MET Oncogene Targeting for Cancer Immunotherapy
Abstract
:1. Introduction
2. Overview of MET-Targeting Molecules
3. MET-Targeting Antibodies to Induce Immune-Mediated Cancer Cell Death
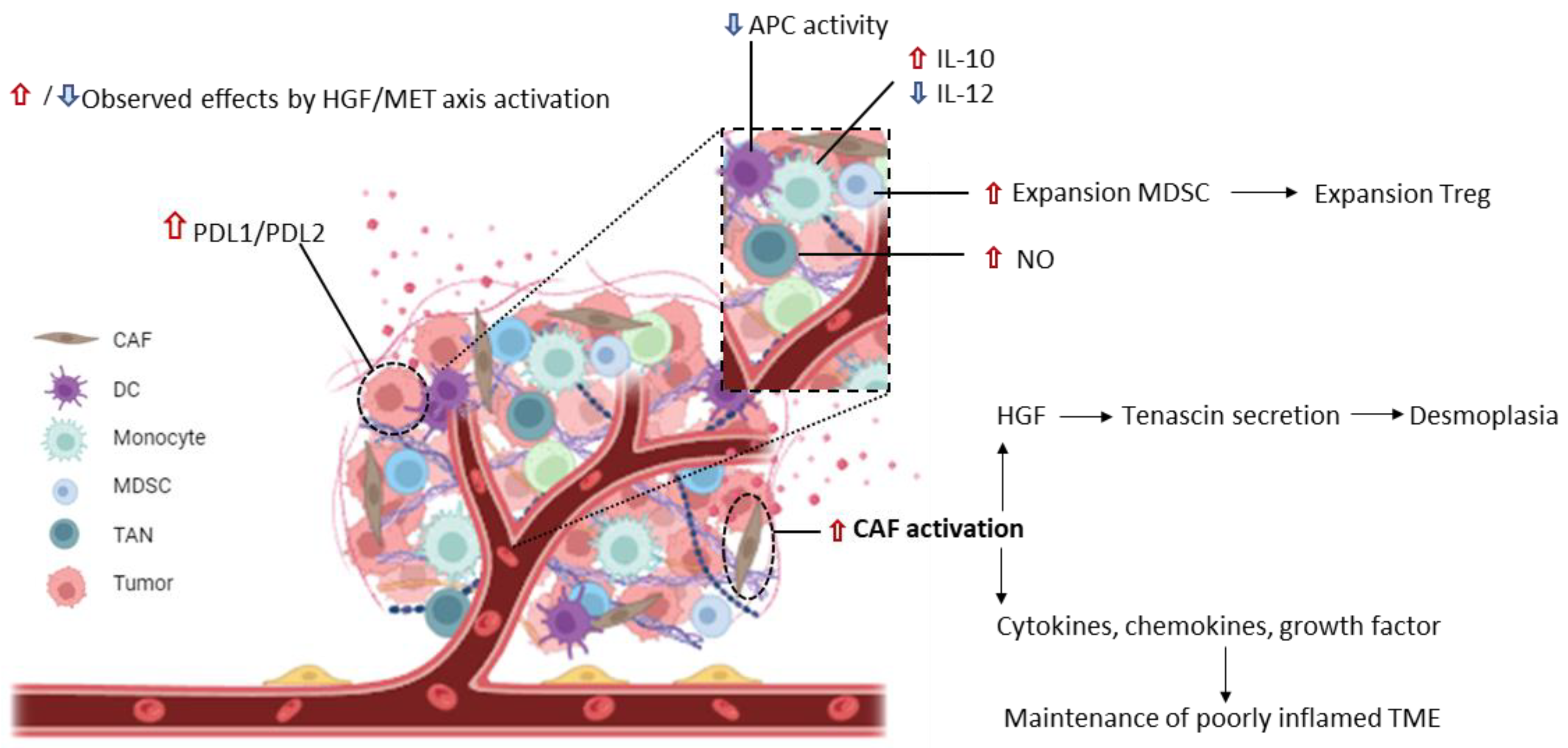
4. Inhibition of MET Signaling to Modulate Cancer Immune Tolerance
5. MET as a Target for Cellular Immunotherapy
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dean, M.; Park, M.; Le Beau, M.M.; Robins, T.S.; Diaz, M.O.; Rowley, J.D.; Blair, D.G.; Vande Woude, G.F. The human met oncogene is related to the tyrosine kinase oncogenes. Nature 1985, 318, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L.; Weidner, K.M.; Vigna, E.; Gaudino, G.; Bardelli, A.; Ponzetto, C.; Narsimhan, R.P.; Hartmann, G.; Zarnegar, R.; Michalopoulos, G.K.; et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991, 10, 2867–2878. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Ponzetto, C.; Di Renzo, M.F.; Cooper, C.S.; Comoglio, P.M. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature 1989, 339, 155–156. [Google Scholar] [CrossRef]
- Peschard, P.; Ishiyama, N.; Lin, T.; Lipkowitz, S.; Park, M. A conserved DpYR motif in the juxtamembrane domain of the Met receptor family forms an atypical c-Cbl/Cbl-b tyrosine kinase binding domain binding site required for suppression of oncogenic activation. J. Biol. Chem. 2004, 279, 29565–29571. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, R.; Longati, P.; Naldini, L.; Vigna, E.; Comoglio, P.M. Identification of the major autophosphorylation site of the Met/hepatocyte growth factor receptor tyrosine kinase. J. Biol. Chem. 1991, 266, 19558–19564. [Google Scholar] [CrossRef] [PubMed]
- Ponzetto, C.; Bardelli, A.; Zhen, Z.; Maina, F.; dalla Zonca, P.; Giordano, S.; Graziani, A.; Panayotou, G.; Comoglio, P.M. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 1994, 77, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Zarnegar, R. Regulation of HGF and HGFR gene expression. EXS 1995, 74, 33–49. [Google Scholar] [PubMed]
- Sonnenberg, E.; Meyer, D.; Weidner, K.M.; Birchmeier, C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J. Cell Biol. 1993, 123, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Trusolino, L.; Comoglio, P.M. Scatter-factor and semaphorin receptors: Cell signalling for invasive growth. Nat. Rev. Cancer 2002, 2, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Birchmeier, C.; Gherardi, E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998, 8, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Rosário, M.; Birchmeier, W. How to make tubes: Signaling by the Met receptor tyrosine kinase. Trends Cell Biol. 2003, 13, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T. Hepatocyte growth factor as mitogen, motogen and morphogen, and its roles in organ regeneration. Princess Takamatsu Symp. 1994, 24, 195–213. [Google Scholar] [PubMed]
- Chmielowiec, J.; Borowiak, M.; Morkel, M.; Stradal, T.; Munz, B.; Werner, S.; Wehland, J.; Birchmeier, C.; Birchmeier, W. c-Met is essential for wound healing in the skin. J. Cell Biol. 2007, 177, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Trusolino, L.; Bertotti, A.; Comoglio, P.M. MET signalling: Principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Birchmeier, C.; Birchmeier, W.; Gherardi, E.; Vande Woude, G.F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003, 4, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Modica, C.; Cortese, M.; Bersani, F.; Lombardi, A.M.; Napoli, F.; Righi, L.; Taulli, R.; Basilico, C.; Vigna, E. Genetic Ablation of the MET Oncogene Defines a Crucial Role of the HGF/MET Axis in Cell-Autonomous Functions Driving Tumor Dissemination. Cancers 2023, 15, 2742. [Google Scholar] [CrossRef] [PubMed]
- Comoglio, P.M.; Trusolino, L.; Boccaccio, C. Known and novel roles of the MET oncogene in cancer: A coherent approach to targeted therapy. Nat. Rev. Cancer 2018, 18, 341–358. [Google Scholar] [CrossRef]
- Recondo, G.; Che, J.; Jänne, P.A.; Awad, M.M. Targeting MET Dysregulation in Cancer. Cancer Discov. 2020, 10, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Luo, J.; Chang, J.; Rekhtman, N.; Arcila, M.; Drilon, A. MET-dependent solid tumours—Molecular diagnosis and targeted therapy. Nat. Rev. Clin. Oncol. 2020, 17, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Smolen, G.A.; Sordella, R.; Muir, B.; Mohapatra, G.; Barmettler, A.; Archibald, H.; Kim, W.J.; Okimoto, R.A.; Bell, D.W.; Sgroi, D.C.; et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc. Natl. Acad. Sci. USA 2006, 103, 2316–2321. [Google Scholar] [CrossRef]
- Lutterbach, B.; Zeng, Q.; Davis, L.J.; Hatch, H.; Hang, G.; Kohl, N.E.; Hatch, H.; Hang, G.; Kohl, N.E.; Gibbs, J.B.; et al. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res. 2007, 67, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.; Hong, L.; Zhang, J.; Heymach, J.; Hong, D.; Le, X. Beyond epidermal growth factor receptor: MET amplification as a general resistance driver to targeted therapy in oncogene-driven non-small-cell lung cancer. ESMO Open 2021, 6, 100319. [Google Scholar] [CrossRef]
- Zhou, J.; Ji, Q.; Li, Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: Underlying mechanisms and reversal strategies. J. Exp. Clin. Cancer Res. 2021, 40, 328. [Google Scholar] [CrossRef] [PubMed]
- Tovar, E.A.; Graveel, C.R. MET in human cancer: Germline and somatic mutations. Ann. Transl. Med. 2017, 5, 205. [Google Scholar] [CrossRef] [PubMed]
- Kong-Beltran, M.; Seshagiri, S.; Zha, J.; Zhu, W.; Bhawe, K.; Mendoza, N.; Holcomb, T.; Pujara, K.; Stinson, J.; Fu, L.; et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res. 2006, 66, 283–289. [Google Scholar] [CrossRef]
- Schrock, A.B.; Frampton, G.M.; Suh, J.; Chalmers, Z.R.; Rosenzweig, M.; Erlich, R.L.; Halmos, B.; Goldman, J.; Forde, P.; Leuenberger, K.; et al. Characterization of 298 Patients with Lung Cancer Harboring MET Exon 14 Skipping Alterations. J. Thorac. Oncol. 2016, 11, 1493–1502. [Google Scholar] [CrossRef]
- Mathieu, L.N.; Larkins, E.; Akinboro, O.; Roy, P.; Amatya, A.K.; Fiero, M.H.; Roy, P.; Amatya, A.K.; Fiero, M.H.; Mishra-Kalyani, P.S.; et al. FDA Approval Summary: Capmatinib and Tepotinib for the Treatment of Metastatic NSCLC Harboring MET Exon 14 Skipping Mutations or Alterations. Clin. Cancer Res. 2022, 28, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Savolitinib: First Approval. Drugs 2021, 81, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Seto, T.; Han, J.Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef]
- Sun, D.; Wu, W.; Wang, L.; Qu, J.; Han, Q.; Wang, H.; Song, S.; Liu, N.; Wang, Y.; Hou, H.; et al. Identification of MET fusions as novel therapeutic targets sensitive to MET inhibitors in lung cancer. J. Transl. Med. 2023, 21, 150. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liao, H.Y.; Zhang, H.H. Roles of MET in human cancer. Clin. Chim. Acta 2022, 525, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Babic, A. Regulation of the MET oncogene: Molecular mechanisms. Carcinogenesis 2016, 37, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, F.; Giovannetti, E.; Saso, L.; Firuzi, O. HGF/MET pathway aberrations as diagnostic, prognostic, and predictive biomarkers in human cancers. Crit. Rev. Clin. Lab. Sci. 2019, 56, 533–566. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Guan, M.; Sun, Z.; Bai, C. High c-Met expression is a negative prognostic marker for colorectal cancer: A meta-analysis. Tumour Biol. 2015, 36, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Szturz, P.; Budíková, M.; Vermorken, J.B.; Horová, I.; Gál, B.; Raymond, E.; de Gramont, A.; Faivre, S. Prognostic value of c-MET in head and neck cancer: A systematic review and meta-analysis of aggregate data. Oral Oncol. 2017, 74, 68–76. [Google Scholar] [CrossRef]
- Ren, J.L.; Wu, H.F.; Wang, W.J.; Hu, G.M.; Gu, B.; Zhang, M.; Wang, Y.X. C-Met as a potential novel prognostic marker in squamous cell carcinoma and adenocarcinoma of esophagus: Evidence from a meta-analysis. Panminerva Med. 2017, 59, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Jiao, X.; Zou, H.; Li, K. Prognostic significance of c-Met in breast cancer: A meta-analysis of 6010 cases. Diagn. Pathol. 2015, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhu, Y.; Wang, Q.; Gao, J.; Li, Y.; Li, Y.; Ge, S.; Shen, L. Prognostic significance of MET amplification and expression in gastric cancer: A systematic review with meta-analysis. PLoS ONE 2014, 9, e84502. [Google Scholar] [CrossRef] [PubMed]
- Comoglio, P.M.; Giordano, S.; Trusolino, L. Drug development of MET inhibitors: Targeting oncogene addiction and expedience. Nat. Rev. Drug Discov. 2008, 7, 504–516. [Google Scholar] [CrossRef] [PubMed]
- de Luca, A.; Arena, N.; Sena, L.M.; Medico, E. Met overexpression confers HGF-dependent invasive phenotype to human thyroid carcinoma cells in vitro. J. Cell Physiol. 1999, 180, 365–371. [Google Scholar] [CrossRef]
- Qian, L.W.; Mizumoto, K.; Inadome, N.; Nagai, E.; Sato, N.; Matsumoto, K.; Nakamura, T.; Tanaka, M. Radiation stimulates HGF receptor/c-Met expression that leads to amplifying cellular response to HGF stimulation via upregulated receptor tyrosine phosphorylation and MAP kinase activity in pancreatic cancer cells. Int. J. Cancer 2003, 104, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Wang, W.; Li, Q.; Matsumoto, K.; Sakurama, H.; Nakamura, T.; Ogino, H.; Kakiuchi, S.; Hanibuchi, M.; Nishioka, Y.; et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008, 68, 9479–9487. [Google Scholar] [CrossRef] [PubMed]
- Pons, E.; Uphoff, C.C.; Drexler, H.G. Expression of hepatocyte growth factor and its receptor c-met in human leukemia-lymphoma cell lines. Leuk. Res. 1998, 22, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, R.; Di Renzo, M.F.; Scotlandi, K.; Baldini, N.; Olivero, M.; Lollini, P.; Cremona, O.; Campanacci, M.; Comoglio, P.M. The Met/HGF receptor is over-expressed in human osteosarcomas and is activated by either a paracrine or an autocrine circuit. Oncogene 1995, 10, 739–749. [Google Scholar] [PubMed]
- Borset, M.; Lien, E.; Espevik, T.; Helseth, E.; Waage, A.; Sundan, A. Concomitant expression of hepatocyte growth factor/scatter factor and the receptor c-MET in human myeloma cell lines. J. Biol. Chem. 1996, 271, 24655–24661. [Google Scholar] [CrossRef]
- Moriyama, T.; Kataoka, H.; Koono, M.; Wakisaka, S. Expression of hepatocyte growth factor/scatter factor and its receptor c-Met in brain tumors: Evidence for a role in progression of astrocytic tumors (Review). Int. J. Mol. Med. 1999, 3, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Narayan, R.N.; Horton, L.; Patel, T.R.; Habib, A.A. The Role of EGFR-Met Interactions in the Pathogenesis of Glioblastoma and Resistance to Treatment. Curr. Cancer Drug Targets 2017, 17, 297–302. [Google Scholar] [CrossRef]
- Kazandjian, D.; Blumenthal, G.M.; Chen, H.Y.; He, K.; Patel, M.; Justice, R.; Keegan, P.; Pazdur, R. FDA approval summary: Crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist 2014, 19, e5–e11. [Google Scholar] [CrossRef] [PubMed]
- Duke, E.S.; Barone, A.K.; Chatterjee, S.; Mishra-Kalyani, P.S.; Shen, Y.L.; Isikwei, E.; Zhao, H.; Bi, Y.; Liu, J.; Rahman, N.A.; et al. FDA Approval Summary: Cabozantinib for Differentiated Thyroid Cancer. Clin. Cancer Res. 2022, 28, 4173–4177. [Google Scholar] [CrossRef]
- Aparicio, T.; Cozic, N.; de la Fouchardière, C.; Meriaux, E.; Plaza, J.; Mineur, L.; Guimbaud, R.; Samalin, E.; Mary, F.; Lecomte, T.; et al. The Activity of Crizotinib in Chemo-Refractory MET-Amplified Esophageal and Gastric Adenocarcinomas: Results from the AcSé-Crizotinib Program. Target. Oncol. 2021, 16, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Otterson, G.A.; Clark, J.W.; Ignatius Ou, S.H.; Weiss, J.; Ades, S.; Shapiro, G.I.; Socinski, M.A.; Murphy, D.A.; Conte, U.; et al. Crizotinib in Patients with MET-Amplified NSCLC. J. Thorac. Oncol. 2021, 16, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.X.; Song, B.B. Gastric cancer patient with c-MET amplification treated with crizotinib after failed multi-line treatment: A case report and literature review. Math. Biosci. Eng. 2019, 16, 5923–5930. [Google Scholar] [CrossRef] [PubMed]
- Lennerz, J.K.; Kwak, E.L.; Ackerman, A.; Michael, M.; Fox, S.B.; Bergethon, K.; Lauwers, G.Y.; Christensen, J.G.; Wilner, K.D.; Haber, D.A.; et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J. Clin. Oncol. 2011, 29, 4803–4810. [Google Scholar] [CrossRef] [PubMed]
- Oddo, D.; Siravegna, G.; Gloghini, A.; Vernieri, C.; Mussolin, B.; Morano, F.; Crisafulli, G.; Berenato, R.; Corti, G.; Volpi, C.C.; et al. Emergence of MET hyper-amplification at progression to MET and BRAF inhibition in colorectal cancer. Br. J. Cancer 2017, 117, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Rochigneux, P.; Thomassin-Piana, J.; Laibe, S.; Brunelle, S.; Salem, N.; Escudier, B.; Vassal, G.; Gravis, G. Long-term efficacy of crizotinib in a metastatic papillary renal carcinoma with MET amplification: A case report and literature review. BMC Cancer 2018, 18, 1159. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Dai, L.; Wang, Y.; Liang, G.; Yang, N.; Chen, M. Responses to crizotinib and disease monitoring with circulating tumor cells in lung adenocarcinoma patient with MET exon 14 skipping mutation: A case report. Medicine 2017, 96, e8744. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Zheng, H.; Yurgelun, M.B.; Abrams, T.A.; Allen, J.N.; Cleary, J.M.; Knowles, M.; Regan, E.; Reardon, A.; Khachatryan, A.; et al. A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer 2017, 123, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Klempner, S.J.; Borghei, A.; Hakimian, B.; Ali, S.M.; Ou, S.I. Intracranial Activity of Cabozantinib in MET Exon 14-Positive NSCLC with Brain Metastases. J. Thorac. Oncol. 2017, 12, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Wang, J.; Xu, P.; Zheng, Y.; Bai, L.; Sun, X.; Li, Z.; Gan, R.; Li, H.; Ke, Z.; et al. A rapid and durable response to cabozantinib in an osimertinib-resistant lung cancer patient with MET D1228N mutation: A case report. Ann. Transl. Med. 2021, 9, 1354. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Tangen, C.; Thompson, I.M.; Balzer-Haas, N.; George, D.J.; Heng, D.Y.C.; Shuch, B.; Stein, M.; Tretiakova, M.; Humphrey, P.; et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: A randomised, open-label, phase 2 trial. Lancet 2021, 397, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Parsons, B.M.; Meier, D.R.; Richmond, C.S.; Gurda, G.T.; Lofgren, K.A.; Burkard, M.E.; Deming, D.A.; Kenny, P.A. Acquisition of Cabozantinib-Sensitive MET D1228N Mutation During Progression on Crizotinib in MET-Amplified Triple-Negative Breast Cancer. Clin. Breast Cancer 2020, 20, e433–e438. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.Y.; Liu, L.S.; Zhang, H.Y.; Lu, C.H.; Guo, X.Y.; Zhang, L.Y.; Yuan, X.B.; Xue, H.H. Responses to crizotinib and cabozantinib in patient with lung adenocarcinoma harboring mesenchymal-epithelial transition factor exon 14 skipping mutation: A case report. Medicine 2021, 100, e24300. [Google Scholar] [CrossRef] [PubMed]
- Torrado, C.; Feng, J.; Faour, E.; Leighl, N.B. Cabozantinib Response in a Patient with NSCLC Harboring Both MET Exon 14 Skipping Mutation and Secondary RET Fusion: A Case Report. JTO Clin. Res. Rep. 2024, 5, 100647. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, M.F.; Ranalli, M.; Shah, N. Clinical efficacy of cabozantinib in two pediatric patients with recurrent renal cell carcinoma. Pediatr. Blood Cancer 2017, 64, e26586. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S. Rilotumumab, a mAb against human hepatocyte growth factor for the treatment of cancer. Curr. Opin. Mol. Ther. 2009, 11, 448–455. [Google Scholar] [PubMed]
- Merchant, M.; Ma, X.; Maun, H.R.; Zheng, Z.; Peng, J.; Romero, M.; Huang, A.; Yang, N.Y.; Nishimura, M.; Greve, J.; et al. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc. Natl. Acad. Sci. USA 2013, 110, E2987–E2996. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.T.; Tebbutt, N.C.; Davidenko, I.; Murad, A.M.; Al-Batran, S.E.; Ilson, D.H.; Tjulandin, S.; Gotovkin, E.; Karaszewska, B.; Bondarenko, I.; et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1467–1482. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Edelman, M.J.; O’Byrne, K.; Paz-Ares, L.; Mocci, S.; Phan, S.; Shames, D.S.; Smith, D.; Yu, W.; Paton, V.E.; et al. Results from the Phase III Randomized Trial of Onartuzumab Plus Erlotinib Versus Erlotinib in Previously Treated Stage IIIB or IV Non-Small-Cell Lung Cancer: METLung. J. Clin. Oncol. 2017, 35, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Bang, Y.J.; Lordick, F.; Alsina, M.; Chen, M.; Hack, S.P.; Bruey, J.M.; Smith, D.; McCaffery, I.; Shames, D.S.; et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin with or without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The METGastric Randomized Clinical Trial. JAMA Oncol. 2017, 3, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Anderson, M.G.; Oleksijew, A.; Vaidya, K.S.; Boghaert, E.R.; Tucker, L.; Zhang, Q.; Han, E.K.; Palma, J.P.; Naumovski, L.; et al. ABBV-399, a c-Met Antibody-Drug Conjugate that Targets Both MET-Amplified and c-Met-Overexpressing Tumors, Irrespective of MET Pathway Dependence. Clin. Cancer Res. 2017, 23, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, J.O.; Yang, K.; Surriga, O.; Nittoli, T.; Kunz, A.; Franklin, M.C.; Delfino, F.J.; Mao, S.; Zhao, F.; Giurleo, J.T.; et al. A Biparatopic Antibody-Drug Conjugate to Treat MET-Expressing Cancers, Including Those that Are Unresponsive to MET Pathway Blockade. Mol. Cancer Ther. 2021, 20, 1966–1976. [Google Scholar] [CrossRef]
- Grandal, M.M.; Havrylov, S.; Poulsen, T.T.; Koefoed, K.; Dahlman, A.; Galler, G.R.; Conrotto, P.; Collins, S.; Eriksen, K.W.; Kaufman, D.; et al. Simultaneous Targeting of Two Distinct Epitopes on MET Effectively Inhibits MET- and HGF-Driven Tumor Growth by Multiple Mechanisms. Mol. Cancer Ther. 2017, 16, 2780–2791. [Google Scholar] [CrossRef] [PubMed]
- Lokker, N.A.; Mark, M.R.; Luis, E.A.; Bennett, G.L.; Robbins, K.A.; Baker, J.B.; Godowski, P.J. Structure-function analysis of hepatocyte growth factor: Identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J. 1992, 11, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, T.T.; Grandal, M.M.; Skartved, N.J.Ø.; Hald, R.; Alifrangis, L.; Koefoed, K.; Lindsted, T.; Fröhlich, C.; Pollmann, S.E.; Eriksen, K.W.; et al. Sym015: A Highly Efficacious Antibody Mixture against MET-Amplified Tumors. Clin. Cancer Res. 2017, 23, 5923–5935. [Google Scholar] [CrossRef] [PubMed]
- Basilico, C.; Hultberg, A.; Blanchetot, C.; de Jonge, N.; Festjens, E.; Hanssens, V.; Osepa, S.I.; De Boeck, G.; Mira, A.; Cazzanti, M.; et al. Four individually druggable MET hotspots mediate HGF-driven tumor progression. J. Clin. Investig. 2014, 124, 3172–3186. [Google Scholar] [CrossRef] [PubMed]
- Hultberg, A.; Morello, V.; Huyghe, L.; De Jonge, N.; Blanchetot, C.; Hanssens, V.; Osepa, S.I.; De Boeck, G.; Mira, A.; Cazzanti, M.; et al. Depleting MET-Expressing Tumor Cells by ADCC Provides a Therapeutic Advantage over Inhibiting HGF/MET Signaling. Cancer Res. 2015, 75, 3373–3383. [Google Scholar] [CrossRef] [PubMed]
- Aftimos, P.; Rolfo, C.; Rottey, S.; Barthélémy, P.; Borg, C.; Park, K.; Oh, D.Y.; Kim, S.W.; De Jonge, N.; Hanssens, V.; et al. The NHance® Mutation-Equipped Anti-MET Antibody ARGX-111 Displays Increased Tissue Penetration and Anti-Tumor Activity in Advanced Cancer Patients. Biomedicines 2021, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Neijssen, J.; Cardoso, R.M.F.; Chevalier, K.M.; Wiegman, L.; Valerius, T.; Anderson, G.M.; Moores, S.L.; Schuurman, J.; Parren, P.W.H.I.; Strohl, W.R.; et al. Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET. J. Biol. Chem. 2021, 296, 100641. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, S.; Lipfert, L.; Chevalier, K.; Bushey, B.S.; Henley, B.; Lenhart, R.; Sendecki, J.; Beqiri, M.; Millar, H.J.; Packman, K.; et al. Amivantamab (JNJ-61186372), an Fc Enhanced EGFR/cMet Bispecific Antibody, Induces Receptor Downmodulation and Antitumor Activity by Monocyte/Macrophage Trogocytosis. Mol. Cancer Ther. 2020, 19, 2044–2056. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tang, K.J.; Cho, B.C.; Liu, B.; Paz-Ares, L.; Cheng, S.; Kitazono, S.; Thiagarajan, M.; Goldman, J.W.; Sabari, J.K.; et al. Amivantamab plus Chemotherapy in NSCLC with EGFR Exon 20 Insertions. N. Engl. J. Med. 2023, 389, 2039–2051. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Felip, E.; Hayashi, H.; Thomas, M.; Lu, S.; Besse, B.; Sun, T.; Martinez, M.; Sethi, S.N.; Shreeve, S.M.; et al. MARIPOSA: Phase 3 study of first-line amivantamab + lazertinib versus osimertinib in EGFR-mutant non-small-cell lung cancer. Future Oncol. 2022, 18, 639–647. [Google Scholar] [CrossRef] [PubMed]
- EGFR-Mutant NSCLC: Spotlight on Amivantamab. Cancer Discov. 2023, 13, OF9. [CrossRef] [PubMed]
- Denis, M.G.; Bennouna, J. Osimertinib for Front-Line Treatment of Locally Advanced or Metastatic EGFR-Mutant NSCLC Patients: Efficacy, Acquired Resistance and Perspectives for Subsequent Treatments. Cancer Manag. Res. 2020, 12, 12593–12602. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Wang, J.; Wang, Y.; Lee, S.H.; Melosky, B.; Shih, J.Y.; Wang, J.; Azuma, K.; Juan-Vidal, O.; Cobo, M.; et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: Primary results from the phase III MARIPOSA-2 study. Ann. Oncol. 2024, 35, 77–90. [Google Scholar] [CrossRef]
- Lee, A.T.M.; Ou, S.I. MARIPOSA-2: Is the treatment worse than the disease? Med 2024, 5, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Albitar, M.; Sudarsanam, S.; Ma, W.; Jiang, S.; Chen, W.; Funari, V.; Blocker, F.; Agersborg, S. Correlation of MET gene amplification and TP53 mutation with PD-L1 expression in non-small cell lung cancer. Oncotarget 2018, 9, 13682–13693. [Google Scholar] [CrossRef] [PubMed]
- Domènech, M.; Muñoz Marmol, A.M.; Mate, J.L.; Estival, A.; Moran, T.; Cucurull, M.; Saigi, M.; Hernandez, A.; Sanz, C.; Hernandez-Gallego, A.; et al. Correlation between PD-L1 expression and MET gene amplification in patients with advanced non-small cell lung cancer and no other actionable oncogenic driver. Oncotarget 2021, 12, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Ahn, S.; Jung, J.; Hyung, S.; Kim, K.M.; Kim, S.T.; Kang, W.K.; Lee, J. Impact of programmed death-ligand 1 (PD-L1) positivity on clinical and molecular features of patients with metastatic gastric cancer. Cancer Med. 2023, 12, 18633–18642. [Google Scholar] [CrossRef] [PubMed]
- Song, K.Y.; Desar, S.; Pengo, T.; Shanley, R.; Giubellino, A. Correlation of MET and PD-L1 Expression in Malignant Melanoma. Cancers 2020, 12, 1847. [Google Scholar] [CrossRef]
- Wang, Q.W.; Sun, L.H.; Zhang, Y.; Wang, Z.; Zhao, Z.; Wang, Z.L.; Wang, K.Y.; Li, G.Z.; Xu, J.B.; Ren, C.Y.; et al. MET overexpression contributes to STAT4-PD-L1 signaling activation associated with tumor-associated, macrophages-mediated immunosuppression in primary glioblastomas. J. Immunother. Cancer 2021, 9, e002451. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Bankel, L.; Hiltbrunner, S.; Rechsteiner, M.; Rüschoff, J.H.; Rushing, E.J.; Britschgi, C.; Curioni-Fontecedro, A. Mutational Landscape and Expression of PD-L1 in Patients with Non-Small Cell Lung Cancer Harboring Genomic Alterations of the MET gene. Target. Oncol. 2022, 17, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Inoue, Y.; Tsuchiya, K.; Karayama, M.; Yamada, H.; Iwashita, Y.; Kawase, A.; Tanahashi, M.; Ogawa, H.; Inui, N.; et al. Elucidation of the relationships of MET protein expression and gene copy number status with PD-L1 expression and the immune microenvironment in non-small cell lung cancer. Lung Cancer 2020, 141, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kammerer-Jacquet, S.F.; Medane, S.; Bensalah, K.; Bernhard, J.C.; Yacoub, M.; Dupuis, F.; Ravaud, A.; Verhoest, G.; Mathieu, R.; Peyronnet, B.; et al. Correlation of c-MET Expression with PD-L1 Expression in Metastatic Clear Cell Renal Cell Carcinoma Treated by Sunitinib First-Line Therapy. Target. Oncol. 2017, 12, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Guisier, F.; Descourt, R.; Babey, H.; Huchot, E.; Falchero, L.; Veillon, R.; Cortot, A.B.; Tissot, C.; Chouaid, C.; Decroisette, C. Brief Report: First-line Pembrolizumab in Metastatic Non-Small Cell Lung Cancer Habouring MET Exon 14 Skipping Mutation and PD-L1 ≥50% (GFPC 01–20 Study). Clin. Lung Cancer 2022, 23, e545–e549. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Liu, X.; Li, A.; Song, L.; Liang, J.; Gao, J.; Tang, X. c-Met up-regulates the expression of PD-L1 through MAPK/NF-κBp65 pathway. J. Mol. Med. 2022, 100, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Saigi, M.; Alburquerque-Bejar, J.J.; Mc Leer-Florin, A.; Pereira, C.; Pros, E.; Romero, O.A.; Baixeras, N.; Esteve-Codina, A.; Nadal, E.; Brambilla, E.; et al. MET-oncogenic and JAK2-inactivating alterations are independent factors that affect regulation of PD-L1 expression in lung cancer. Clin. Cancer Res. 2018, 24, 4579–4587. [Google Scholar] [CrossRef] [PubMed]
- Demuth, C.; Andersen, M.N.; Jakobsen, K.R.; Madsen, A.T.; Sørensen, B.S. Increased PD-L1 expression in erlotinib-resistant NSCLC cells with MET gene amplification is reversed upon MET-TKI treatment. Oncotarget 2017, 8, 68221–68229. [Google Scholar] [CrossRef]
- Martin, V.; Chiriaco, C.; Modica, C.; Acquadro, A.; Cortese, M.; Galimi, F.; Perera, T.; Gammaitoni, L.; Aglietta, M.; Comoglio, P.M.; et al. Met inhibition revokes IFNγ-induction of PD-1 ligands in MET-amplified tumours. Br. J. Cancer 2019, 120, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Huang, X.; Zhang, G.; Liang, T. Combinational blockade of MET and PD-L1 improves pancreatic cancer immunotherapeutic efficacy. J. Exp. Clin. Cancer Res. 2021, 40, 279. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, J.M.; Pauken, K.E. Localization, tissue biology and T cell state—Implications for cancer immunotherapy. Nat. Rev. Immunol. 2023, 23, 807–823. [Google Scholar] [CrossRef] [PubMed]
- Modica, C.; Olivero, M.; Zuppini, F.; Milan, M.; Basilico, C.; Vigna, E. HGF/MET Axis Induces Tumor Secretion of Tenascin-C and Promotes Stromal Rewiring in Pancreatic Cancer. Cancers 2021, 13, 3519. [Google Scholar] [CrossRef] [PubMed]
- Yen, B.L.; Yen, M.L.; Hsu, P.J.; Liu, K.J.; Wang, C.J.; Bai, C.H.; Sytwu, H.K. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Rep. 2013, 1, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Bonanno, G.; Procoli, A.; Mariotti, A.; de Ritis, D.G.; Curti, A.; Danese, S.; Pessina, G.; Pandolfi, S.; Natoni, F.; et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood 2006, 108, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Glodde, N.; Bald, T.; van den Boorn-Konijnenberg, D.; Nakamura, K.; O’Donnell, J.S.; Szczepanski, S.; Brandes, M.; Eickhoff, S.; Das, I.; Shridhar, N.; et al. Reactive Neutrophil Responses Dependent on the Receptor Tyrosine Kinase c-MET Limit Cancer Immunotherapy. Immunity 2017, 47, 789–802.e9. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Chiriaco, C.; Donini, C.; Cortese, M.; Ughetto, S.; Modica, C.; Martinelli, I.; Proment, A.; Vitali, L.; Fontani, L.; Casucci, M.; et al. Efficacy of CAR-T immunotherapy in MET overexpressing tumors not eligible for anti-MET targeted therapy. J. Exp. Clin. Cancer Res. 2022, 41, 309. [Google Scholar] [CrossRef] [PubMed]
- Thayaparan, T.; Petrovic, R.M.; Achkova, D.Y.; Zabinski, T.; Davies, D.M.; Klampatsa, A.; Parente-Pereira, A.C.; Whilding, L.M.; van der Stegen, S.J.; Woodman, N.; et al. CAR T-cell immunotherapy of MET-expressing malignant mesothelioma. Oncoimmunology 2017, 6, e1363137. [Google Scholar] [CrossRef] [PubMed]
- Tchou, J.; Zhao, Y.; Levine, B.L.; Zhang, P.J.; Davis, M.M.; Melenhorst, J.J.; Kulikovskaya, I.; Brennan, A.L.; Liu, X.; Lacey, S.F.; et al. Safety and Efficacy of Intratumoral Injections of Chimeric Antigen Receptor (CAR) T Cells in Metastatic Breast Cancer. Cancer Immunol. Res. 2017, 5, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Guo, J.; Li, T.; Jia, L.; Tang, X.; Zhu, J.; Tang, Q.; Feng, Z. c-Met-targeted chimeric antigen receptor T cells inhibit hepatocellular carcinoma cells in vitro and in vivo. J. Biomed. Res. 2021, 36, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Mori, J.I.; Adachi, K.; Sakoda, Y.; Sasaki, T.; Goto, S.; Matsumoto, H.; Nagashima, Y.; Matsuyama, H.; Tamada, K. Anti-tumor efficacy of human anti-c-met CAR-T cells against papillary renal cell carcinoma in an orthotopic model. Cancer Sci. 2021, 112, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Huo, Q.; Lv, J.; Zhang, J.; Huang, H.; Hu, H.; Zhao, Y.; Zhang, X.; Wang, Y.; Zhou, Y.; Qiu, J.; et al. c-Met is a chimeric antigen receptor T-cell target for treating recurrent nasopharyngeal carcinoma. Cytotherapy 2023, 25, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Brentjens, R.; Rivière, I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.H.; Kim, Y.; Lee, D.Y.; Choi, S.U.; Lee, H.K.; Park, C.H. c-Met-Specific Chimeric Antigen Receptor T Cells Demonstrate Anti-Tumor Effect in c-Met Positive Gastric Cancer. Cancers 2021, 13, 5738. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, C.; Mori, T.; Sakabe, T.; Ariyama, Y.; Shinomiya, T.; Date, K.; Hagiwara, A.; Yamaguchi, T.; Takahashi, T.; Nakamura, Y.; et al. Gains, losses, and amplifications of genomic materials in primary gastric cancers analyzed by comparative genomic hybridization. Genes Chromosomes Cancer 1999, 24, 299–305. [Google Scholar] [CrossRef]
- Martinelli, I.; Modica, C.; Chiriaco, C.; Basilico, C.; Hughes, J.M.; Corso, S.; Giordano, S.; Comoglio, P.M.; Vigna, E. hOA-DN30: A highly effective humanized single-arm MET antibody inducing remission of “MET-addicted” cancers. J. Exp. Clin. Cancer Res. 2022, 41, 112. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Su, W.C.; Schuler, M.; Nam, D.H.; Lim, W.T.; Bauer, T.M.; Azaro, A.; Poon, R.T.P.; Hong, D.; Lin, C.C.; et al. Phase 1 study of capmatinib in MET-positive solid tumor patients: Dose escalation and expansion of selected cohorts. Cancer Sci. 2020, 111, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Karaszewska, B.; Kang, Y.K.; Chung, H.C.; Shankaran, V.; Siena, S.; Go, N.F.; Yang, H.; Schupp, M.; Cunningham, D. A Multicenter Phase II Study of AMG 337 in Patients with MET-Amplified Gastric/Gastroesophageal Junction/Esophageal Adenocarcinoma and Other MET-Amplified Solid Tumors. Clin. Cancer Res. 2019, 25, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Corso, S.; Isella, C.; Bellomo, S.E.; Apicella, M.; Durando, S.; Migliore, C.; Ughetto, S.; D’Errico, L.; Menegon, S.; Moya-Rull, D.; et al. A Comprehensive PDX Gastric Cancer Collection Captures Cancer Cell-Intrinsic Transcriptional MSI Traits. Cancer Res. 2019, 79, 5884–5896. [Google Scholar] [CrossRef]
- Yuan, X.; Sun, Z.; Yuan, Q.; Hou, W.; Liang, Q.; Wang, Y.; Mo, W.; Wang, H.; Yu, M. Dual-function chimeric antigen receptor T cells targeting c-Met and PD-1 exhibit potent anti-tumor efficacy in solid tumors. Investig. New Drugs 2021, 39, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, T.; Guo, J.; Wang, J.; Jia, L.; Shi, X.; Yang, T.; Jiao, R.; Wei, X.; Feng, Z.; et al. Bispecific c-Met/PD-L1 CAR-T Cells Have Enhanced Therapeutic Effects on Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 546586. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Gu, Y.M.; Zhang, F.; Zhang, Z.C.; Zhang, Y.T.; He, Y.D.; Wang, L.; Zhou, N.; Tang, F.T.; Liu, H.J.; et al. Construction of PD1/CD28 chimeric-switch receptor enhances anti-tumor ability of c-Met CAR-T in gastric cancer. Oncoimmunology 2021, 10, 1901434. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Weekes, C.D.; Nemunaitis, J.; Ramanathan, R.K.; Heist, R.S.; Morgensztern, D.; Angevin, E.; Bauer, T.M.; Yue, H.; Motwani, M.; et al. First-in-Human Phase I, Dose-Escalation and -Expansion Study of Telisotuzumab Vedotin, an Antibody–Drug Conjugate Targeting c-Met, in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2018, 36, 3298–3306. [Google Scholar] [CrossRef]
- Camidge, D.R.; Morgensztern, D.; Heist, R.S.; Barve, M.; Vokes, E.; Goldman, J.W.; Hong, D.S.; Bauer, T.M.; Strickler, J.H.; Angevin, E.; et al. Phase I Study of 2- or 3-Week Dosing of Telisotuzumab Vedotin, an Antibody-Drug Conjugate Targeting c-Met, Monotherapy in Patients with Advanced Non-Small Cell Lung Carcinoma. Clin. Cancer Res. 2021, 27, 5781–5792. [Google Scholar]
- Camidge, D.R.; Barlesi, F.; Goldman, J.W.; Morgensztern, D.; Heist, R.; Vokes, E.; Spira, A.; Angevin, E.; Su, W.-C.; Hong, D.S.; et al. Phase Ib Study of Telisotuzumab Vedotin in Combination With Erlotinib in Patients With c-Met Protein-Expressing Non-Small-Cell Lung Cancer. J. Clin. Oncol 2023, 41, 1105. [Google Scholar] [CrossRef]
- Camidge, D.R.; Barlesi, F.; Goldman, J.W.; Morgensztern, D.; Heist, R.; Vokes, E.; Angevin, E.; Hong, D.S.; Rybkin, I.I.; Barve, M.; et al. A Phase 1b Study of Telisotuzumab Vedotin in Combination with Nivolumab in Patients With NSCLC. JTO Clin. Res. Rep. 2021, 3, 100262. [Google Scholar] [CrossRef]
- Brazel, D.; Nagasaka, M. MARIPOSA: Can Amivantamab and Lazertinib Replace Osimertinib in the Front-Line Setting? Cancer 2024, 15, 41–47. [Google Scholar]
- Shah, P.D.; Huang, A.C.; Xu, X.; Orlowski, R.; Amaravadi, R.K.; Schuchter, L.M.; Zhang, P.; Tchou, J.; Matlawski, T.; Cervini, A.; et al. Phase I Trial of Autologous RNA-electroporated cMET-directed CAR T Cells Administered Intravenously in Patients with Melanoma and Breast Carcinoma. Cancer Res. Commun 2023, 3, 821–829. [Google Scholar]


| Antibody | Structure | Epitope on MET | HGF Displacement | MET Downregulation | MET Shedding | Antibody-Dependent Immune Functions | Antibody Drug Conjugated | Clinical Trial * |
|---|---|---|---|---|---|---|---|---|
| Onartuzumab (OA-5D5, MetMab) | Monovalent IgG1/k | SEMA (blades 4/5/6) | Yes | No | No | No | No | Ph III |
| Telisotuzumab (ABT-700) | Bivalent IgG1/k | IPT-1 | Yes + inhibition of receptor dimerization | Yes | Not reported | No | Teliso-V (ABBV-399) conjugated with Vedotin | Ph III (Telisotuzumab) Ph III (Teliso-V) |
| Emibetuzumab (LY 2875358) | Bivalent IgG4/k | SEMA (blades 2/3) | Yes | Yes | No | No | No | Ph II |
| Amivantamab (JNJ-61186372) | Bispecific (MET/EGFR) IgG1/k | SEMA (blade2) | Yes | Yes | Not reported | ADCC ADCP ADCT | No | Ph III |
| ARGX-111 | Bivalent IgG1/k | SEMA (blades 2/3) | Yes | Yes | No | ADCC | No | Ph I |
| Sym015 (1:1 mix of Hu9006 and Hu9338) | Bivalent mAbs, IgG1/k | SEMA (blade 2, blade 3) | Yes | Yes | Not reported | ADCC CDC | No | Ph i/II |
| SAIT-301 | Bivalent IgG2/k | SEMA (MET α-chain) | Yes | Yes | No | No | Ph I | |
| REGN-5093 (METxMET) | Biparatopic IgG1,IgG3/k | SEMA (blade 3, blades 5/6) | Yes | Yes + inhibition of surface receptor recycling | Not reported | No | REGN-5093-M114 (METxMET-M114) conjugated with a maytansin derivative | Ph I/II (REGN-5093) Ph I/II (REGN-5093-M114) |
| hOA-DN30 | Monovalent IgG1/k | IPT-4 | No | No | Yes | No | No | IND completed; clinical trial expected in 2024 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, A.M.; Sangiolo, D.; Vigna, E. MET Oncogene Targeting for Cancer Immunotherapy. Int. J. Mol. Sci. 2024, 25, 6109. https://doi.org/10.3390/ijms25116109
Lombardi AM, Sangiolo D, Vigna E. MET Oncogene Targeting for Cancer Immunotherapy. International Journal of Molecular Sciences. 2024; 25(11):6109. https://doi.org/10.3390/ijms25116109
Chicago/Turabian StyleLombardi, Andrea Maria, Dario Sangiolo, and Elisa Vigna. 2024. "MET Oncogene Targeting for Cancer Immunotherapy" International Journal of Molecular Sciences 25, no. 11: 6109. https://doi.org/10.3390/ijms25116109





