8 ± 2.45 | Ethyl oleate, Lecithin/Tween 80, Water | Prevent degradation process of curcumin, enhance the skin permeation | [240] |
| O/W ME | 27.3 ± 2 | Capryol 90, Cremophor RH 40, Transcutol P | Enhance the oral bioavailability | [241] |
| O/W NE | 174.44 | HI-Cap 100, Sunflower oil, Water | Enhance solubility of curcumin | [242] |
| O/W ME | 8 | Ethyl butyrate, Pluronic surfactant F127, Fetal bovine serum, Sodium Caprylate | Enhance hepatoprotective, neuroprotective and anti-cancer effects of curcumin | [243] |
| O/W NE | 100–900 | Cottonseed oil, Ethanol, Glycerol, Poloxamer, Tween 20, SDS DTAB | Enhance antioxidant efficacy of curcumin | [244] |
| O/W NE | 372 | Pluronic 127, Olive Oil, Water | Inhibit MDA-MB-231 breast cancer cell | [245] |
| O/W NE | 141.6 ± 15.4 | WPC-70, Tween 80, MCT-60, Water | Increase hydrophilicity and bioaccessibility of curcumin | [246] |
| O/W NE | 218 | Span 20 saturated MCT, Monostearin, water, Tween 20 | Improve oral bioavailability of curcumin | [247] |
| O/W NE | 26.76 ± 0.9 | PEG 400/Tween 80/Water | Accelerate the skin tissue regeneration process | [248] |
| O/W NE | 105 | Soybean oil, MCT, Lecithin, Benzyl alcohol, Sodium Oleate | Improve plasma resistance of curcumin | [249] |
| O/W NE | 102–131 | PEG-660 stearate, castor oil, purified fish oil, egg lecithin, water | Allow intranasal administration of curcumin | [250] |
| O/W NE | 130 | Tween 80, Ethanol, Propylen Glycol, Myrrh Oil | Improve anti-inflammatory effects of curcumin | [251] |
| O/W ME | 10.9 | Soybean Oil, Tween 80, Ethanol, Water | Enhance curcumin potential to inhibit growth of colon cancer cells HT-29 | [252] |
| O/W NE | 85 ± 1.5 | Glyceryl monooleate, Cremophor PH40, Polyethylene glycol 400, Water | Improve curcumin permeability through skin and protect curcumin from chemical degradation | [253] |
| O/W NE | 34.52 ± 2 | MCT, Cremophor RH 40, Glycerol, Water | Enhance cellular cytotoxicity, cellular uptake, cell cycle arrest and apoptosis against prostate cancer cells | [254] |
| O/W ME | 66.74 ± 3.46 | Capmul MCM, Accenon CC: Transcutol P, Aqueous polycarbaphil | Improve transnasal delivery of curcumin | [255] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. https://doi.org/10.3390/ijms24108874
Ciuca MD, Racovita RC. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. International Journal of Molecular Sciences. 2023; 24(10):8874. https://doi.org/10.3390/ijms24108874
Chicago/Turabian StyleCiuca, Maria D., and Radu C. Racovita. 2023. "Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions" International Journal of Molecular Sciences 24, no. 10: 8874. https://doi.org/10.3390/ijms24108874
APA StyleCiuca, M. D., & Racovita, R. C. (2023). Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. International Journal of Molecular Sciences, 24(10), 8874. https://doi.org/10.3390/ijms24108874






