A Systematic Review on the Continuous Cropping Obstacles and Control Strategies in Medicinal Plants
Abstract
:1. Introduction
| Plant Name | Plant Adaptive Reaction | Reference |
|---|---|---|
| Pinellia ternata | Plant growth decline, reductions in photosynthetic pigments, soluble sugar, yield, and alkaloids | [22] |
| Panax ginseng | Heavy metal and allelochemicals’ accumulation, imbalance in the rhizosphere micro-ecosystem, deterioration of physical and chemical properties, and soil-borne disease | [8] |
| Lilium lancifolium | Accumulation of pathogenic bacteria in the rhizosphere, causing severe root rot disease | [23] |
| Lepidium meyeni | Reduced N, P, K contents, soil organic matter, dry and fresh weight | [9] |
| Andrographis paniculata | Increased bacterial diversity, Acidobacteria and Zygomycota phyla and fungal genus Mortierella, alongside decreased fungal diversity and bacterial genus Pseudolabrys | [10] |
| Rehmannia glutinosa L. | Reduction in root activity, chlorophyll contents, and leaf size, and loss of ATPase activity in roots, growth rate, and photosynthetic efficiency | [13,24] |
| Coffea arabica L. | Increased major soil-borne diseases and yield decline | [25] |
| Angelica sinensis (Oliv.) Diels | Increased SS, EL, proline and MDA, alongside decreased CAT, SOD, POD, yield, chlorophyll contents, plant growth, photosynthetic rate, and essential oil contents | [26] |
| Panax notoginseng (Burk.) | Decreased production and tuber quality, and increased seedling mortality rate | [27] |
| Pogostemon cablin (Benth.) | Oxidative stress, nutritional deficiency, core metabolic disorder, and genotoxicity | [28] |
| Codonopsis tangshen (Oliv.) | Increased total soluble protein, MDA, and CAT activity, alongside reduced SOD, Chl. a (chlorophyll a), Chl. b (chlorophyll b), and total Chl. (total chlorophyll) | [29] |
| Panax Ginseng C.A. Mayer | Yellow spot disease on leaves and stems, and serious fibrous abscission in roots (rust rot and root rot) | [30] |
| Salvia miltiorrhiza Bge. | Loss of fresh weight, lipophilic, lithospermic acid, root length and diameter, salvianolic acid B, hydrophilic contents, and significant yield decline | [31] |
| Coptis chinensis Franch. | Decreased Proteobacteria and alpha diversity, increased Acedobacteria, change in rhizosphere species, and pH slightly acidic | [32] |
| Radix pseudostellariae L. | Reduction in acid protease and chitinase, alongside increased sucrose, urease, protocatechuic acid, and cellulose | [33] |
| Panax quinquefolium | Reduced soil pH, alpha fungal diversity, and bacterial diversity | [34] |
| Panax quinquefolius L. | Increased NO3-N, soil salt, and NH4+-N, alongside decreased cellulose activity, soil pH, alkaline phosphate, and C: N ratio | [21] |
2. Methods
3. Results and Discussion
3.1. Causes of Continuous Cropping Obstacles
3.1.1. Soil Microbial Community
3.1.2. Nutrient Availability
Soil Organic Matter and Organic Carbon Contents
Carbon (C) and Nitrogen (N) Cycling and Mineralization
Nutrient Deficiencies
Physiochemical Properties of Soil
3.1.3. Allelopathic Effects
3.1.4. Others
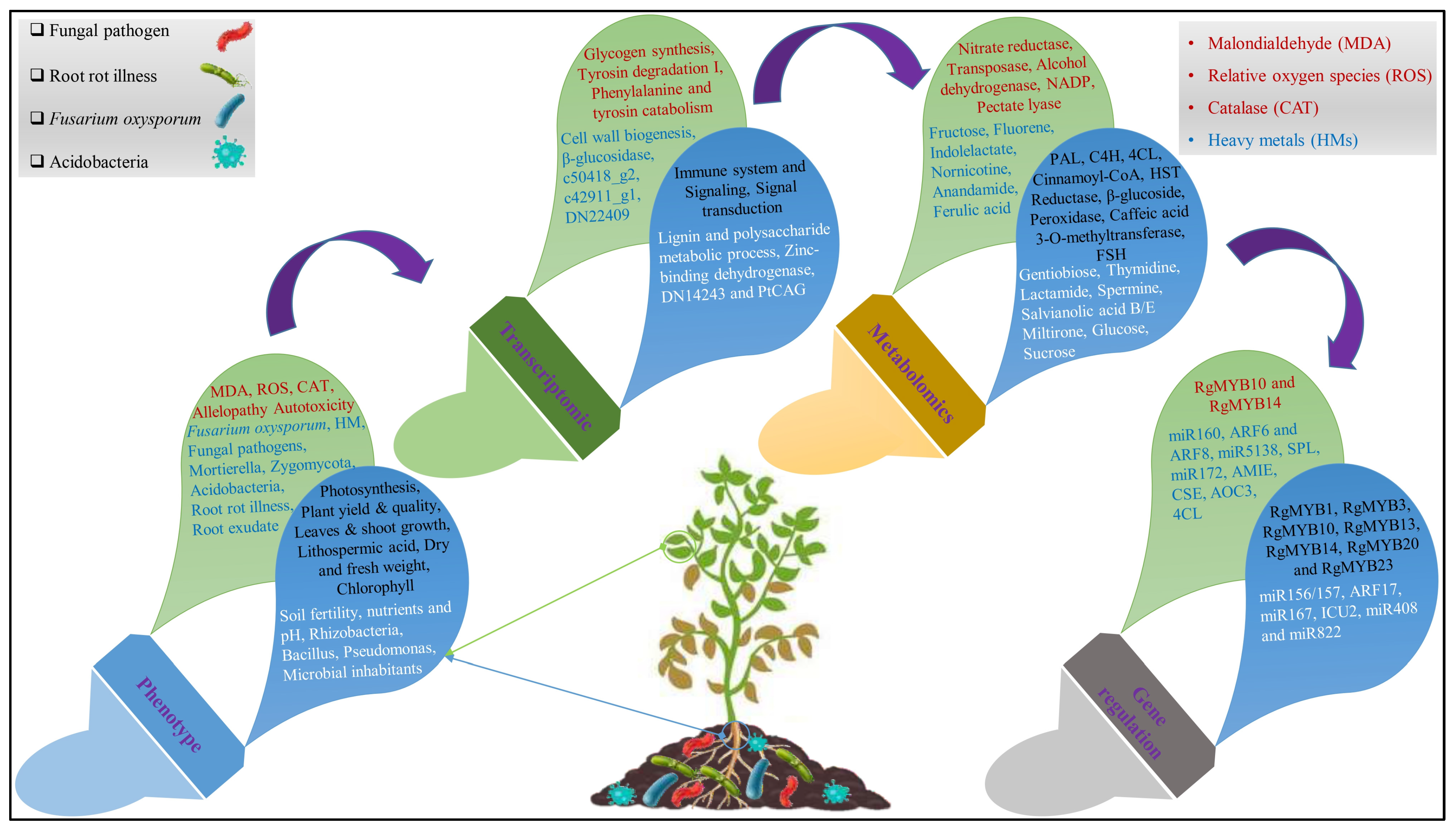
3.2. Molecular Mechanisms
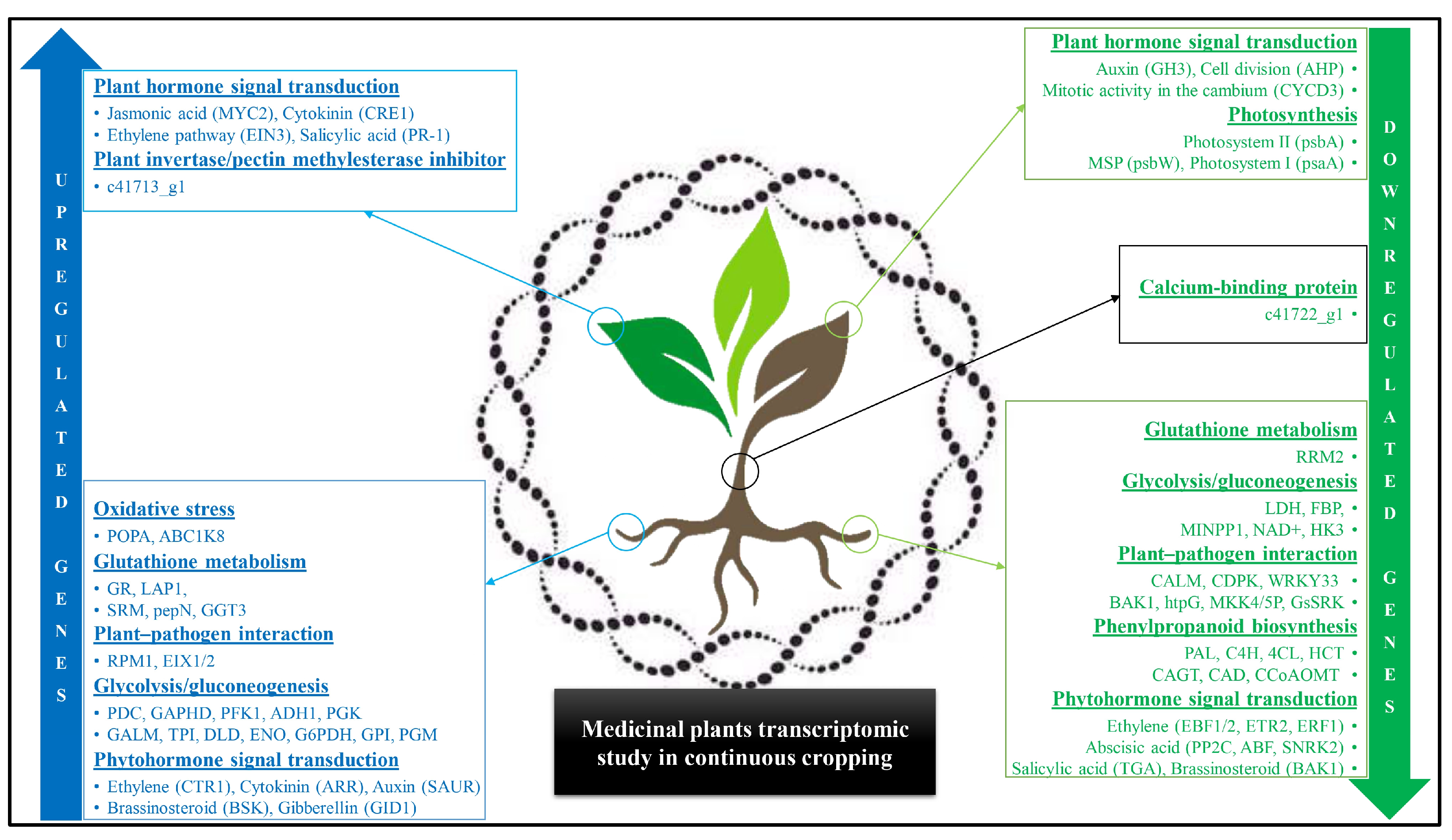
3.2.1. Transcriptomic Analysis
3.2.2. Metabolomics Analysis
3.2.3. Gene Regulation Network and miRNA Study
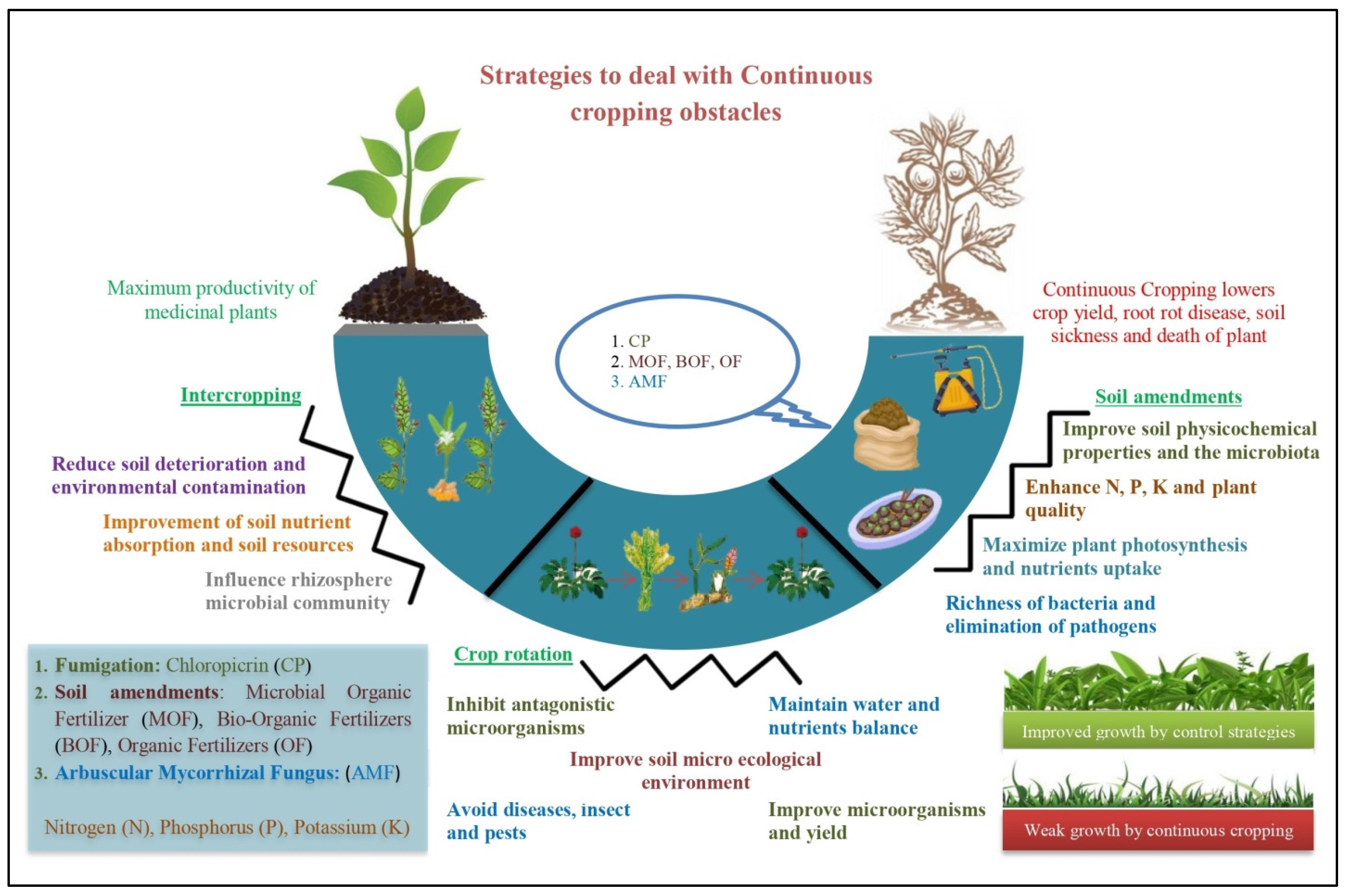
3.3. Strategy to Deal with Continuous Cropping Obstacles
3.3.1. Soil Amendments
3.3.2. Crop Rotation
3.3.3. Intercropping
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alami, M.M.; Pang, Q.; Gong, Z.; Yang, T.; Tu, D.; Zhen, O.; Yu, W.; Alami, M.J.; Wang, X. Continuous cropping changes the composition and diversity of bacterial communities: A meta-analysis in nine different fields with different plant cultivation. Agriculture 2021, 11, 1224. [Google Scholar] [CrossRef]
- Wacal, C.; Ogata, N.; Sasagawa, D.; Handa, T.; Basalirwa, D.; Acidri, R.; Ishigaki, T.; Yamamoto, S.; Nishihara, E. Seed yield, crude protein and mineral nutrient contents of sesame during a two-year continuous cropping on upland field converted from a paddy. Field Crops Res. 2019, 240, 125–133. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Cruse-Sanders, J.; Chamberlain, J.L.; Ferreira, S.; Young, J.A. Explaining harvests of wild-harvested herbaceous plants: American ginseng as a case study. Biol. Conserv. 2019, 231, 139–149. [Google Scholar] [CrossRef]
- Wu, L.K.; Chen, J.; Wu, H.M.; Wang, J.Y.; Wu, Y.H.; Lin, S.; Khan, M.U.; Zhang, Z.Y.; Lin, W.X. Effects of consecutive monoculture of Pseudostellaria heterophylla on soil fungal community as determined by pyrosequencing. Sci. Rep. 2016, 6, 26601. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Sun, Y.; Liu, Q.M.; Li, Z.; Wei, M.C.; Zhao, F.R. Causes of continuous cropping obstacles and biological control of Panax quinquefolium L. J. Chin. Med. Mat. 2016, 39, 2665–2667. [Google Scholar]
- Li, L.; Jiang, J.L. Research advances in allelopathic autotoxicity and continuous cropping obstacle of American ginseng. Mol. Plant Breed. 2018, 16, 4436–4443. [Google Scholar]
- Wang, G.; Ren, Y.; Bai, X.; Su, Y.; Han, J. Contributions of beneficial microorganisms in soil remediation and quality improvement of medicinal plants. Plants 2022, 11, 3200. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dong, L.L.; Xu, J.; Chen, J.W.; Li, X.W.; Chen, S.L. Progress in improvement of continuous monoculture cropping problem in Panax ginseng by controlling soil-borne disease management. China J. Chin. Mater. Med. 2016, 41, 3890–3896. [Google Scholar]
- Wang, S.; Dong, L.Q.; Luo, Y.Y.; Jia, W.J.; Qu, Y. Characterization of rhizosphere microbial communities in continuous cropping maca (Lepidium meyenii) red soil, Yunnan, China. Arch. Agron. Soil Sci. 2019, 66, 805–818. [Google Scholar] [CrossRef]
- Li, J.R.; Chen, X.Z.; Li, S.M.; Zuo, Z.M.; Zhan, R.T.; He, R. Variations of rhizospheric soil microbial communities in response to continuous Andrographis paniculata cropping practices. Bot. Stud. 2020, 61, 18. [Google Scholar] [CrossRef]
- Zhou, X.; Li, C.; Liu, L.; Zhao, J.; Zhang, J.; Cai, Z.; Huang, X. Control of Fusarium wilt of lisianthus by reassembling the microbial community in infested soil through reductive soil disinfestation. Microbiol. Res. 2019, 220, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, S.M.; Rather, G.A.; Sharma, A.; Lattoo, S.K. In perspective: Potential medicinal plant resources of Kashmir Himalayas, their domestication and cultivation for commercial exploitation. J. Appl. Res. Med. Aromat. Plants 2018, 8, 10–25. [Google Scholar] [CrossRef]
- Yin, W.; Du, J.; Li, J.; Zhang, Z. Effects of continuous cropping obstacle on growth of Rehmannia glutinosa. China J. Chin. Mater. Medica 2009, 34, 18–21. [Google Scholar]
- Luo, L.; Guo, C.; Wang, L.; Zhang, J.; Deng, L.; Luo, K.; Huang, H.; Liu, Y.; Mei, X.; Zhu, S. Negative plant-soil feedback driven by re-assemblage of the rhizosphere microbiome with the growth of Panax notoginseng. Front. Microbiol. 2019, 10, 1597. [Google Scholar] [CrossRef] [Green Version]
- Bao, L.; Liu, Y.; Ding, Y.; Shang, J.; Wei, Y.; Tan, Y.; Zi, F. Interactions between phenolic acids and microorganisms in rhizospheric soil from continuous cropping of Panax notoginseng. Front. Microbiol. 2022, 13, 791603. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Lin, S.; Chu, L.; Gao, J.; Azeem, S.; Lin, W. Insight into structure dynamics of soil microbiota mediated by the richness of replanted Pseudostellaria heterophylla. Sci. Rep. 2016, 6, 26175. [Google Scholar] [CrossRef]
- Wu, L.K.; Chen, J.; Xiao, Z.G.; Zhu, X.C.; Wang, J.Y.; Wu, H.M.; Wu, Y.H.; Zhang, Z.Y.; Lin, W.X. Barcoded pyrosequencing reveals a shift in the bacterial community in the rhizosphere and rhizoplane of Rehmannia glutinosa under consecutive monoculture. Int. J. Mol. Sci. 2018, 19, 850. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.K.; Wu, H.M.; Chen, J.; Wang, J.Y.; Lin, W.X. Microbial community structure and its temporal changes in Rehmannia glutinosa rhizospheric soils monocultured for different years. Eur. J. Soil Biol. 2016, 72, 1–5. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Belay, A.; Claassens, A.S.; Wehner, F.C. Soil nutrient contents, microbial properties and maize yield under long-term legume-based crop rotation and fertilization: A comparison of residual effect of manure and NPK fertilizers. S. Afr. J. Plant Soil 2002, 19, 104–110. [Google Scholar] [CrossRef]
- Liu, N.; Shao, C.; Sun, H.; Liu, Z.; Guan, Y.; Wu, L.; Zhang, L.; Pan, X.; Zhang, Z.; Zhang, Y.; et al. Arbuscular mycorrhizal fungi biofertilizer improves American ginseng (Panax quinquefolius L.) growth under the continuous cropping regime. Geoderma 2020, 363, 114155. [Google Scholar] [CrossRef]
- An, Y.; Yang, D.; Li, X.; Jin, X.J. Study on the effect and physiological mechanism of continuous cropping obstruction of Pinellia ternata. Acta Agric. Borealioccident. Sin. 2018, 27, 1017–1022. [Google Scholar]
- Dai, L.; Singh, S.K.; Gong, H.; Tang, Y.; Peng, Z.; Zhang, J.; Wu, D.; Zhang, H.; He, D. Rhizospheric microbial consortium of Lilium lancifolium Thunb. causes lily root rot under continuous cropping system. Front. Microbiol. 2022, 13, 981615. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Niu, M.M.; Zheng, H.Y.; Wang, J.M.; Wu, L.K.; Li, Z.F.; Zhang, Z.Y. Effect of continuous cropping of Rehmannia on its morphological and physiological characteristics. J. Chin. Med. Mater. 2013, 36, 691–695. [Google Scholar]
- Zhao, Q.; Xiong, W.; Xing, Y.; Sun, Y.; Lin, X.; Dong, Y. Long-term coffee monoculture alters soil chemical properties and microbial communities. Sci. Rep. 2018, 8, 6116. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, E.; Wang, H.; Lang, D. Effects of continuous cropping obstacle on growth of Angelica sinensis and its mechanism. China J. Chin. Mater. Medica 2010, 35, 1231–1234. [Google Scholar]
- Tan, Y.; Cui, Y.; Li, H.; Kuang, A.; Li, X.; Wei, Y.; Ji, X. Rhizospheric soil and root endogenous fungal diversity and composition in response to continuous Panax notoginseng cropping practices. Microbiol. Res. 2017, 194, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.P.; Cao, S.J.; Wu, Y.G.; Ye, Z.C.; Zhang, C.; Yao, G.L.; Yu, J.; Yang, D.M.; Zhang, J.F. Integrated analysis of physiological, mRNA sequencing and miRNA sequencing data reveals a specific mechanism for the response to continuous cropping obstacles in Pogostemon cablin roots. Front. Plant Sci. 2022, 13, 853110. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, M.; Zhou, W.; Ai, L.; You, J.; Liu, H.; You, J.; Wang, H.; Wassie, M.; Wang, M.; et al. Transcriptome analysis reveals novel insights into the continuous cropping induced response in Codonopsis tangshen, a medicinal herb. Plant Physiol. Biochem. 2019, 141, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.L.; Cheng, L.Y.; Zhang, T.; Li, Q.; Zhan, Y.; Yan, N.; Wang, E.P.; Chen, C.B. Identification of ginseng (Panax ginseng C.A. Mayer) continuous cropping obstacle responsive miRNAs and their target genes. Appl. Ecol. Environ. Res. 2023, 21, 1025–1041. [Google Scholar] [CrossRef]
- Liu, H.; Niu, M.; Zhu, S.; Zhang, F.; Liu, Q.; Liu, Y.; Liu, R.H.; Zhang, Y.P. Effect study of continuous monoculture on the quality of Salvia miltiorrhiza Bge roots. BioMed Res. Int. 2020, 4284385. [Google Scholar] [CrossRef] [PubMed]
- Alami, M.M.; Xue, J.; Ma, Y.; Zhu, D.; Gong, Z.; Shu, S.; Wang, X. Structure, diversity, and composition of bacterial communities in rhizospheric soil of Coptis chinensis Franch under continuously cropped fields. Diversity 2020, 12, 57. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Wu, L.; Zhu, Q.; Wang, J.; Qin, X.; Xu, J.; Kong, L.; Chen, J.; Lin, S.; Umar, K.M.; et al. The role of organic acids on microbial deterioration in the Radix pseudostellariae rhizosphere under continuous monoculture regimes. Sci. Rep. 2017, 7, 3497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Fan, S.; Qin, J.; Dai, J.; Zhao, F.; Gao, L.; Lian, X.; Shang, W.; Xu, X.; Hu, X. Changes in the microbiome in the soil of an American ginseng continuous plantation. Front. Plant Sci. 2020, 11, 572199. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Singh, J. Long-term effects of continuous cropping and different nutrient management practices on the distribution of organic nitrogen in soil under rice-wheat system. Plant Soil Environ. 2014, 60, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, A.J.; DeBruyn, J.M.; Allen, F.L.; Radosevich, M.; Owens, P.R. Microbial community structure is affected by cropping sequences and poultry litter under long-term no-tillage. Soil Biol. Biochem. 2017, 114, 210–219. [Google Scholar] [CrossRef]
- Van Wyk, D.A.; Adeleke, R.; Rhode, O.H.; Bezuidenhout, C.C.; Mienie, C. Ecological guild and enzyme activities of rhizosphere soil microbial communities associated with Bt-maize cultivation under field conditions in north west province of South Africa. J. Basic Microbiol. 2017, 57, 781–792. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [Green Version]
- Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.; Chen, D.; Wei, H.; Saleem, M. Continuous cropping alters multiple biotic and abiotic indicators of soil health. Soil Syst. 2020, 4, 59. [Google Scholar] [CrossRef]
- Enwall, K.; Nyberg, K.; Bertilsson, S.; Cederlund, H.; Stenström, J.; Hallin, S. Long-term impact of fertilization on activity and composition of bacterial communities and metabolic guilds in agricultural soil. Soil Biol. Biochem. 2007, 39, 106–115. [Google Scholar] [CrossRef]
- Klein, E.; Katan, J.; Gamliel, A. Soil suppressiveness by organic amendment to Fusarium disease in cucumber: Effect on pathogen and host. Phytoparasitica 2016, 44, 239–249. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.Z.; Liu, Q.Z.; Zhang, Y.T.; Li, X.Y.; Li, H.Q.; Li, W.H. Phase changes of continuous cropping obstacles in strawberry (Fragaria × ananassa Duch.) production. Appl. Soil Ecol. 2020, 155, 103626. [Google Scholar] [CrossRef]
- Urashima, Y.; Sonoda, T.; Fujita, Y.; Uragami, A. Application of PCR-denaturing-gradient gel electrophoresis (DGGE) method to examine microbial community structure in asparagus fields with growth inhibition due to continuous cropping. Microbes Environ. 2012, 27, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santhanam, R.; Luu, V.T.; Weinhold, A.; Goldberg, J.; Oh, Y.; Baldwin, I.T. Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc. Natl. Acad. Sci. USA 2015, 112, E5013–E5020. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Picone, N.; Pol, A.; Mesman, R.; van Kessel, M.A.; Cremers, G.; van Gelder, A.H.; van Alen, T.A.; Jetten, M.S.; Lücker, S.; Op den Camp, H.J. Ammonia oxidation at pH 2.5 by a new gammaproteobacterial ammonia-oxidizing bacterium. ISME J. 2021, 15, 1150–1164. [Google Scholar] [CrossRef]
- Smith, E.G.; Zentner, R.P.; Campbell, C.A.; Lemke, R.; Brandt, K. Long term crop rotation effects on production, grain quality, profitability, and risk in the northern great plains. J. Agron. 2017, 109, 957–967. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.; Jones, H.G.; Karley, A.J.; et al. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef]
- Naresh, R.K.; Chandra, M.S.; Baliyan, A.; Kumar, B.N.; Kanaujiya, P.K.; Pathak, S.O.; Kumar, N. Carbon Dynamics in Climate Smart Agriculture Precision Land Leveling Practices on Topsoil Microbial Community Changes and Soil Organic Carbon in Cereal Based Cropping Systems of Sub-Tropical India: A Review. Int. J. Plant Soil Sci. 2021, 33, 53–66. [Google Scholar] [CrossRef]
- Karunakaran, G.; Suriyaprabha, R.; Manivasakan, P.; Yuvakkumar, R.; Rajendran, V.; Prabu, P.; Kannan, N. Effect of nanosilica and silicon sources on plant growth promoting rhizobacteria, soil nutrients and maize seed germination. IET Nanobiotechnol. 2013, 7, 70–77. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, A.; Dick, W.A. Bacterial community diversity in soil under two tillage practices as determined by pyrosequencing. Microb. Ecol. 2015, 70, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Cayetano, R.D.A.; Park, J.; Kim, G.B.; Jung, J.H.; Kim, S.H. Enhanced anaerobic digestion of waste-activated sludge via bioaugmentation strategy—Phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt2) analysis through hydrolytic enzymes and possible linkage to system performance. Bioresour. Technol. 2021, 332, 125014. [Google Scholar] [CrossRef] [PubMed]
- Nunan, N.; Schmidt, H.; Raynaud, X. The ecology of heterogeneity: Soil bacterial communities and C dynamics. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlen, D.L.; Hurley, E.G.; Andrews, S.S.; Cambardella, C.A.; Meek, D.W.; Duffy, M.D.; Mallarino, A.P. Crop rotation effects on soil quality at three northern corn/soybean belt locations. Agron. J. 2006, 98, 484–495. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Bora, S.; Thrall, P.H.; Richardson, A.E. Soil C and N as causal factors of spatial variation in extracellular enzyme activity across grassland-woodland ecotones. Appl. Soil Ecol. 2016, 105, 1–8. [Google Scholar] [CrossRef]
- Ashagrie, Y.; Zech, W.; Guggenberger, G.; Mamo, T. Soil aggregation, and total and particulate organic matter following conversion of native forests to continuous cultivation in Ethiopia. Soil Tillage Res. 2007, 94, 101–108. [Google Scholar] [CrossRef]
- Borjesson, G.; Bolinder, M.A.; Kirchmann, H.; Kätterer, T. Organic carbon stocks in topsoil and subsoil in long-term ley and cereal monoculture rotations. Biol. Fertil. Soils 2018, 54, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Hati, K.M.; Swarup, A.; Dwivedi, A.K.; Misra, A.K.; Bandyopadhyay, K.K. Changes in soil physical properties and organic carbon status at the topsoil horizon of a vertisol of central India after 28 years of continuous cropping, fertilization and manuring. Agric. Ecosyst. Environ. 2007, 119, 127–134. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Allen, F.L.; Wight, J.P.; Saxton, A.M.; Tyler, D.D.; Sams, C.E. Soil organic carbon sequestration rates under crop sequence diversity, bio-covers, and no-tillage. Soil Sci. Soc. Am. J. 2014, 78, 1726–1733. [Google Scholar] [CrossRef]
- Wacal, C.; Ogata, N.; Basalirwa, D.; Sasagawa, D.; Ishigaki, T.; Handa, T.; Kato, M.; Tenywa, M.M.; Masunaga, T.; Yamamoto, S.; et al. Imbalanced soil chemical properties and mineral nutrition in relation to growth and yield decline of sesame on different continuously cropped upland fields converted paddy. Agronomy 2019, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- Fiorini, A.; Boselli, R.; Maris, S.C.; Santelli, S.; Ardenti, F.; Capra, F.; Tabaglio, V. May conservation tillage enhance soil C and N accumulation without decreasing yield in intensive irrigated croplands? Results from an eight-year maize monoculture. Agric. Ecosyst. Environ. 2020, 296, 106926. [Google Scholar] [CrossRef]
- Katterer, T.; Bolinder, M.A.; Andrén, O.; Kirchmann, H.; Menichetti, L. Roots contribute more to refractory soil organic matter than above-ground crop residues, as revealed by a long-term field experiment. Agric. Ecosyst. Environ. 2011, 141, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Liptzin, D.; Norris, C.E.; Cappellazzi, S.B.; Mac, B.G.; Cope, M.; Greub, K.L.; Rieke, E.L.; Tracy, P.W.; Aberle, E.; Ashworth, A.; et al. An evaluation of carbon indicators of soil health in long-term agricultural experiments. Soil Biol. Biochem. 2022, 172, 108708. [Google Scholar] [CrossRef]
- Aparicio, V.; Costa, J.L. Soil quality indicators under continuous cropping systems in the Argentinean Pampas. Soil Tillage Res. 2007, 96, 155–165. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Ciescinska, B. Effect of crop rotation and long term fertilization on the carbon and glomalin content in the soil. J. Cent. Eur. Agric. 2012, 13, 814–821. [Google Scholar] [CrossRef]
- Mahal, N.; Osterholz, W.; Miguez, F.; Poffenbarger, H.; Sawyer, J.; Olk, D.; Archontoulis, S.; Castellano, M. Nitrogen fertilizer suppresses mineralization of soil organic matter in maize agroecosystems. Front. Ecol. Evol. 2019, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Gentry, L.E.; Below, F.E.; David, M.B.; Bergerou, J.A. Source of the soybean N credit in maize production. Plant Soil 2001, 236, 175–184. [Google Scholar] [CrossRef]
- Gentry, L.F.; Ruffo, M.L.; Below, F.E. Identifying factors controlling the continuous corn yield penalty. Agron. J. 2013, 105, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Agegnehu, G.; Lakew, B.; Nelson, P.N. Cropping sequence and nitrogen fertilizer effects on the productivity and quality of malting barley and soil fertility in the Ethiopian highlands. Arch. Agron. Soil Sci. 2014, 60, 1261–1275. [Google Scholar] [CrossRef] [Green Version]
- Raguet, P.; Cade-Menun, B.; Mollier, A.; Abdi, D.; Ziadi, N.; Karam, A.; Morel, C. Mineralization and speciation of organic phosphorus in a sandy soil continuously cropped and phosphorus-fertilized for 28 years. Soil Biol. Biochem. 2023, 178, 108938. [Google Scholar] [CrossRef]
- Hussain, M.Z.; Bhardwaj, A.K.; Basso, B.; Robertson, G.P.; Hamilton, S.K. Nitrate leaching from continuous corn, perennial grasses, and poplar in the US Midwest. J. Environ. Qual. 2019, 48, 1849–1855. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, J.E.; Willson, T.C.; Kizilkaya, K.; Parker, E.; Harwood, R.R. Enhancing the mineralizable nitrogen pool through substrate diversity in long term cropping systems. Soil Sci. Soc. Am. J. 2001, 65, 1442–1447. [Google Scholar] [CrossRef]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. Crop yield, plant nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on Nitisols. Soil Tillage Res. 2016, 160, 1–13. [Google Scholar] [CrossRef]
- Jiao, X.L.; Zhang, X.S.; Lu, X.H.; Qin, R.; Bi, Y.M.; Gao, W.W. Effects of maize rotation on the physicochemical properties and microbial communities of American ginseng cultivated soil. Sci. Rep. 2019, 9, 8615. [Google Scholar] [CrossRef] [Green Version]
- Studdert, G.A.; Monterubbianesi, M.G.; Domínguez, G.F. Use of RothC to simulate changes of organic carbon stock in the arable layer of a Mollisol of the southeastern Pampas under continuous cropping. Soil Tillage Res. 2011, 117, 191–200. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Owens, P.R.; Allen, F.L. Long-term cropping systems management influences soil strength and nutrient cycling. Geoderma 2020, 361, 114062. [Google Scholar] [CrossRef]
- Domínguez, G.F.; Diovisalvi, N.V.; Studdert, G.A.; Monterubbianesi, M.G. Soil organic C and N fractions under continuous cropping with contrasting tillage systems on mollisols of the southeastern Pampas. Soil Tillage Res. 2009, 102, 93–100. [Google Scholar] [CrossRef]
- Zhalnina, K.; Dias, R.; de Quadros, P.D.; Davis-Richardson, A.; Camargo, F.A.; Clark, I.M.; McGrath, S.P.; Hirsch, P.R.; Triplett, E.W. Soil pH determines microbial diversity and composition in the park grass experiment. Microb. Ecol. 2015, 69, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Bowles, T.M.; Acostamartinez, V.; Calderon, F.J.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil. Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Wang, S.; Ren, J.; Huang, T.; Qiao, G.; Hu, H.; Li, W.; Wu, F.; Pan, K. Evaluation of soil enzyme activities and microbial communities in tomato continuous cropping soil treated with jerusalem artichoke residues. Commun. Soil Sci. Plant Anal. 2018, 49, 2727–2740. [Google Scholar]
- Rubio, V.; Quincke, A.; Ernst, O. Deep tillage and nitrogen do not remediate cumulative soil deterioration effects of continuous cropping. Agron. J. 2021, 113, 5584–5596. [Google Scholar] [CrossRef]
- Jones, A.R.; Orton, T.G.; Dalal, R.C. The legacy of cropping history reduces the recovery of soil carbon and nitrogen after conversion from continuous cropping to permanent pasture. Agric. Ecosyst. Environ. 2016, 216, 166–176. [Google Scholar] [CrossRef]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant allelochemicals: Agronomic, nutritional and ecological relevance in the soil system. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Ullah, S.; Xu, Y.; Liao, C.; Li, W.; Cheng, F.; Ye, S.; Yang, M. Continuous planting Eucalyptus plantations in subtropical China: Soil phenolic acid accumulation and adsorption physiognomies. Front. For. Glob. Chang. 2023, 6, 1135029. [Google Scholar] [CrossRef]
- Kumar, S.; Jakhar, S.R.; Dahiya, S.; Jangir, C.K.; Meena, R.S. Soil sickness and productivity from ecological aspects. J. Pharmacogn. Phytochem. 2017, 6, 827–831. [Google Scholar]
- Yeasmin, R.; Nakamatsu, K.; Matsumoto, H.; Motoki, S.; Nishihara, E.; Yamamoto, S. Inference of allelopathy and autotoxicity to varietal resistance of asparagus (Asparagus officinalis L.). Aust. J. Crop Sci. 2014, 8, 251–256. [Google Scholar]
- Hu, Y.S.; Wu, K.; Li, C.X.; Sun, F.L.; Jia, X.C. Effects of phenolic compounds on the growth of Cucumis sativus seedlings and Fusarium oxysporum hypha. Chin. J. Ecol. 2007, 26, 1738–1742. [Google Scholar]
- Yuan, F.; Zhang, C.L.; Shen, Q.R. Effect and mechanism of phenol compounds in alleviating cucumber Fusarium wilt. Sci. Agric. Sin. 2004, 34, 545–551. [Google Scholar]
- Chen, P.; Hou, Y.; Zhuge, Y.; Wei, W.; Huang, Q. The effects of soils from different forest types on the growth of the invasive plant Phytolacca americana. Forests 2019, 10, 492. [Google Scholar] [CrossRef] [Green Version]
- Topps, D.; Khabir, M.I.U.; Abdelmagid, H.; Jackson, T.; Iqbal, J.; Robertson, B.K.; Pervaiz, Z.H.; Saleem, M. Impact of cover crop monocultures and mixtures on organic carbon contents of soil aggregates. Soil Syst. 2021, 5, 43. [Google Scholar] [CrossRef]
- Ali, A.; Imran Ghani, M.; Li, Y.; Ding, H.; Meng, H.; Cheng, Z. Hiseq base molecular characterization of soil microbial community, diversity structure, and predictive functional profiling in continuous cucumber planted soil affected by diverse cropping systems in an intensive greenhouse region of northern China. Int. J. Mol. Sci. 2019, 20, 2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.K.; Yan, Z.Q.; Li, X.Z.; Jin, H.; Yang, X.Y.; Xie, M.; Su, A.X.; Qin, B. Characterization of allelochemicals from the rhizosphere soil of Pinellia ternate (Thnub.) and their inhibition activity on protective enzymes. Appl. Soil Ecol. 2018, 125, 301–306. [Google Scholar] [CrossRef]
- Tagele, S.B.; Kim, R.H.; Jeong, M.; Jung, D.R.; Lee, D.; Shin, J.H. An optimized biofumigant improves pepper yield without exerting detrimental effects on soil microbial diversity. Chem. Biol. Technol. Agric. 2022, 9, 99. [Google Scholar] [CrossRef]
- Briar, S.S.; Wichman, D.; Reddy, G.V. Plant-parasitic nematode problems in organic agriculture. J. Sustain. Agric. 2016, 9, 107–122. [Google Scholar]
- Murage, E.W.; Karanja, N.K.; Smithson, P.C.; Woomer, P.L. Diagnostic indicators of soil quality in productive and non-productive smallholders’ fields of Kenya’s Central Highlands. Agric. Ecosyst. Environ. 2000, 79, 1–8. [Google Scholar] [CrossRef]
- Higo, M.; Sato, R.; Serizawa, A.; Takahashi, Y.; Gunji, K.; Tatewaki, Y.; Isobe, K. Can phosphorus application and cover cropping alter arbuscular mycorrhizal fungal communities and soybean performance after a five-year phosphorus-unfertilized crop rotational system? PeerJ 2018, 6, e4606. [Google Scholar] [CrossRef] [Green Version]
- Edwards, D.; Batley, J.; Snowdon, R.J. Accessing complex crop genomes with next-generation sequencing. Theor. Appl. Genet. 2013, 126, 1–11. [Google Scholar] [CrossRef]
- Wu, B.; Long, Q.; Gao, Y.; Wang, Z.; Shao, T.; Liu, Y.; Li, Y.; Ding, W. Comprehensive characterization of a time-course transcriptional response induced by autotoxins in Panax ginseng using RNA-Seq. BMC Genom. 2015, 16, 1010. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; He, L.; Wu, Y.; Ma, W.; Chen, H.; Ye, Z. Comparative proteomic analysis of Pogostemon cablin leaves after continuous cropping. Protein Expr. Purif. 2018, 152, 13–22. [Google Scholar] [CrossRef]
- Xu, Y.X.; Liu, J.J.; Liu, X.F.; Li, H.; Yang, Z.; Wang, H.B.; Huang, X.Y.; Lan, L.; An, Y.T.; Li, L.J.; et al. Continuous cropping of alfalfa (Medicago sativa L.) reduces bacterial diversity and simplifies cooccurrence networks in aeolian sandy soil. Soil Ecol. Lett. 2021, 4, 131–143. [Google Scholar] [CrossRef]
- Zhou, W.; Jiang, X.; Tan, X.; Li, D.; Wang, H.; You, J.; Li, X.; Zhang, M. Transcriptome analysis provides novel insights into the soil amendments induced response in continuously cropped Codonopsis tangshen. Front. Plant Sci. 2022, 13, 972804. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, Y.; Yan, Y.; Qin, S.; He, H.; Mao, R.; Liang, Z. Dynamic analysis of physiological indices and transcriptome profiling revealing the mechanisms of the allelopathic effects of phenolic acids on Pinellia ternata. Front. Plant Sci. 2022, 13, 1039507. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Zhan, R.; He, R. Transcriptome profiling reveals metabolic alteration in Andrographis paniculata in response to continuous cropping. Ind. Crops Prod. 2019, 137, 585–596. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R. Patchouli (Pogostemon cablin Benth.): Botany, agrotechnology and biotechnological aspects. Ind. Crops Prod. 2016, 87, 161–176. [Google Scholar] [CrossRef]
- Yan, W.; Liu, X.; Cao, S.; Yu, J.; Zhang, J.; Yao, G.; Yang, H.; Yang, D.; Wu, Y. Molecular basis of Pogostemon cablin responding to continuous cropping obstacles revealed by integrated transcriptomic, miRNA and metabolomic analyses. Ind. Crops Prod. 2023, 200, 116862. [Google Scholar] [CrossRef]
- Lim, E.K.; Doucet, C.J.; Li, Y.; Elias, L.; Worrall, D.; Spencer, S.P.; Ross, J.; Bowles, D.J. The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4- hydroxybenzoic acid and other benzoates. J. Biol. Chem. 2002, 277, 586–592. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.H.; Ding, Y.Q.; Nie, Y.X.; Wang, X.J.; An, Y.Q.; Roessner, U.; Walker, R.; Du, B.; Bai, J.G. Plant metabolomics integrated with transcriptomics and rhizospheric bacterial community indicates the mitigation effects of Klebsiella oxytoca P620 on p-hydroxybenzoic acid stress in cucumber. J. Hazard. Mater. 2021, 415, 125756. [Google Scholar] [CrossRef]
- Yan, W.; Cao, S.; Liu, X.; Yao, G.; Yu, J.; Zhang, J.; Bian, T.; Yu, W.; Wu, Y. Combined physiological and transcriptome analysis revealed the response mechanism of Pogostemon cablin roots to p-hydroxybenzoic acid. Front. Plant Sci. 2022, 13, 980745. [Google Scholar] [CrossRef]
- Mashego, M.R.; Rumbold, K.; De Mey, M.; Vandamme, E.; Soetaert, W.; Heijnen, J.J. Microbial metabolomics: Past, present and future methodologies. Biotechnol. Lett. 2007, 29, 1–16. [Google Scholar] [CrossRef]
- Collins, S.L.; Koo, I.; Peters, J.M.; Smith, P.B.; Patterson, A.D. Current Challenges and Recent Developments in Mass Spectrometry–Based Metabolomics. Annu. Rev. Anal. Chem. 2021, 14, 467–487. [Google Scholar] [CrossRef]
- Meng, X.; Huang, X.; Li, Q.; Wang, E.; Chen, C. Application of UPLC-Orbitrap-HRMS targeted metabolomics in screening of allelochemicals and model plants of ginseng. J. Plant Physiol. 2023, 285, 153996. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, L.; Gao, J.; Niu, F.; Duan, X.; Yin, L.; Tian, W. Identification of autotoxic compounds from Atractylodes macrocephala Koidz and preliminary investigations of their influences on immune system. J. Plant Physiol. 2018, 230, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Song, J.; Luo, H.; Zhang, Y.; Li, Q.; Zhu, Y.; Xu, J.; Li, Y.; Song, C.; Wang, B.; et al. Analysis of the genome sequence of the medicinal plant Salvia miltiorrhiza. Mol. Plant 2016, 9, 949–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Cui, L.; Zhou, B.; Wang, X.; Guo, L.; Liu, W. Visualizing the spatial distribution and alteration of metabolites in continuously cropped Salvia miltiorrhiza Bge using MALDI-MSI. J. Pharm. Anal. 2022, 12, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Ma, S.; Li, L.; Wang, D.; Liu, W.; Liu, F.; Guo, L.; Wang, X. Visualizing the distributions and spatiotemporal changes of metabolites in Panax notoginseng by MALDI mass spectrometry imaging. J. Ginseng Res. 2021, 45, 726–733. [Google Scholar] [CrossRef]
- Li, L.; Jiang, J.L. Analysis of specific metabolites in rhizosphere soil of Panax quinquefolius L. with root rot diseases based on metabolomics. Med. Plant Res. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Tu, N.; Hu, Y.; Jin, C.; Luo, Y.; Liu, A.; Zhang, X. Integrated transcriptome and microRNA profiles analysis reveals molecular mechanisms underlying the consecutive monoculture problem of Polygonatum odoratum. Cell. Mol. Biol. 2020, 66, 47–52. [Google Scholar] [CrossRef]
- Pagano, L.; Rossi, R.; Paesano, L.; Marmiroli, N.; Marmiroli, M. miRNA regulation and stress adaptation in plants. Environ. Exp. Bot. 2021, 184, 104369. [Google Scholar] [CrossRef]
- Hayzadeh, M.; Turktas, M.; Khawar, K.M.; Unver, T. miR408 overexpression causes increased drought tolerance in chickpea. Gene 2015, 555, 186–193. [Google Scholar] [CrossRef]
- Kang, Y.C.; Yang, X.Y.; Liu, Y.H.; Shi, M.F.; Zhang, W.N.; Fan, Y.L.; Yao, Y.; Zhang, J.; Qin, S. Integration of mRNA and miRNA analysis reveals the molecular mechanism of potato (Solanum tuberosum L.) response to alkali stress. Int. J. Biol. Macromol. 2021, 182, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Garcia, C.; Ahmed, S.S.S.J.; Ramalingam, S.; Selvaraj, D.; Srivastava, A.; Paul, S.; Sharma, A. Identification of microRNAs from medicinal plant Murraya koenigii by high-throughput sequencing and their functional implications in secondary metabolite biosynthesis. Plan. Theory 2022, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W. Plant diversity and conservation in China: Planning a strategic bio resource for a sustainable future. Bot. J. Linn. Soc. 2011, 166, 282–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.; Gupta, S.C.; Chander, Y.; Singh, A.K. Antibiotic use in agriculture and its impact on the terrestrial environment. Adv. Agron. 2005, 87, 1–54. [Google Scholar]
- Yang, Y.; Chen, X.; Chen, J.; Xu, H.; Li, J.; Zhang, Z. Differential miRNA expression in Rehmannia glutinosa plants subjected to continuous cropping. BMC Plant Biol. 2011, 11, 53. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Jiang, N.H.; Song, W.L.; Ma, C.H.; Yang, S.C.; Chen, J.W. De novo sequencing and transcriptome analysis of Pinellia ternata identify the candidate genes involved in the biosynthesis of benzoic acid and ephedrine. Front. Plant Sci. 2016, 7, 1209. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Jia, T.; Chen, X. The ‘how’ and ‘where’ of plant micro RNAs. New Phytol. 2017, 216, 1002–1017. [Google Scholar] [CrossRef] [Green Version]
- Funikov, S.Y.; Zatcepina, O.G. Regulation of microRNA activity in stress. Mol. Biol. 2017, 51, 496–505. [Google Scholar] [CrossRef]
- Chen, P.; Li, H.Q.; Li, X.Y.; Zhou, X.H.; Zhang, X.X.; Zhang, A.S.; Liu, Q.Z. Transcriptomic analysis provides insight into defensive strategies in response to continuous cropping in strawberry (Fragaria× ananassa Duch.) plants. BMC Plant Biol. 2022, 22, 476. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, W.; Zhu, X.; Liu, L.; He, Z.; Yang, S.; Liang, Z.; Yan, X.; He, Y.; Liu, Y. Identification and characterization of Salvia miltiorrhizain miRNAs in response to replanting disease. PLoS ONE 2016, 11, e0159905. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Sun, D.; Wang, R.; An, Y. Integration of transcriptomics and metabolomics reveals the responses of sugar beet to continuous cropping obstacle. Front. Plant Sci. 2021, 12, 711333. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Zhou, Y.; Chen, Y.; Jin, C.; Hu, Y. Integrated analysis of microRNA and RNA-seq reveals phenolic acid secretion metabolism in continuous cropping of Polygonatum odoratum. Plants 2023, 12, 943. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Li, M.J.; Yi, Y.J.; Li, R.F.; Dong, C.; Zhang, Z.Y. The root transcriptome of Achyranthes bidentata and the identification of the genes involved in the replanting benefit. Plant Cell Rep. 2018, 37, 611–625. [Google Scholar] [CrossRef]
- Lopes, E.A.; Canedo, E.J.; Gomes, V.A.; Vieira, B.S.; Parreira, D.F.; Neves, W. Anaerobic soil disinfestation for the management of soilborne pathogens: A review. Appl. Soil Ecol. 2022, 174, 104408. [Google Scholar] [CrossRef]
- Jayaraman, S.; Naorem, A.K.; Lal, R.; Dalal, R.C.; Sinha, N.K.; Patra, A.K.; Chaudhari, S.K. Disease-suppressive soils beyond food Production: A critical review. Soil Sci. Plant Nutr. 2021, 21, 1437–1465. [Google Scholar] [CrossRef] [PubMed]
- DuPont, S.T.; Hewavitharana, S.S.; Mazzola, M. Field scale application of Brassica seed meal and anaerobic soil disinfestation for the control of apple replant disease. Appl. Soil Ecol. 2021, 166, 104076. [Google Scholar] [CrossRef]
- Givannini, D.; Brandi, F.; Lanteri, A.P.; Lazzeri, L.; Maltoni, M.L.; Matteo, R.; Minuto, A.; Sbrighi, P.; Stagno, F.; Baruzzi, G. Non-chemical soil fumigation for sustainable strawberry production in southern Italy. Agronomy 2021, 11, 1678. [Google Scholar] [CrossRef]
- Gupta, K.K.; Aneja, K.R.; Rana, D. Current status of cow dung as a bioresource for sustainable development. Bioresour. Bioprocess. 2016, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Mazzola, M. Field evaluation of reduced rate brassicaceae seed meal amendment and rootstock genotype on the microbiome and control of apple replant disease. Phytopathology 2019, 109, 1378–1391. [Google Scholar] [CrossRef]
- Pu, R.; Wang, P.; Guo, L.; Li, M.; Cui, X.; Wang, C.; Liu, Y.; Yang, Y. The remediation effects of microbial organic fertilizer on soil microorganisms after chloropicrin fumigation. Ecotoxicol. Environ. Saf. 2022, 231, 113188. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Huang, B.; Song, Z.; Ren, L.; Hao, B.; Liu, J.; Zhu, J.; Fang, W.; Yan, D.; et al. Organic fertilizer improves soil fertility and restores the bacterial community after 1,3-dichloropropene fumigation. Sci. Total Environ. 2020, 738, 140345. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhou, D.; Wei, H.; Wu, S.; Xie, B. Alleviating soil degradation caused by watermelon continuous cropping obstacle: Application of urban waste compost. Chemosphere 2021, 262, 128387. [Google Scholar] [CrossRef]
- Chen, W.; Teng, Y.; Li, Z.; Liu, W.; Ren, W.; Luo, Y.; Christie, P. Mechanisms by which organic fertilizer and effective microbes mitigate peanut continuous cropping yield constraints in a red soil of south China. Appl. Soil Ecol. 2018, 128, 23–34. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, N.; Li, Y.; Zhu, C.; Qu, B.; Liu, H.; Li, R.; Bai, Y.; Shen, Q.; Falcao, S.J. Bio-organic soil amendment promotes the suppression of Ralstonia solanacearum by inducing changes in the functionality and composition of rhizosphere bacterial communities. New Phytol. 2022, 235, 1558–1574. [Google Scholar] [CrossRef]
- Liu, J.J.; Yao, Q.; Li, Y.S.; Zhang, W.; Mi, G.; Chen, X.L.; Yu, Z.H.; Wang, G.H. Continuous cropping of soybean alters the bulk and rhizospheric soil fungal communities in a mollisol of Northeast PR China. Land Degrad. Dev. 2019, 30, 1725–1738. [Google Scholar] [CrossRef]
- Duran, P.; Thiergart, T.; Garrido-Oter, R.; Agler, M.; Kemen, E.; Schulze-Lefert, P.; Hacquard, S. Microbial interkingdom interactions in roots promote Arabidopsis survival. Cell 2018, 175, 973–983. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.; Liu, Z.; Hu, X.; Yu, Z.; Li, Y.; Chen, X.; Li, L.; Jin, J.; Wang, G. Chitin amendments eliminate the negative impacts of continuous cropping obstacles on soil properties and microbial assemblage. Front. Plant Sci. 2022, 13, 1067618. [Google Scholar] [CrossRef]
- Mbarki, S.; Cerdà, A.; Brestic, M.; Mahendra, R.; Abdelly, C.; Pascual, J.A. Vineyard compost supplemented with Trichoderma Harzianum T78 improve saline soil quality. Land Degrad. Dev. 2017, 28, 1028–1037. [Google Scholar] [CrossRef]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Gill, S.S.; Gill, R.; Trivedi, D.K.; Anjum, N.A.; Sharma, K.K.; Ansari, M.W.; Ansari, A.A.; Johri, A.K.; Prasad, R.; Pereira, E.; et al. Piriformospora indica: Potential and significance in plant stress tolerance. Front. Microbiol. 2016, 7, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuccio, E.E.; Hodge, A.; Pett-Ridge, J.; Herman, D.J.; Weber, P.K.; Firestone, M.K. An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ. Microbiol. 2013, 15, 1870–1881. [Google Scholar] [CrossRef]
- Rodriguez-Caballero, G.; Caravaca, F.; Fernandez-Gonzalez, A.J.; Alguacil, M.M.; Fernandez-Lopez, M.; Roldan, A. Arbuscular mycorrhizal fungi inoculation mediated changes in rhizosphere bacterial community structure while promoting revegetation in a semiarid ecosystem. Sci. Total Environ. 2017, 584, 838–848. [Google Scholar] [CrossRef]
- Ek, P.; Chatue, G.C.; Wakam, L.N.; Kouipou, R.M.T.; Fokou, P.V.T.; Boyom, F.F. Mycorrhiza consortia suppress the fusarium root rot (Fusarium solani f. sp Phaseoli) in common bean (Phaseolus vulgaris L.). Biol. Control 2016, 103, 240–250. [Google Scholar] [CrossRef]
- Dong, L.; Xu, J.; Zhang, L.; Cheng, R.; Wei, G.; Su, H.; Yang, J.; Qian, J.; Xu, R.; Chen, S. Rhizospheric microbial communities are driven by Panax ginseng at different growth stages and biocontrol bacteria alleviates replanting mortality. Acta Pharm. Sin. B 2018, 8, 272–282. [Google Scholar] [CrossRef]
- El-Khallal, S.M. Induction and modulation of resistance in tomato plants against Fusarium wilt disease by bioagent fungi (Arbuscular mycorrhiza) and/or hormonal elicitors (Jasmonic acid & salicylic acid): 2-changes in the antioxidant enzymes, phenolic compounds and pathogen related-proteins. Aust. J. Basic Appl. Sci. 2007, 1, 717–732. [Google Scholar]
- Ghani, M.I.; Ali, A.; Atif, M.J.; Ali, M.; Amin, B.; Cheng, Z. Arbuscular mycorrhizal fungi and dry raw garlic stalk amendment alleviate continuous monocropping growth and photosynthetic declines in eggplant by bolstering its antioxidant system and accumulation of osmolytes and secondary metabolites. Front. Plant Sci. 2022, 13, 849521. [Google Scholar] [CrossRef] [PubMed]
- Koocheki, A.; Nassiri, M.; Alimoradi, L.; Ghorbani, R. Effect of cropping systems and crop rotations on weeds. Agron. Sustain. Dev. 2009, 29, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Sokolowski, A.C.; McCormick, B.P.; De Grazia, J.; Wolski, J.E.; Rodríguez, H.A.; Rodríguez-Frers, E.P.; Gagey, M.C.; Debelis, S.P.; Paladino, I.R.; Barrios, M.B. Tillage and no-tillage effects on physical and chemical properties of an Argiaquoll soil under long-term crop rotation in Buenos Aires, Argentina. Int. Soil Water Conserv. Res. 2020, 8, 185–194. [Google Scholar] [CrossRef]
- Chongtham, I.R.; Bergkvist, G.; Watson, C.A.; Sandström, E.; Bengtsson, J.; Öborn, I. Factors influencing crop rotation strategies on organic farms with different time periods since conversion to organic production. Biol. Agric. Hortic. 2016, 33, 14–27. [Google Scholar] [CrossRef]
- Chamberlain, L.A.; Bolton, M.L.; Cox, M.S.; Suen, G.; Conley, S.P.; Ane, J.M. Crop rotation, but not cover crops, influenced soil bacterial community composition in a corn-soybean system in southern Wisconsin. Appl. Soil Ecol. 2020, 154, 103603. [Google Scholar] [CrossRef]
- Hati, K.M.; Swarup, A.; Singh, D.; Misra, A.K.; Ghosh, P.K. Long-term continuous cropping, fertilization, and manuring effects on physical properties and organic carbon content of a sandy loam soil. Soil Res. 2006, 44, 487–495. [Google Scholar] [CrossRef]
- Hilton, S.; Bennett, A.J.; Keane, G.; Bending, G.D.; Chandler, D.; Stobart, R.; Mills, P. Impact of shortened crop rotation of oilseed rape on soil and rhizosphere microbial diversity in relation to yield decline. PLoS ONE 2013, 8, e59859. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Grandy, A.S.; Atkinson, E.E.; Marin-Spiotta, E.; McDaniel, M.D. Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecol. Lett. 2015, 18, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Bhattacharyya, R.; Das, T.K.; Sharma, A.R.; Ghosh, A.; Das, S.; Jha, P. Crop rotation and residue management effects on soil enzyme activities, glomalin and aggregate stability under zero tillage in the Indo-Gangetic Plains. Soil Tillage Res. 2018, 184, 291–300. [Google Scholar] [CrossRef]
- Balota, E.L.; Colozzi-Filho, A.; Andrade, D.S.; Dick, R.P. Microbial biomass in soils under different tillage and crop rotation systems. Biol. Fertil. Soils 2003, 38, 15–20. [Google Scholar] [CrossRef]
- Teresa, D.; Angela, D.; Pedro, M.A. Accounting for soil biotic effects on soil health and crop productivity in the design of crop rotations. J. Sci. Food Agric. 2015, 95, 447–454. [Google Scholar]
- Soman, C.; Li, D.F.; Wander, M.M.; Kent, A.D. Long-term fertilizer and crop rotation treatments differentially affect soil bacterial community structure. Plant Soil 2016, 413, 145–159. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Wei, M.; Wang, Y.; Liang, Z.; Xia, P. Appropriate crop rotation alleviates continuous cropping barriers by changing rhizosphere microorganisms in Panax notoginseng. Rhizosphere 2022, 23, 100568. [Google Scholar] [CrossRef]
- Zhou, X.G.; Liu, J.; Wu, F.Z. Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth. Plant Soil 2017, 415, 507–520. [Google Scholar] [CrossRef]
- Bedoussac, L.; Journet, E.P.; Hauggaard-Nielsen, H.; Naudin, C.; Corre-Hellou, G.; Jensen, E.S.; Prieur, L.; Justes, E. Ecological principles underlying the increase of productivity achieved by cereal-grain legume intercrops in organic farming. A review. Agron. Sustain. Dev. 2015, 35, 911–935. [Google Scholar] [CrossRef]
- Singh, R.K.; Lui, E.; Wright, D.; Taylor, A.; Bakovic, M. Alcohol extract of North American ginseng (Panax quinquefolius) reduces fatty liver, dyslipidemia, and other complications of metabolic syndrome in a mouse model. Can. J. Physiol. Pharmacol. 2017, 95, 1046–1057. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.N.; Sun, Y.M.; Wang, E.T.; Yang, J.S.; Yuan, H.L.; Scow, K.M. Effects of intercropping and Rhizobial inoculation on the ammonia oxidizing microorganisms in rhizospheres of maize and faba bean plants. Appl. Soil Ecol. 2015, 85, 76–85. [Google Scholar] [CrossRef]
- Hontoria, C.; García-González, I.; Quemada, M.; Roldán, A.; Alguacil, M.M. The cover crop determines the AMF community composition in soil and in roots of maize after a ten-year continuous crop rotation. Sci. Total Environ. 2019, 660, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Wuest, S.B.; Gollany, H.T. Soil organic carbon and nitrogen after application of nine organic amendments. Soil Sci. Soc. Am. J. 2013, 77, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Akinnifesi, F.K.; Makumba, W.; Kwesiga, F.R. Sustainable maize production using gliricidia/maize intercropping in southern Malawi. Exp. Agric. 2006, 42, 441–457. [Google Scholar] [CrossRef] [Green Version]
- Faheem, M.; Raza, W.; Zhong, W.; Nan, Z.; Shen, Q.; Xu, Y. Evaluation of the biocontrol potential of Streptomyces goshikiensis YCXU against Fusarium oxysporum f. sp. niveum. Biol. Control 2015, 81, 101–110. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, S.D.; Patel, R.K.; Srivastava, K.; Nath, V. Studies on feasibility of intercropping under litchi based cropping system. Ecoscan Spec. 2014, 6, 285–289. [Google Scholar]

| Plant Name | Study Type | Upregulated Genes | Downregulated Genes | References |
|---|---|---|---|---|
| Rehmannia glutinosa | miRNA identification | miR160 (Auxin response), ARF6 and ARF8 (adventitious roots regulator), miR5138, SPL, miR172. | miR156/157, ARF17, miR167, ICU2 (flowering), miR408, miR822. | [125] |
| Panax ginseng | Transcriptomics analysis | Mitotic spindle elongation, enzyme inhibitor activity, centrosome cycle and duplication, carboxylesterase and pectinesterase activity, c41713_g1 (Invertase/pectin methylesterase inhibitor in leaves). | Photosynthesis, ion binding, cellulose metabolic process, polysaccharide and lignin metabolic process, C8930_g1 (Zinc binding dehydrogenase in roots), c41722_g1 (calcium-binding protein in the stem). | [99] |
| Pogostemon cablin | Transcriptomics analysis | AP2/ERF-ERF, bHLH, sesquiterpene synthase activity (GO: 0010334), exo alpha bergamotene biosynthesis (GO: 0045339), farnesyl diphosphate catabolic process (GO: 0045339), FAD binding (GO: 0071949). | MYB, HSF, bZIP, GARP-G2-Like, HB-HD-ZIP, water channel activity (go: 0015250), protein complex oligomerization (GO: 0051259), response to hydrogen peroxide (GO: 0042542), oligopeptide transport (GO: 0006857). | [28] |
| Codonopsis tangshen | Transcriptomics analysis | MYC2 (α-linolenic acid metabolism), EIN3 (ubiquitin mediated proteolysis), PR-1 (phenylalanine metabolism), CRE1 (zeatin biosynthesis), phenylalanine catabolism, tyrosine degradation I, glycogen synthesis, tyrosine catabolism, AP2, EREBP, WRKY, photosynthesis (PERK8, RAP2-4, DnaJ). | Immune system, signal transduction, mitotic activity, cell division, psaA (photosystem I), psbA (photosystem II), psbW (MSP), AHP (zeatin biosynthesis), GH3 (cell enlargement plant growth), CYCD3 (cell division), MYB, MADS, Jumonji family, trihelix, photosynthesis (BPS1, apoprotein, SR34A). | [29] |
| Fragaria ananassa | Transcriptomics analysis | Peroxidase activity (GO: 0004601), CNCGS, PR1 and WRKY transcription factors, cell wall (GO: 0005618), lignin catabolic process (GO: 0046274), heme and copper ion binding, response to virus (GO: 0009615). | FaWRKY33, nutrient transport and synthesis, glycogen phosphorylase activity, ammonium transmembrane transport (GO: 0072488), water transport (GO: 0006833), starch biosynthetic process (GO: 0019252). | [129] |
| Salvia miltiorrhiza | miRNA identification | miR156, smi-miR156a-1, miR396, miR319, pab-miR160a-like, smi-miR164a-1, miR166, leaf (miR031, miR021a, miR028), Stem (miR025a), miR165a-3p-like. | Root (miR031), NAC100-like (i5833_g1_i1), SPL13, athb-14-like (CL132Contig4), root growth ARF18-like, GRF3-like, athb-14-like. | [130] |
| Beta vulgaris | Metabolomics analysis | Terephthalic acid, 1,5-anhydroglucitol, fluorene, 3,4-dihydroxypyridine, anandamide, lactitol, salicylaldehyde, nornicotine, fructose, indolelactate, dihydroxyacetone. | Xylose, tyramine, gentiobiose, glucose, sucrose, lactamide, thymidine, neohesperidin, gentiobiose, pyridoxal phosphate, 5-alpha-dihydroprogesterone, cuminic alcohol, phytanic acid, valine. | [131] |
| Andrographis paniculata | Metabolomics analysis | Transposase, alcohol dehydrogenase (NADP+), nitrate reductase, pectate lyase, peptidylprolyl isomerase, NADP dependent sorbitol 6-phosphate dehydrogenase, β-glucoside gene, mevalonate 5-dinhophate decarboxylase (MVD, EC 4.1.1.33), isoflavonoid biosynthesis (1.1.4.1.3.21), apigenin (1.1.4.1.3.21), caffeic acid (6.2.1.12), Sinapyl alcohol (1.11.1.7), phenylalanine (4.3.1.24). | Flavonoids (Ko00941), terpenoids (Ko00900) and phenylpropanoid (Ko00940) biosynthesis, PAL, C4H, 4CL, Cinnamoyl-CoA reductase (CCR, EC 1.2.1.44), β-glucoside, HST gene (shikimate O-hydroxycinnamoyl transferase, EC 2.3.1.133), peroxidase (EC 1.11.1.7), caffeic acid 3-O-methyltransferase gene (COMT, EC 2.1.1.68), FSH gene, phosphomevalonate kinase (PMK, EC 2.7.4.2). | [104] |
| Polygonatum odoratum | miRNA identification | Nitrogen metabolism, phenylalanine, phenylpropanoid biosynthesis, tyrosine and tryptophan biosynthesis, phenylacetate synthesis (AOC3), AMIE, CSE, TYRAAT, 4CL, CYP98A, AOC3, ALDO, ASP5, HCT, PGD, SCRK, ADT, Ath-miR172a, novel_130, ath-miR172c, tcc-miR172d. | DNA replication, plant hormone signal transduction, brassinosteroid biosynthesis, syringyl lignin formation (EC: 1.11.1.7) CCR, CAD, and COMT, FBP, RPE, SORD, PGD, GLGC, GPI, E3.2.1.4, AROK, AROC, MAlZ, TYDC, GPD, E1.10.3.1, sbi-miR172f, osa-miR528-5p, mtr-miR2673a, mtr-miR2673a (EC: 1.11.1.7). | [132] |
| Salvia miltiorrhiza | Metabolomics analysis | Phenylalanine, ferulic acid. | Pehtasaccharide, Dihydrotanshinone I, spermine, salvianolic acid B/E, miltirone, spermidine, tanshinone II A, dehydromiltirone, tetrasaccharide, dehydrotanshinone IIA. | [115] |
| Pinellia ternata | Transcriptomic analysis | DN22409, DN59405, DN150689, DN14287, DN139642, 1,4-β-D-xylan synthase, DHAR, cell wall formation, β-glucosidase, APX, AOX. | PtCAG, PtSRK2, PtCCoAMT, phenylpropanoid biosynthesis, DN14243, DN11615, C4H, PtCSLD5, PtSS. | [103] |
| Pogostemon cablin | miRNA identification | pab-mir160c, ahy-mir408-3p, osa-mir397b, aof-mir398, novel20_master, mdm-mir1511, mdm-mir397a, stu-mir408a-3p, osa-mir397b, ahy-mir408–3p, stu-mir397–5p, aof-mir398, smo-mir408, mdm-mir397a, smi-mir12112, novel9_star, sly mir398a. | osa-miR397b, ahy-miR408–3p, mdm-miR397a, aof-miR398. | [106] |
| Achyranthes bidentata | Transcriptomic analysis | Gluconeogenesis (DLD, ENO, G6PDH, PGK, PGM, GPI, GALM, TPI, ADH1), glutathione metabolism (GR, LAP1, pepN, GGT3, SRM), plant–pathogen interaction (EIX1/2, RPM1, PR1, ETI, CAD, CERK1), signal transduction (SAUR, ARR, GID1, BSK, BZR1/2, CYCD3, CTR1, PR1, ARF, TIR1, AUX/IAA). | Glutathione metabolism (RRM2), gluconeogenesis (LDH, FBP, HK3, MINPP1, NAD+), plant-pathogen interaction (CALM, CDPK, WRKY33, BAK1, htpG, MKK4/5P, GsSRK), signal transduction (TGA, ERF1, ETR2, EIN3, EBF1/2, SNRK2, ABF, PP2C, BAK1, GH3), phenylpropanoid biosynthesis (PAL, C4H, 4CL, CCoAOMT, CAGT, HCT). | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeeshan Ul Haq, M.; Yu, J.; Yao, G.; Yang, H.; Iqbal, H.A.; Tahir, H.; Cui, H.; Liu, Y.; Wu, Y. A Systematic Review on the Continuous Cropping Obstacles and Control Strategies in Medicinal Plants. Int. J. Mol. Sci. 2023, 24, 12470. https://doi.org/10.3390/ijms241512470
Zeeshan Ul Haq M, Yu J, Yao G, Yang H, Iqbal HA, Tahir H, Cui H, Liu Y, Wu Y. A Systematic Review on the Continuous Cropping Obstacles and Control Strategies in Medicinal Plants. International Journal of Molecular Sciences. 2023; 24(15):12470. https://doi.org/10.3390/ijms241512470
Chicago/Turabian StyleZeeshan Ul Haq, Muhammad, Jing Yu, Guanglong Yao, Huageng Yang, Hafiza Amina Iqbal, Hassam Tahir, Hongguang Cui, Ya Liu, and Yougen Wu. 2023. "A Systematic Review on the Continuous Cropping Obstacles and Control Strategies in Medicinal Plants" International Journal of Molecular Sciences 24, no. 15: 12470. https://doi.org/10.3390/ijms241512470
APA StyleZeeshan Ul Haq, M., Yu, J., Yao, G., Yang, H., Iqbal, H. A., Tahir, H., Cui, H., Liu, Y., & Wu, Y. (2023). A Systematic Review on the Continuous Cropping Obstacles and Control Strategies in Medicinal Plants. International Journal of Molecular Sciences, 24(15), 12470. https://doi.org/10.3390/ijms241512470








