Sperm Meiotic Segregation Analysis of Reciprocal Translocations Carriers: We Have Bigger FISH to Fry
Abstract
:1. Introduction
2. Results
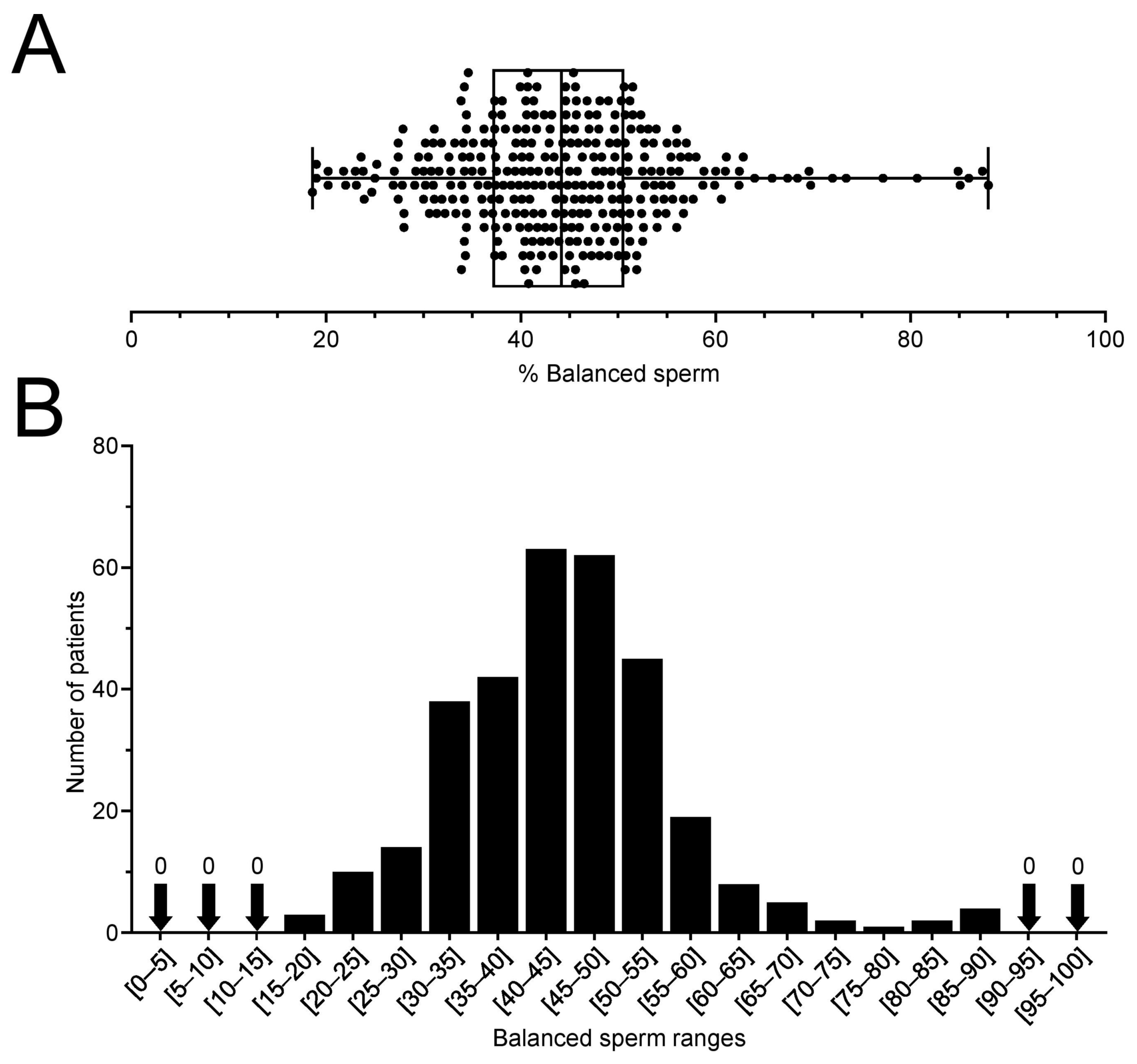
2.1. RT Carriers Produce between 20 and 90% of Balanced Sperm
2.2. Abnormal Sperm Parameters Do Not Correlate with Unbalanced Chromosome Content
2.3. RT Carriers Display Less Balanced Sperm When an Acrocentric Chromosome Is Involved in the Translocation, Especially Chromosome 22
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Literature Search
4.3. Sperm Technique
4.4. FISH Scoring
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clementini, E.; Palka, C.; Iezzi, I.; Stuppia, L.; Guanciali-Franchi, P.; Tiboni, G. Prevalence of chromosomal abnormalities in 2078 infertile couples referred for assisted reproductive techniques. Hum. Reprod. 2005, 20, 437–442. [Google Scholar] [CrossRef]
- Martin, R. Cytogenetic determinants of male fertility. Hum. Reprod. Updat. 2008, 14, 379–390. [Google Scholar] [CrossRef]
- Magli, M.C.; Crippa, A.; Benincasa, M.; Terzuoli, G.; Azzena, S.; Maresca, L.; Albanese, C.; Colombo, F.; Ferraretti, A.P.; Gianaroli, L. Sperm chromosome abnormalities in patients with normal karyotype and in translocation carriers: Clinical relevance for assisted reproductive technology. Reprod. Biomed. Online 2020, 41, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.; Pertile, M.; Norris, H.; Hale, L.; Baker, H. Chromosome translocations in couples with in-vitro fertilization implantation failure. Hum. Reprod. 1999, 14, 2097–2101. [Google Scholar] [CrossRef]
- Mau-Holzmann, U. Somatic chromosomal abnormalities in infertile men and women. Cytogenet. Genome Res. 2005, 111, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Donker, R.B.; Vloeberghs, V.; Groen, H.; Tournaye, H.; A Van Ravenswaaij-Arts, C.M.; Land, J.A. Chromosomal abnormalities in 1663 infertile men with azoospermia: The clinical consequences. Hum. Reprod. 2017, 32, 2574–2580. [Google Scholar] [CrossRef]
- Benet, J.; Oliver-Bonet, M.; Cifuentes, P.; Templado, C.; Navarro, J. Segregation of chromosomes in sperm of reciprocal translocation carriers: A review. Cytogenet. Genome Res. 2005, 111, 281–290. [Google Scholar] [CrossRef]
- Scriven, P.N.; Handyside, A.H.; Ogilvie, C.M. Chromosome translocations: Segregation modes and strategies for preimplantation genetic diagnosis. Prenat Diagn. 1998, 18, 1437–1449. [Google Scholar] [CrossRef]
- Kouru, K.H.; Malmgren, H.; White, I.; Sanchez, A.R.; Lundberg, E.S. Meiotic segregation analyses of reciprocal translocations in spermatozoa and embryos: No support for predictive value regarding PGD outcome. Reprod. Biomed. Online 2017, 34, 645–652. [Google Scholar] [CrossRef]
- Xie, P.; Hu, L.; Peng, Y.; Tan, Y.-Q.; Luo, K.; Gong, F.; Lu, G.; Lin, G. Risk Factors Affecting Alternate Segregation in Blastocysts From Preimplantation Genetic Testing Cycles of Autosomal Reciprocal Translocations. Front. Genet. 2022, 13, 880208. [Google Scholar] [CrossRef]
- Martinez, G.; Garcia, C. Sexual selection and sperm diversity in primates. Mol. Cell Endocrinol. 2020, 518, 110974. [Google Scholar] [CrossRef]
- Ko, D.S.; Cho, J.W.; Park, S.Y.; Kim, J.Y.; Koong, M.K.; Song, I.O.; Kang, I.S.; Lim, C.K. Clinical outcomes of preimplantation genetic diagnosis (PGD) and analysis of meiotic segregation modes in reciprocal translocation carriers. Am. J. Med. Genet. Part A 2010, 152A, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, D.; Zhu, Y.; Jiang, W.; Xia, M.; Li, J.; Yan, J.; Chen, Z.-J. Interaction of acrocentric chromosome involved in translocation and sex of the carrier influences the proportion of alternate segregation in autosomal reciprocal translocations. Hum. Reprod. 2018, 34, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, X.; Wang, J.; Li, R.; Ding, C.; Cai, B.; Zhou, C.; Xu, Y. Effect of carriers’ sex on meiotic segregation patterns and chromosome stability of reciprocal translocations. Reprod. Biomed. Online 2021, 43, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Zhang, S.; Zhu, S.; Wu, J.; Xiao, M.; Zhou, J.; Fu, J.; Sun, Y.; Xu, C.; Sun, X. Conventional ICSI improves the euploid embryo rate in male reciprocal translocation carriers. J. Assist. Reprod. Genet. 2020, 38, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, C. Reciprocal translocation and Robertsonian translocation in relation to semen parameters: A retrospective study and systematic review. Andrologia 2021, 54, e14262. [Google Scholar] [CrossRef]
- Zenagui, R.; Bernicot, I.; Ranisavljevic, N.; Haquet, E.; Ferrieres-Hoa, A.; Pellestor, F.; Anahory, T. Inheritance of imbalances in recurrent chromosomal translocation t(11;22): Clarification by PGT-SR and sperm-FISH analysis. Reprod. Biomed. Online 2019, 39, 40–48. [Google Scholar] [CrossRef]
- Vozdova, M.; Oracova, E.; Kasikova, K.; Prinosilova, P.; Rybar, R.; Horinova, V.; Gaillyova, R.; Rubes, J. Balanced chromosomal translocations in men: Relationships among semen parameters, chromatin integrity, sperm meiotic segregation and aneuploidy. J. Assist. Reprod. Genet. 2013, 30, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, H.; Inagaki, H.; Ohye, T.; Kogo, H.; Tsutsumi, M.; Kato, T.; Tong, M.; Emanuel, B. The constitutional t(11;22): Implications for a novel mechanism responsible for gross chromosomal rearrangements. Clin. Genet. 2010, 78, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, A.; Ahdad, N.; Hesters, L.; Brisset, S.; Romana, S.; Tosca, L.; Tachdjian, G.; Frydman, N. Chromosomal translocations and semen quality: A study on 144 male translocation carriers. Reprod. Biomed. Online 2019, 38, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, R.; Yu, Y.; Zhu, H.; Li, L.; Yang, X.; Hu, X.; Liu, R. Non-Robertsonian translocations involving chromosomes 13, 14, or 15 in male infertility. Medicine 2019, 98, e14730. [Google Scholar] [CrossRef]
- Hajlaoui, A.; Slimani, W.; Kammoun, M.; Sallem, A.; Braham, S.; Bibi, M.; Saad, A.; Mougou-Zerelli, S. Sperm fluorescent in situ hybridisation study of interchromosomal effect in six Tunisian carriers of reciprocal and Robertsonian translocations. Andrologia 2018, 50, e12949. [Google Scholar] [CrossRef]
- Pellestor, F.; Anahory, T.; Lefort, G.; Puechberty, J.; Liehr, T.; Hedon, B.; Sarda, P. Complex chromosomal rearrangements: Origin and meiotic behavior. Hum. Reprod. Updat. 2011, 17, 476–494. [Google Scholar] [CrossRef]
- Morel, F.; Laudier, B.; Guérif, F.; Couet, M.; Royère, D.; Roux, C.; Bresson, J.; Amice, V.; De Braekeleer, M.; Douet-Guilbert, N. Meiotic segregation analysis in spermatozoa of pericentric inversion carriers using fluorescence in-situ hybridization. Hum. Reprod. 2006, 22, 136–141. [Google Scholar] [CrossRef]
- Vialard, F.; Delanete, A.; Clement, P.; Simon-Bouy, B.; Aubriot, F.X.; Selva, J. Sperm chromosome analysis in two cases of paracentric inversion. Fertil. Steril. 2007, 87, 418.e1–418.e5. [Google Scholar] [CrossRef]
- Caer, E.; Perrin, A.; Douet-Guilbert, N.; Amice, V.; De Braekeleer, M.; Morel, F. Differing mechanisms of meiotic segregation in spermatozoa from three carriers of a pericentric inversion of chromosome 8. Fertil. Steril. 2008, 89, 1637–1640. [Google Scholar] [CrossRef]
- Bhatt, S.; Moradkhani, K.; Mrasek, K.; Puechberty, J.; Manvelyan, M.; Hunstig, F.; Lefort, G.; Weise, A.; Lespinasse, J.; Sarda, P.; et al. Breakpoint mapping and complete analysis of meiotic segregation patterns in three men heterozygous for paracentric inversions. Eur. J. Hum. Genet. 2008, 17, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xu, C.; Sun, Y.; Wang, L.; Chen, S.; Jin, F. Different segregation patterns in five carriers due to a pericentric inversion of chromosome 1. Syst Biol Reprod Med. 2014, 60, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.L.; Warburton, D. Detection of Chromosomal Aberrations in Clinical Practice: From Karyotype to Genome Sequence. Annu. Rev. Genom. Hum. Genet. 2015, 16, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Beliveau, B.J. An open source 16-channel fluidics system for automating sequential fluorescent in situ hybridization (FISH)-based imaging. Hardwarex 2022, 12, e00343. [Google Scholar] [CrossRef]
- Kao, K.-J.; Tai, C.-H.; Chang, W.-H.; Yeh, T.-S.; Chen, T.-C.; Lee, G.-B. A fluorescence in situ hybridization (FISH) microfluidic platform for detection of HER2 amplification in cancer cells. Biosens. Bioelectron. 2015, 69, 272–279. [Google Scholar] [CrossRef]
- Kwasny, D.; Vedarethinam, I.; Shah, P.; Dimaki, M.; Silahtaroglu, A.; Tumer, Z.; Svendsen, W.E. Advanced microtechnologies for detection of chromosome abnormalities by fluorescent in situ hybridization. Biomed. Microdevices 2012, 14, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Beliveau, B.J.; Joyce, E.F.; Apostolopoulos, N.; Yilmaz, F.; Fonseka, C.Y.; McCole, R.B.; Chang, Y.; Li, J.B.; Senaratne, T.N.; Williams, B.R.; et al. Versatile design and synthesis platform for visualizing genomes with Oligopaint FISH probes. Proc. Natl. Acad. Sci. USA 2012, 109, 21301–21306. [Google Scholar] [CrossRef] [PubMed]
- Beliveau, B.J.; Apostolopoulos, N.; Wu, C. Visualizing Genomes with Oligopaint FISH Probes. Curr. Protoc. Mol. Biol. 2014, 105, 14.23.1–14.23.20. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, W.; Bates, M.; Zhuang, X. Three-Dimensional Super-Resolution Imaging by Stochastic Optical Reconstruction Microscopy. Science 2008, 319, 810–813. [Google Scholar] [CrossRef]
- Bates, M.; Huang, B.; Dempsey, G.T.; Zhuang, X. Multicolor Super-Resolution Imaging with Photo-Switchable Fluorescent Probes. Science 2007, 317, 1749–1753. [Google Scholar] [CrossRef]
- Eklund, A.S.; Ganji, M.; Gavins, G.; Seitz, O.; Jungmann, R. Peptide-PAINT Super-Resolution Imaging Using Transient Coiled Coil Interactions. Nano Lett. 2020, 20, 6732–6737. [Google Scholar] [CrossRef]
- Jungmann, R.; Avendaño, M.S.; Woehrstein, J.B.; Dai, M.; Shih, W.M.; Yin, P. Multiplexed 3D cellular super-resolution imaging with DNA-PAINT and Exchange-PAINT. Nat. Methods 2014, 11, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cottle, W.T.; Wang, H.; Feng, X.A.; Mallon, J.; Gavrilov, M.; Bailey, S.; Ha, T. Genome oligopaint via local denaturation fluorescence in situ hybridization. Mol. Cell 2021, 81, 1566–1577.e8. [Google Scholar] [CrossRef] [PubMed]
- WHO Laboratory Manual for the Examination and Processing of Human Semen. Sixth Edition. Available online: https://www.who.int/publications/i/item/9789240030787 (accessed on 4 January 2023).
- Lamotte, A.; Martinez, G.; Devillard, F.; Hograindleur, J.-P.; Satre, V.; Coutton, C.; Harbuz, R.; Amblard, F.; Lespinasse, J.; Benchaib, M.; et al. Is sperm FISH analysis still useful for Robertsonian translocations? Meiotic analysis for 23 patients and review of the literature. Basic Clin. Androl. 2018, 28, 5. [Google Scholar] [CrossRef]
- Martinez, G.; Gillois, P.; Le Mitouard, M.; Borye, R.; Esquerré-Lamare, C.; Satre, V.; Bujan, L.; Hennebicq, S. FISH and tips: A large scale analysis of automated versus manual scoring for sperm aneuploidy detection. Basic Clin. Androl. 2013, 23, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Patient | Age | Translocation | Segregation (%) | Number of Counted Cells | PMRS (%) | Sperm Parameters | Reproductive History | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alternate | Adjacent1 | Adjacent2 | 3:1 ech | 3:1 ter | Others | Concentration (×106/mL) | Motility (%) | Morphology (% Normal) | ||||||
| P01 | 31 | (1;4) (p21;q13) | 31.36 | 14.40 | 14.05 | 9.76 | 23.75 | 7.14 | 1722 | 0.11 | 25 | 40 | 27 | Spontaneous abortions/Repeated miscarriages |
| P02 | 35 | (1;6) (q42;p22) | 52.73 | 25.39 | 5.47 | 10.16 | 6.25 | 0.00 | 1024 | 21.15 | 50 | 60 | 26 | Spontaneous abortions/Repeated miscarriages |
| P03 | 49 | (1;11) (p34;q24) | 49.67 | 36.14 | 1.74 | 7.47 | 4.98 | 0.00 | 1606 | 22.79 | 90 | 45 | 2 | Infertility |
| P04 | - | (1;11) (q12;q24) | 57.23 | 26.42 | 0.00 | 5.03 | 10.69 | 0.63 | 1590 | 0.49 | nc | nc | nc | Familial study |
| P05 | - | (2;4) (q24;p15.1) | 42.19 | 29.86 | 10.68 | 9.32 | 7.95 | 0.00 | 2190 | 2.71 | 52 | 70 | 52 | Infertility |
| P06 | 31 | (2;7) (p25;p15.1) | 36.19 | 30.08 | 2.33 | 12.30 | 15.40 | 3.70 | 2487 | 27.06 | 1.8 | 60 | 2 | Infertility |
| P07 | 33 | (2;10) (q21;q24) | 36.00 | 23.00 | 7.00 | 21.00 | 13.00 | 0.00 | 1000 | 0.60 | 2.6 | 50 | 4 | Infertility |
| P08 | 28 | (2;12) (q14.2;q15) | 46.99 | 33.11 | 6.76 | 3.71 | 3.63 | 5.80 | 2588 | 0.23 | 8 | 60 | 19 | Infertility |
| P09 | 36 | (3;11) (p24;p12) | 62.43 | 21.27 | 6.91 | 5.80 | 3.59 | 0.00 | 1448 | 10.44 | 90 | 65 | 17 | Spontaneous abortions/Repeated miscarriages |
| P10 | 35 | (3;12) (q13.1;q13) | 50.19 | 27.63 | 13.62 | 5.45 | 3.11 | 0.00 | 1028 | 0.74 | 150 | 70 | 18 | Infertility |
| P11 | 30 | (4;8) (q31.1;p21) | 47.14 | 31.31 | 3.70 | 12.79 | 5.05 | 0.00 | 1188 | 10.34 | 15.6 | 65 | 17 | Infertility |
| P12 | 29 | (4;10) (q21;q24) | 37.50 | 27.00 | 7.25 | 13.25 | 7.25 | 7.75 | 1600 | 1.29 | 67 | 50 | 32 | Spontaneous abortions/Repeated miscarriages |
| P13 | 38 | (4;14) (p14;q32) | 33.86 | 15.91 | 4.77 | 14.09 | 31.36 | 0.00 | 1760 | 31.54 | 1.6 | 40 | 8 | Infertility |
| P14 | 29 | (4;17) (p16.1;q21.3) | 34.42 | 20.41 | 14.21 | 14.01 | 14.92 | 5.58 | 1970 | 17.86 | 10 | 55 | 10 | Spontaneous abortions/Repeated miscarriages |
| P15 | - | (4;21) (q28;q21) | 50.25 | 35.47 | 4.43 | 2.96 | 5.42 | 1.48 | 1218 | - | 15 | 20 | 6 | Spontaneous abortions/Repeated miscarriages |
| P16 | 26 | (5;18) (p15.1;q12.3) | 46.08 | 21.97 | 21.46 | 4.83 | 5.34 | 0.31 | 1966 | 14.58 | 105 | 65 | 26 | Familial study |
| P17 | 29 | (6;12) (p25;p13) | 46.83 | 39.98 | 1.90 | 5.45 | 3.90 | 1.95 | 2001 | 32.24 | 50 | 60 | 18 | Infertility |
| P18 | 36 | (6;15) (p12;p13) | 65.80 | 23.42 | 0.74 | 4.09 | 4.83 | 1.12 | 1614 | 29.03 | 11.6 | 55 | 3 | Infertility |
| P19 | 31 | (6;15) (q12;q21) | 39.93 | 37.92 | 5.37 | 9.73 | 7.05 | 0.00 | 1788 | 4.29 | 22 | 70 | 23 | Spontaneous abortions/Repeated miscarriages |
| P20 | 30 | (6;18) (p21.1;q23) | 40.51 | 30.55 | 9.15 | 7.94 | 8.83 | 4.35 | 2481 | 18.31 | 37 | 50 | 16 | Familial study |
| P21 | 28 | (6;18) (p21.1;q23) | 27.83 | 27.50 | 6.32 | 8.06 | 19.44 | 10.85 | 1502 | 18.31 | 56 | 40 | 18 | Familial study |
| P22 | 32 | (7;9) (p14;q21) | 42.11 | 23.98 | 9.65 | 13.45 | 10.82 | 0.00 | 1710 | - | 15 | 60 | 5 | Infertility |
| P23 | 36 | (8;16) (p12;q23) | 31.84 | 23.38 | 3.48 | 21.39 | 19.90 | 0.00 | 1005 | 16.35 | 1 | 15 | 3 | Infertility |
| P24 | 34 | (8;16) (p12;q23) | 45.32 | 31.72 | 7.85 | 7.25 | 7.85 | 0.00 | 1655 | 16.35 | 42 | 45 | 17 | Familial study |
| P25 | 43 | (8;20) (p12;q12) | 56.41 | 13.46 | 23.72 | 3.21 | 3.21 | 0.00 | 1560 | 12.17 | 12 | 35 | 7 | Spontaneous abortions/Repeated miscarriages |
| P26 | 30 | (9;11) (p24;q23) | 42.49 | 35.42 | 6.67 | 4.55 | 5.09 | 5.77 | 2789 | 25.74 | 84 | 50 | 12 | Infertility |
| P27 | 56 | (9;17) (p22;q23) | 52.31 | 27.17 | 2.31 | 11.27 | 6.94 | 0.00 | 1730 | 18.74 | 3.5 | 50 | 13 | Infertility |
| P28 | 43 | (9;17) (q22;q21) | 41.60 | 30.47 | 2.59 | 13.49 | 7.60 | 4.24 | 1697 | 5.40 | 102 | 30 | 20 | Infertility |
| P29 | 34 | (10;17) (q11.2;q25) | 44.63 | 27.48 | 10.03 | 9.52 | 7.52 | 2.10 | 1954 | 14.58 | 33 | 65 | 19 | Familial study |
| P30 | 29 | (10;17) (p15;q12) | 69.75 | 11.37 | 11.49 | 2.70 | 3.52 | 1.17 | 1706 | 4.86 | 40 | 60 | 14 | Spontaneous abortions/Repeated miscarriages |
| P31 | 30 | (13;15) (q32;q22) | 33.95 | 34.15 | 4.86 | 10.88 | 11.43 | 4.72 | 1461 | 9.65 | 0.9 | 50 | 20 | Spontaneous abortions/Repeated miscarriages |
| P32 | 40 | (13;18) (q14;q22) | 43.91 | 34.51 | 7.76 | 5.70 | 4.62 | 3.49 | 1947 | 17.35 | 45 | 35 | 4 | Infertility |
| P33 | - | (14;17) (q22;p11.2) | 53.07 | 20.11 | 8.38 | 10.61 | 7.82 | 0.00 | 1790 | 11.44 | 27.5 | 20 | 11 | Infertility |
| P34 | 43 | (8;16) (p22;p12) | 43.61 | 21.93 | 29.07 | 0.80 | 4.01 | 0.50 | 2002 | - | 60 | 20 | 35 | Infertility |
| P35 | 41 | (2;13) (q33;q14) | 41.65 | 45.90 | 3.27 | 2.58 | 2.58 | 4.06 | 2019 | - | 24 | 42 | 36 | Infertility |
| P36 | 32 | (8;19) (p23;p11) | 53.70 | 29.26 | 9.65 | 3.62 | 1.46 | 1.71 | 1989 | - | 76 | 59 | 8 | Infertility |
| P37 | 32 | (12;15) (q14;q25) | 43.18 | 37.83 | 8.88 | 3.19 | 2.31 | 4.62 | 2038 | - | 31.3 | 43 | 25 | Spontaneous abortions/Repeated miscarriages |
| P38 | 44 | (8;20) (q24.3;q11) | 47.60 | 41.95 | 4.57 | 1.73 | 0.70 | 2.61 | 2145 | - | 127.7 | 49 | 17 | Infertility |
| P39 | 34 | (4;5) (q35;q22) | 53.40 | 44.90 | 0.35 | 0.20 | 0.35 | 0.45 | 2025 | - | 48.1 | 68 | 19 | Spontaneous abortions/Repeated miscarriages |
| P40 | 36 | (1;2) (p22;p14) | 49.66 | 24.54 | 12.68 | 2.93 | 7.93 | 1.82 | 2082 | - | 11 | 56 | 7 | Infertility |
| P41 | 34 | (2;4) (p13;q27) | 36.23 | 42.31 | 11.32 | 3.10 | 2.25 | 4.80 | 2004 | - | 145 | 56 | 35 | Infertility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Llano, E.; Perrin, A.; Morel, F.; Devillard, F.; Harbuz, R.; Satre, V.; Amblard, F.; Bidart, M.; Hennebicq, S.; Brouillet, S.; et al. Sperm Meiotic Segregation Analysis of Reciprocal Translocations Carriers: We Have Bigger FISH to Fry. Int. J. Mol. Sci. 2023, 24, 3664. https://doi.org/10.3390/ijms24043664
Del Llano E, Perrin A, Morel F, Devillard F, Harbuz R, Satre V, Amblard F, Bidart M, Hennebicq S, Brouillet S, et al. Sperm Meiotic Segregation Analysis of Reciprocal Translocations Carriers: We Have Bigger FISH to Fry. International Journal of Molecular Sciences. 2023; 24(4):3664. https://doi.org/10.3390/ijms24043664
Chicago/Turabian StyleDel Llano, Edgar, Aurore Perrin, Frédéric Morel, Françoise Devillard, Radu Harbuz, Véronique Satre, Florence Amblard, Marie Bidart, Sylviane Hennebicq, Sophie Brouillet, and et al. 2023. "Sperm Meiotic Segregation Analysis of Reciprocal Translocations Carriers: We Have Bigger FISH to Fry" International Journal of Molecular Sciences 24, no. 4: 3664. https://doi.org/10.3390/ijms24043664
APA StyleDel Llano, E., Perrin, A., Morel, F., Devillard, F., Harbuz, R., Satre, V., Amblard, F., Bidart, M., Hennebicq, S., Brouillet, S., Ray, P. F., Coutton, C., & Martinez, G. (2023). Sperm Meiotic Segregation Analysis of Reciprocal Translocations Carriers: We Have Bigger FISH to Fry. International Journal of Molecular Sciences, 24(4), 3664. https://doi.org/10.3390/ijms24043664






