DNA Methylation Biomarkers for Young Children with Idiopathic Autism Spectrum Disorder: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Research
2.2. Eligibility Criteria
- Studies focused on DNA methylation
- Studies including individuals with a well-established diagnosis of idiopathic ASD
- DNA methylation analyses in children with a mean age ≤ 8 years
- Studies performed on easily available peripheral tissue (i.e., peripheral blood, saliva, buccal swabs)
- Availability of the full text of the paper
- Articles published in languages other than English
- Reviews and/or meta-analyses
- Studies performed on DNA from the placenta or cord blood
- Non-peer-reviewed studies
- In vitro and in silico studies or studies using animal models
2.3. Assessing the Risk of Bias, Outcome Assessment, Quality Scoring, and Statistical Methods
3. Results
3.1. Study Selection
3.2. Summary of the Included Studies
3.3. DNA Methylation Investigation Techniques
3.4. Characteristics of Participants
3.5. Main DNA Methylation Findings
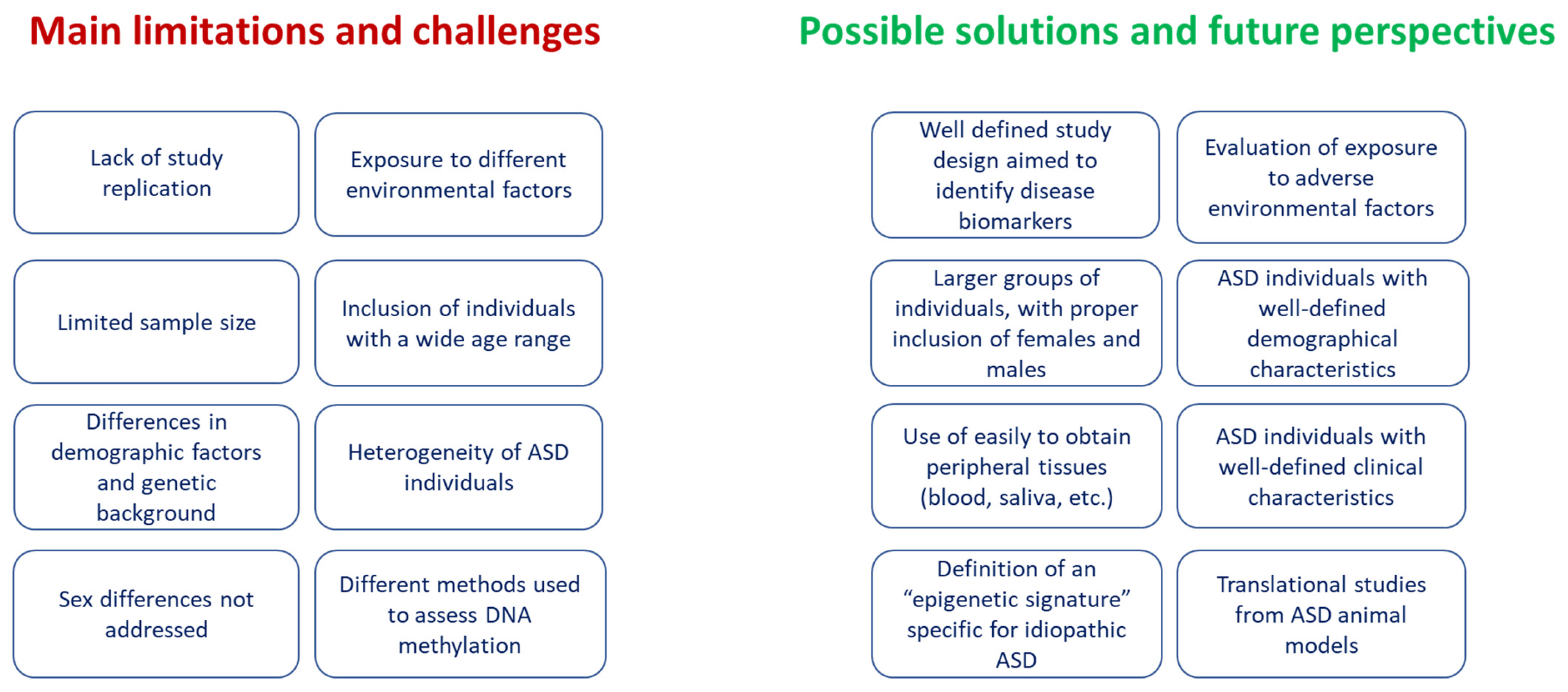
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9 (Suppl. S1), S55–S65. [Google Scholar] [CrossRef]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Dai, Y.G.; Fein, D.A.; Robins, D.L. Characteristics of toddlers with early versus later diagnosis of autism spectrum disorder. Autism 2021, 25, 416–428. [Google Scholar] [CrossRef]
- Shulman, C.; Esler, A.; Morrier, M.J.; Rice, C.E. Diagnosis of Autism Spectrum Disorder Across the Lifespan. Child Adolesc. Psychiatr. Clin. N. Am. 2020, 29, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Solmi, M.; Michelini, G.; Bellato, A.; Blanner, C.; Canozzi, A.; Eudave, L.; Farhat, L.C.; Højlund, M.; Köhler-Forsberg, O.; et al. Candidate diagnostic biomarkers for neurodevelopmental disorders in children and adolescents: A systematic review. World Psychiatry 2023, 22, 129–149. [Google Scholar] [CrossRef]
- Yoon, S.H.; Choi, J.; Lee, W.J.; Do, J.T. Genetic and Epigenetic Etiology Underlying Autism Spectrum Disorder. J. Clin. Med. 2020, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Tick, B.; Bolton, P.; Happé, F.; Rutter, M.; Rijsdijk, F. Heritability of autism spectrum disorders: A meta-analysis of twin studies. J. Child Psychol. Psychiatry 2016, 57, 585–595. [Google Scholar] [CrossRef]
- Ornoy, A.; Weinstein-Fudim, L.; Ergaz, Z. Genetic Syndromes, Maternal Diseases and Antenatal Factors Associated with Autism Spectrum Disorders (ASD). Front. Neurosci. 2016, 10, 316. [Google Scholar] [CrossRef]
- Genovese, A.; Butler, M.G. Clinical Assessment; Genetics; and Treatment Approaches in Autism Spectrum Disorder (ASD). Int. J. Mol. Sci. 2020, 21, 4726. [Google Scholar] [CrossRef]
- Abrahams, B.S.; Arking, D.E.; Campbell, D.B.; Mefford, H.C.; Morrow, E.M.; Weiss, L.A.; Menashe, I.; Wadkins, T.; Banerjee-Basu, S.; Packer, A. SFARI Gene 2.0: A community-driven knowledgebase for the autism spectrum disorders (ASDs). Mol. Autism 2013, 4, 36. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Wu, Q.; Yang, X.; Huang, A.Y.; Zhang, J.; Ye, A.Y.; Dou, Y.; Yan, L.; Zhou, W.Z.; et al. AutismKB 2.0: A knowledgebase for the genetic evidence of autism spectrum disorder. Database 2018, 2018, bay106. [Google Scholar] [CrossRef]
- Jiang, C.C.; Lin, L.S.; Long, S.; Ke, X.Y.; Fukunaga, K.; Lu, Y.M.; Han, F. Signalling pathways in autism spectrum disorder: Mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 2022, 7, 229. [Google Scholar] [CrossRef]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism spectrum disorder. Nat. Rev. Dis. Prim. 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Cheroni, C.; Caporale, N.; Testa, G. Autism spectrum disorder at the crossroad between genes and environment: Contributions; convergences; and interactions in ASD developmental pathophysiology. Mol. Autism 2020, 11, 69. [Google Scholar] [CrossRef]
- Bölte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.; Migliore, L.; Muratori, F. Pregnancy risk factors related to autism: An Italian case-control study in mothers of children with autism spectrum disorders (ASD); their siblings and of typically developing children. J. Dev. Orig. Health Dis. 2018, 9, 442–449. [Google Scholar] [CrossRef]
- Portela, A.; Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef]
- Yasuda, Y.; Matsumoto, J.; Miura, K.; Hasegawa, N.; Hashimoto, R. Genetics of autism spectrum disorders and future direction. J. Hum. Genet. 2023, 68, 193–197. [Google Scholar] [CrossRef]
- Mossink, B.; Negwer, M.; Schubert, D.; Nadif Kasri, N. The emerging role of chromatin remodelers in neurodevelopmental disorders: A developmental perspective. Cell. Mol. Life Sci. 2021, 78, 2517–2563. [Google Scholar] [CrossRef]
- Chin, E.W.M.; Goh, E.L.K. MeCP2 Dysfunction in Rett Syndrome and Neuropsychiatric Disorders. Methods Mol. Biol. 2019, 2011, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.W.; Jiang, Y.H. DNA Methylation and Susceptibility to Autism Spectrum Disorder. Annu. Rev. Med. 2019, 70, 151–166. [Google Scholar] [CrossRef]
- LaSalle, J.M. Epigenomic signatures reveal mechanistic clues and predictive markers for autism spectrum disorder. Mol. Psychiatry 2023. [Google Scholar] [CrossRef]
- Williams, L.A.; LaSalle, J.M. Future Prospects for Epigenetics in Autism Spectrum Disorder. Mol. Diagn. Ther. 2022, 26, 569–579. [Google Scholar] [CrossRef]
- Jin, Y.; Allen, E.G.; Jin, P. Cell-free DNA methylation as a potential biomarker in brain disorders. Epigenomics 2022, 14, 369–374. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Cutler, P.; Melnyk, S.; Jernigan, S.; Janak, L.; Gaylor, D.W.; Neubrander, J.A. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 2004, 80, 1611–1617. [Google Scholar] [CrossRef]
- Almstrup, K.; Lindhardt Johansen, M.; Busch, A.S.; Hagen, C.P.; Nielsen, J.E.; Petersen, J.H.; Juul, A. Pubertal development in healthy children is mirrored by DNA methylation patterns in peripheral blood. Sci. Rep. 2016, 6, 28657. [Google Scholar] [CrossRef]
- Han, L.; Zhang, H.; Kaushal, A.; Rezwan, F.I.; Kadalayil, L.; Karmaus, W.; Henderson, A.J.; Relton, C.L.; Ring, S.; Arshad, S.H.; et al. Changes in DNA methylation from pre- to post-adolescence are associated with pubertal exposures. Clin. Epigenet. 2019, 11, 176. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Robinson, W.P.; Price, E.M. The human placental methylome. Cold Spring Harb. Perspect. Med. 2015, 5, a023044. [Google Scholar] [CrossRef]
- Morin, A.M.; Gatev, E.; McEwen, L.M.; MacIsaac, J.L.; Lin, D.T.S.; Koen, N.; Czamara, D.; Räikkönen, K.; Zar, H.J.; Koenen, K.; et al. Maternal blood contamination of collected cord blood can be identified using DNA methylation at three CpGs. Clin. Epigenetics 2017, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2019. Available online: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort (accessed on 25 March 2023).
- Alshamrani, A.A.; Alshehri, S.; Alqarni, S.S.; Ahmad, S.F.; Alghibiwi, H.; Al-Harbi, N.O.; Alqarni, S.A.; Al-Ayadhi, L.Y.; Attia, S.M.; Alfardan, A.S.; et al. DNA Hypomethylation Is Associated with Increased Inflammation in Peripheral Blood Neutrophils of Children with Autism Spectrum Disorder: Understanding the Role of Ubiquitous Pollutant Di(2-ethylhexyl) Phthalate. Metabolites 2023, 13, 458. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.V.; Sheppard, B.; Windham, G.C.; Schieve, L.A.; Schendel, D.E.; Croen, L.A.; Chopra, P.; Alisch, R.S.; Newschaffer, C.J.; Warren, S.T.; et al. Case-control meta-analysis of blood DNA methylation and autism spectrum disorder. Mol. Autism 2018, 9, 40. [Google Scholar] [CrossRef]
- Aspra, Q.; Cabrera-Mendoza, B.; Morales-Marín, M.E.; Márquez, C.; Chicalote, C.; Ballesteros, A.; Aguilar, M.; Castro, X.; Gómez-Cotero, A.; Balboa-Verduzco, A.M.; et al. Epigenome-Wide Analysis Reveals DNA Methylation Alteration in ZFP57 and Its Target RASGFR2 in a Mexican Population Cohort with Autism. Children 2022, 9, 462. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Vishweswaraiah, S.; Aydas, B.; Mishra, N.K.; Yilmaz, A.; Guda, C.; Radhakrishna, U. Artificial intelligence analysis of newborn leucocyte epigenomic markers for the prediction of autism. Brain Res. 2019, 1724, 146457. [Google Scholar] [CrossRef]
- Elagoz Yuksel, M.; Yuceturk, B.; Karatas, O.F.; Ozen, M.; Dogangun, B. The altered promoter methylation of oxytocin receptor gene in autism. J. Neurogenet. 2016, 30, 280–284. [Google Scholar] [CrossRef]
- Gallo, R.; Stoccoro, A.; Cagiano, R.; Nicolì, V.; Ricciardi, R.; Tancredi, R.; Trovato, R.; Santorelli, F.M.; Calderoni, S.; Muratori, F.; et al. Correlation among maternal risk factors; gene methylation and disease severity in females with autism spectrum disorder. Epigenomics 2022, 14, 175–185. [Google Scholar] [CrossRef]
- García-Ortiz, M.V.; de la Torre-Aguilar, M.J.; Morales-Ruiz, T.; Gómez-Fernández, A.; Flores-Rojas, K.; Gil-Campos, M.; Martin-Borreguero, P.; Ariza, R.R.; Roldán-Arjona, T.; Perez-Navero, J.L. Analysis of Global and Local DNA Methylation Patterns in Blood Samples of Patients with Autism Spectrum Disorder. Front. Pediatr. 2021, 9, 685310. [Google Scholar] [CrossRef]
- Gui, A.; Jones, E.J.H.; Wong, C.C.Y.; Meaburn, E.; Xia, B.; Pasco, G.; Lloyd-Fox, S.; Charman, T.; Bolton, P.; Johnson, M.H.; et al. Leveraging epigenetics to examine differences in developmental trajectories of social attention: A proof-of-principle study of DNA methylation in infants with older siblings with autism. Infant Behav. Dev. 2020, 60, 101409. [Google Scholar] [CrossRef]
- Hannon, E.; Schendel, D.; Ladd-Acosta, C.; Grove, J.; iPSYCH-Broad ASD Group; Hansen, C.S.; Andrews, S.V.; Hougaard, D.M.; Bresnahan, M.; Mors, O.; et al. Elevated polygenic burden for autism is associated with differential DNA methylation at birth. Genome Med. 2018, 10, 19. [Google Scholar] [CrossRef]
- Hu, Z.; Ying, X.; Huang, L.; Zhao, Y.; Zhou, D.; Liu, J.; Zhong, J.; Huang, T.; Zhang, W.; Cheng, F.; et al. Association of human serotonin receptor 4 promoter methylation with autism spectrum disorder. Medicine 2020, 99, e18838. [Google Scholar] [CrossRef] [PubMed]
- Jasoliya, M.; Gu, J.; AlOlaby, R.R.; Durbin-Johnson, B.; Chedin, F.; Tassone, F. Profiling Genome-Wide DNA Methylation in Children with Autism Spectrum Disorder and in Children with Fragile X Syndrome. Genes 2022, 13, 1795. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, Z.; Wang, Y.; Li, X.; Yang, X.; Zhan, X.; Huang, Y.; Gao, Z.; Zhang, M.; Sun, C.; et al. Genome-Wide DNA Methylation Analysis Reveals Epigenetic Pattern of SH2B1 in Chinese Monozygotic Twins Discordant for Autism Spectrum Disorder. Front. Neurosci. 2019, 13, 712. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, S.; Fuchs, G.J.; Schulz, E.; Lopez, M.; Kahler, S.G.; Fussell, J.J.; Bellando, J.; Pavliv, O.; Rose, S.; Seidel, L.; et al. Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. J. Autism Dev. Disord. 2012, 42, 367–377. [Google Scholar] [CrossRef]
- Neri de Souza Reis, V.; Tahira, A.C.; Daguano Gastaldi, V.; Mari, P.; Portolese, J.; Feio Dos Santos, A.C.; Lisboa, B.; Mari, J.; Caetano, S.C.; Brunoni, D.; et al. Environmental Influences Measured by Epigenetic Clock and Vulnerability Components at Birth Impact Clinical ASD Heterogeneity. Genes 2021, 12, 1433. [Google Scholar] [CrossRef]
- Stoccoro, A.; Gallo, R.; Calderoni, S.; Cagiano, R.; Muratori, F.; Migliore, L.; Grossi, E.; Coppedè, F. Artificial neural networks reveal sex differences in gene methylation; and connections between maternal risk factors and symptom severity in autism spectrum disorder. Epigenomics 2022, 14, 1181–1195. [Google Scholar] [CrossRef]
- Wang, X.; Liang, S.; Sun, Y.; Li, H.; Endo, F.; Nakao, M.; Saitoh, N.; Wu, L. Analysis of estrogen receptor β gene methylation in autistic males in a Chinese Han population. Metab. Brain Dis. 2017, 32, 1033–1042. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Chai, X.; Liu, J. The association between ST8SIA2 gene and behavioral phenotypes in children with autism spectrum disorder. Front. Behav. Neurosci. 2022, 16, 929878. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, C.; Yu, H.; Zhang, W.; Cheng, F.; Yu, H.; Zhou, D.; Li, B.; Liu, J.; Dai, J.; et al. Association between the methylation of six apoptosis-associated genes with autism spectrum disorder. Mol. Med. Rep. 2018, 18, 4629–4634. [Google Scholar] [CrossRef]
- Kurdyukov, S.; Bullock, M. DNA Methylation Analysis: Choosing the Right Method. Biology 2016, 5, 3. [Google Scholar] [CrossRef]
- Zhou, Z.; Lunetta, K.L.; Smith, A.K.; Wolf, E.J.; Stone, A.; Schichman, S.A.; McGlinchey, R.E.; Milberg, W.P.; Miller, M.W.; Logue, M.W. Correction for multiple testing in candidate-gene methylation studies. Epigenomics 2019, 11, 1089–1105. [Google Scholar] [CrossRef]
- Ladd-Acosta, C.; Hansen, K.D.; Briem, E.; Fallin, M.D.; Kaufmann, W.E.; Feinberg, A.P. Common DNA methylation alterations in multiple brain regions in autism. Mol. Psychiatry 2014, 19, 862–871. [Google Scholar] [CrossRef]
- Dall’Aglio, L.; Muka, T.; Cecil, C.A.M.; Bramer, W.M.; Verbiest, M.M.P.J.; Nano, J.; Hidalgo, A.C.; Franco, O.H.; Tiemeier, H. The role of epigenetic modifications in neurodevelopmental disorders: A systematic review. Neurosci. Biobehav. Rev. 2018, 94, 17–30. [Google Scholar] [CrossRef]
- Berko, E.R.; Suzuki, M.; Beren, F.; Lemetre, C.; Alaimo, C.M.; Calder, R.B.; Ballaban-Gil, K.; Gounder, B.; Kampf, K.; Kirschen, J.; et al. Mosaic epigenetic dysregulation of ectodermal cells in autism spectrum disorder. PLoS Genet. 2014, 10, e1004402. [Google Scholar] [CrossRef]
- Wong, C.C.; Meaburn, E.L.; Ronald, A.; Price, T.S.; Jeffries, A.R.; Schalkwyk, L.C.; Plomin, R.; Mill, J. Methylomic analysis of monozygotic twins discordant for autism spectrum disorder and related behavioural traits. Mol. Psychiatry 2014, 19, 495–503. [Google Scholar] [CrossRef]
- Voineagu, I.; Wang, X.; Johnston, P.; Lowe, J.K.; Tian, Y.; Horvath, S.; Mill, J.; Cantor, R.M.; Blencowe, B.J.; Geschwind, D.H. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011, 474, 380–384. [Google Scholar] [CrossRef]
- Nguyen, A.; Rauch, T.A.; Pfeifer, G.P.; Hu, V.W. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J. 2010, 24, 3036–3051. [Google Scholar] [CrossRef]
- Jossin, Y. Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation. Biomolecules 2020, 10, 964. [Google Scholar] [CrossRef]
- Lintas, C.; Sacco, R.; Persico, A.M. Differential methylation at the RELN gene promoter in temporal cortex from autistic and typically developing post-puberal subjects. J. Neurodev. Disord. 2016, 8, 18. [Google Scholar] [CrossRef]
- Zhubi, A.; Chen, Y.; Guidotti, A.; Grayson, D.R. Epigenetic regulation of RELN and GAD1 in the frontal cortex (FC) of autism spectrum disorder (ASD) subjects. Int. J. Dev. Neurosci. 2017, 62, 63–72. [Google Scholar] [CrossRef]
- Gomez-Fernandez, A.; de la Torre-Aguilar, M.J.; Gil-Campos, M.; Flores-Rojas, K.; Cruz-Rico, M.D.; Martin-Borreguero, P.; Perez-Navero, J.L. Children With Autism Spectrum Disorder With Regression Exhibit a Different Profile in Plasma Cytokines and Adhesion Molecules Compared to Children Without Such Regression. Front. Pediatr. 2018, 6, 264. [Google Scholar] [CrossRef] [PubMed]
- Ferri, S.L.; Abel, T.; Brodkin, E.S. Sex Differences in Autism Spectrum Disorder: A Review. Curr. Psychiatry Rep. 2018, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Migliore, L.; Nicolì, V.; Stoccoro, A. Gender Specific Differences in Disease Susceptibility: The Role of Epigenetics. Biomedicines 2021, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Hu, V.W.; Hong, Y.; Xu, M.; Shu, H.T. Altered DNA methylation in a severe subtype of idiopathic autism: Evidence for sex differences in affected metabolic pathways. Autism 2021, 25, 887–910. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Saad, K.; Chirumbolo, S.; Kern, J.K.; Geier, D.A.; Geier, M.R.; Urbina, M.A. Immune dysfunction and neuroinflammation in autism spectrum disorder. Acta Neurobiol. Exp. 2016, 76, 257–268. [Google Scholar] [CrossRef]
- Meyza, K.Z.; Blanchard, D.C. The BTBR mouse model of idiopathic autism—Current view on mechanisms. Neurosci. Biobehav. Rev. 2017, 76 Pt A, 99–110. [Google Scholar] [CrossRef]
- Nadeem, A.; Al-Harbi, N.O.; Ahmad, S.F.; Alhazzani, K.; Attia, S.M.; Alsanea, S.; Alhoshani, A.; Mahmood, H.M.; Alfardan, A.S.; Bakheet, S.A. Exposure to the plasticizer, Di-(2-ethylhexyl) phthalate during juvenile period exacerbates autism-like behavior in adult BTBR T + tf/J mice due to DNA hypomethylation and enhanced inflammation in brain and systemic immune cells. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110249. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Dammering, F.; Martins, J.; Dittrich, K.; Czamara, D.; Rex-Haffner, M.; Overfeld, J.; de Punder, K.; Buss, C.; Entringer, S.; Winter, S.M.; et al. The pediatric buccal epigenetic clock identifies significant ageing acceleration in children with internalizing disorder and maltreatment exposure. Neurobiol. Stress 2021, 15, 100394. [Google Scholar] [CrossRef]
- Oblak, L.; van der Zaag, J.; Higgins-Chen, A.T.; Levine, M.E.; Boks, M.P. A systematic review of biological; social and environmental factors associated with epigenetic clock acceleration. Ageing Res. Rev. 2021, 69, 101348. [Google Scholar] [CrossRef]
- Berdasco, M.; Esteller, M. Clinical epigenetics: Seizing opportunities for translation. Nat. Rev. Genet. 2019, 20, 109–127. [Google Scholar] [CrossRef]
- Aref-Eshghi, E.; Kerkhof, J.; Pedro, V.P.; France, G.D.; Barat-Houari, M.; Ruiz-Pallares, N.; Andrau, J.C.; Lacombe, D.; Van-Gils, J.; Fergelot, P.; et al. Evaluation of DNA Methylation Episignatures for Diagnosis and Phenotype Correlations in 42 Mendelian Neurodevelopmental Disorders. Am. J. Hum. Genet. 2021, 108, 1161–1163. [Google Scholar] [CrossRef]
- Sadikovic, B.; Levy, M.A.; Kerkhof, J.; Aref-Eshghi, E.; Schenkel, L.; Stuart, A.; McConkey, H.; Henneman, P.; Venema, A.; Schwartz, C.E.; et al. Clinical epigenomics: Genome-wide DNA methylation analysis for the diagnosis of Mendelian disorders. Genet. Med. 2021, 23, 1065–1074. [Google Scholar] [CrossRef]
- Wong, C.C.Y.; Smith, R.G.; Hannon, E.; Ramaswami, G.; Parikshak, N.N.; Assary, E.; Troakes, C.; Poschmann, J.; Schalkwyk, L.C.; Sun, W.; et al. Genome-wide DNA methylation profiling identifies convergent molecular signatures associated with idiopathic and syndromic autism in post-mortem human brain tissue. Hum. Mol. Genet. 2019, 28, 2201–2211. [Google Scholar] [CrossRef]


| Reference | Tissue Information | Sample Size | ASD Age (Range or Mean ± SD) | ASD Diagnosis and Severity | Methodology for DNA Methylation Analysis | Genomic Region Analysed | Main Findings |
|---|---|---|---|---|---|---|---|
| Alshamrani et al., 2023 [33] | Peripheral blood | 28 ASD and 24 TD | 7.5 ± 2.9 | DSM-5, CARS | ELISA assay | Global methylation | Global DNA hypomethylation in ASD subjects. |
| Andrews et al., 2018 [34] | Peripheral blood | 453 ASD and 515 TD | Between 3 and 5 years | ADOS, ADI-R | Illumina 450 K array | Genome-wide | No CpG sites reached EWAS threshold significance. Most DMPs were associated with the CENPM, FENDRR, SNRNP200, PGLYRP4, EZH1, DIO3, and CCDC181 genes. |
| Aspra et al., 2022 [35] | Buccal cells | 27 ASD and 15 TD | 5.2 ± 1.9 years | ADI-R, SRS | Illumina 450 K array | Genome-wide | The hypermethylation of DMR is associated with the ZFP57, CPXM2, and NRIP2 genes. The hypomethylation of DMRs is associated with the RASGRF2, GSTT1, FAIM, and SOX7 genes. |
| Bahado-Singh et al., 2019 [36] | Neonatal dried blood spots | 14 ASD and 10 TD | At birth (29 h–79 h after birth) | DSM-IV classification | Illumina 450 K array | Genome-wide | CpG methylation changes were found in 230 loci, associated with 249 genes, including some previously associated with ASD (EIF4E, FYN, SHANK1, and VIM). The best predictive CpG sites were associated with seven genes: NAV2, OXCT1, LOC389033, MYL9, ALS2CR4, C19orf73, and ASCL2. |
| Elagoz Yuksel et al., 2016 [37] | Peripheral blood | 27 ASD and 39 TD | Between 22 and 94 months | DSM-IV-TR, CARS | MSRE-PCR | OXTR gene | Higher frequency of OXTR promoter hypomethylation in ASD. |
| Gallo et al., 2022 [38] | Peripheral blood | 42 ASD | 4.8 ± 2.0 years | DSM-5, ADOS-2 | MS-HRM | MECP2, OXTR, HTR1A, RELN, BCL-2 and EN-2 genes | High maternal gestational weight gain associated with increased BDNF methylation. Lack of maternal folic acid supplementation and low RELN methylation associated with higher severity of ASD. |
| García-Ortiz et al., 2021 [39] | Peripheral blood | 53 ASD and 45 TD | 43.7 ± 11.2 months | DSM-5, ADI-R, ADOS-2 | Pyrosequencing | LINE-1 regions, NCAM1 and NGF genes | Decreased LINE-1 and increased NCAM1 methylation in ASD; increased NGF methylation in ASD with mental regression during the first two years of life compared to TD and to ASD without mental regression. |
| Gui et al., 2020 [40] | Buccal swabs | 51 infants, 10 of which developed ASD | Between 8 months and 2 years | MSEL, ADOS, ADI-R | Illumina 450 K array | Genome-wide | No global DNA methylation levels differences between children with and without ASD. The most associated CpG to ASD resided in TUFT1, CYCS, SND1 and CACNA2D1 genes. |
| Hannon et al., 2018 [41] | Neonatal dried blood spot | 629 ASD and 634 TD | 6.08 ± 3.24 days | WHO-ICD-10 diagnosis codes | Illumina 450 K array | Genome-wide | No CpG sites reach EWAS threshold significance. The most associated CpG to ASD resided in RALY gene. Significant association between increased polygenic burden for autism and methylomic variation at two specific loci in chromosome 8 close to FAM167A and RP1L1 genes, respectively. |
| Hu et al., 2020 [42] | Peripheral blood | 61 ASD and 66 TD | 4.02 ± 2.83 years | DSM-5, CARS, ABC | qMSP | HTR4 promoter gene | Decreased HTR4 methylation in ASD. The difference was significant in males, but not in females. Higher methylation in females ASD compared to males ASD. No differences between females and males TD subjects. |
| Jasoliya et al., 2022 [43] | Peripheral blood | 23 ASD, 23 FXSA, and 11 TD | Between 2 and 6 years | DSM-5, ADOS | EPIC array | Genome-wide | Two genes, PAK2 and FANCD2 differentially methylated between ASD and TD |
| Liang et al., 2019 [44] | Peripheral blood | 5 pairs of ASD-discordant monozygotic twins; 5 pairs of ASD concordant ASD monozygotic twins; 30 pairs of sporadic patients with age- and sex-matched controls | Twins = 5.7 years Sporadic ASD = 4.46 | DSM-5; ADOS | Illumina 450 K array, RRBS, pyrosequencing | Genome-wide, SH2B1 gene. | Identified 2397 DMRs between discordant twins, including the SH2B1 gene. Methylation of SH2B1 increased in ASD-discordant monozygotic twins compared to concordant monozygotic twins and in sporadic ASD compared to control subjects. |
| Melnyk et al., 2012 [45] | Peripheral blood | 68 ASD, 54 TD, 40 unaffected siblings | 5.8 ± 2.1 years | DSM-IV, ADOS, CARS | HPLC | Global methylation | Decreased global methylation in ASD compared to TD and siblings. |
| Neri de Souza Reis et al., 2021 [46] | Peripheral blood | 67 ASD | 4.7 ± 1.3 years | DSM-IV; ICD-10; ADI-R, CARS; VABS | Illumina 450 K array | Epigenetic clock | Parental and intrauterine ASD risk factors moderated by age acceleration associated with Vineland total score. Moreover, authors calculate the epigenetic clock as a proxy of post-natal stress exposure, finding that children had a higher biological age than chronological age. |
| Stoccoro et al., 2022 [47] | Peripheral blood | 58 ASD | 4.35 ± 1.79 years | ADOS-2 | MS-HRM | MECP2, OXTR, HTR1A, RELN, BCL-2 and EN-2 genes | Sex-related methylation differences: methylation levels of MECP2, HTR1A, and OXTR genes were connected to females, and those of EN2, BCL2, and RELN genes to males. Various maternal factors, including a lack of folic acid supplementation, were associated with high disease severity. BDNF methylation was associated with various ASD risk factors. |
| Wang et al., 2017 [48] | Peripheral blood | 54 ASD and 54 TD | 4.24 ± 0.98 years | DSM-IV, CARS, ABC | BSP | ESR2 gene | Eight CpG sites were hypermethylated in ASD. Four CpG sites were positively associated with severe symptoms |
| Yang et al., 2022 [49] | Peripheral blood | 30 ASD and 30 TD | Between 2 and 6 years | DSM-5, ADI-R | Pyrosequencing | ST8SIA2 gene | Methylation levels of two CpG sites of the ST8SIA2 gene were higher in ASD than in TD. One of these CpG sites positively correlated with the stereotyped behaviors of ASD children. |
| Zhao et al., 2018 [50] | Peripheral blood | 42 ASD and 26 TD | 4.07 ± 2.78 years | DSM-5, CARS, ABC | qMSP | TGFB1, BAX, IGFBP3, PRKCB, PSEN2, CCL2 | Methylation levels of TGFB1 were decreased in ASD compared to TD. TGFB1 methylation was positively associated with the interaction ability score. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoccoro, A.; Conti, E.; Scaffei, E.; Calderoni, S.; Coppedè, F.; Migliore, L.; Battini, R. DNA Methylation Biomarkers for Young Children with Idiopathic Autism Spectrum Disorder: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 9138. https://doi.org/10.3390/ijms24119138
Stoccoro A, Conti E, Scaffei E, Calderoni S, Coppedè F, Migliore L, Battini R. DNA Methylation Biomarkers for Young Children with Idiopathic Autism Spectrum Disorder: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(11):9138. https://doi.org/10.3390/ijms24119138
Chicago/Turabian StyleStoccoro, Andrea, Eugenia Conti, Elena Scaffei, Sara Calderoni, Fabio Coppedè, Lucia Migliore, and Roberta Battini. 2023. "DNA Methylation Biomarkers for Young Children with Idiopathic Autism Spectrum Disorder: A Systematic Review" International Journal of Molecular Sciences 24, no. 11: 9138. https://doi.org/10.3390/ijms24119138
APA StyleStoccoro, A., Conti, E., Scaffei, E., Calderoni, S., Coppedè, F., Migliore, L., & Battini, R. (2023). DNA Methylation Biomarkers for Young Children with Idiopathic Autism Spectrum Disorder: A Systematic Review. International Journal of Molecular Sciences, 24(11), 9138. https://doi.org/10.3390/ijms24119138








