Astroglial Dysfunctions in Mood Disorders and Rodent Stress Models: Consequences on Behavior and Potential as Treatment Target
Abstract
:1. Introduction
2. Astroglia Are Affected by Stress
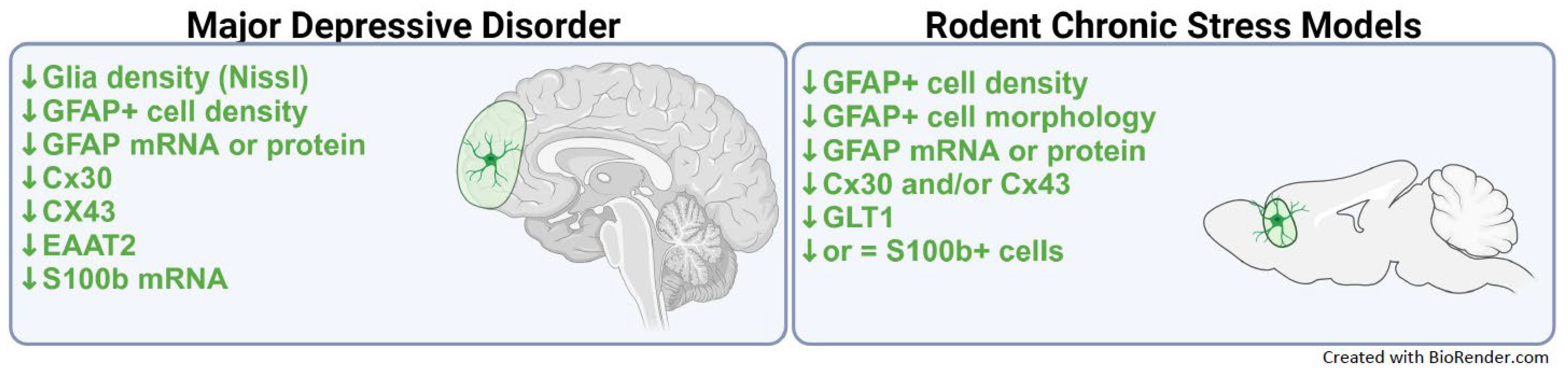
3. Astroglial Dysfunction in Mood Disorders
3.1. Evidence of Astroglial Dysfunction in MDD
3.2. Evidence of Astroglial Dysfunction in PTSD
4. Astroglial Changes in Animal Models of Stress
5. Effect of Astrocyte Manipulations on Emotion-Related Behavior
6. Targeting Astrocytes for Treatment
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saint-Martin, M.; Goda, Y. Astrocyte-Synapse Interactions and Cell Adhesion Molecules. FEBS J. 2022, 290, 3512–3526. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Hussaini, S.M.Q.; Jang, M.H. New Roles for Old Glue: Astrocyte Function in Synaptic Plasticity and Neurological Disorders. Int. Neurourol. J. 2018, 22, S106–S114. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Goldman, S.A.; Nedergaard, M. Heterogeneity of Astrocytic Form and Function. Methods Mol. Biol. 2012, 814, 23–45. [Google Scholar] [CrossRef]
- Endo, F.; Kasai, A.; Soto, J.S.; Yu, X.; Qu, Z.; Hashimoto, H.; Gradinaru, V.; Kawaguchi, R.; Khakh, B.S. Molecular Basis of Astrocyte Diversity and Morphology across the CNS in Health and Disease. Science 2022, 378, eadc9020. [Google Scholar] [CrossRef]
- Qian, Z.; Qin, J.; Lai, Y.; Zhang, C.; Zhang, X. Large-Scale Integration of Single-Cell RNA-Seq Data Reveals Astrocyte Diversity and Transcriptomic Modules across Six Central Nervous System Disorders. Biomolecules 2023, 13, 692. [Google Scholar] [CrossRef] [PubMed]
- Westergard, T.; Rothstein, J.D. Astrocyte Diversity: Current Insights and Future Directions. Neurochem. Res. 2020, 45, 1298–1305. [Google Scholar] [CrossRef]
- Bugiani, M.; Plug, B.C.; Man, J.H.K.; Breur, M.; van der Knaap, M.S. Heterogeneity of White Matter Astrocytes in the Human Brain. Acta Neuropathol. 2022, 143, 159–177. [Google Scholar] [CrossRef]
- Köhler, S.; Winkler, U.; Hirrlinger, J. Heterogeneity of Astrocytes in Grey and White Matter. Neurochem. Res. 2021, 46, 3–14. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yi, M.; Zhou, F.; He, W.; Yang, A.; Qiu, M.; Huang, H. S100B Is Selectively Expressed by Gray Matter Protoplasmic Astrocytes and Myelinating Oligodendrocytes in the Developing CNS. Mol. Brain 2021, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, K.K. Glial Fibrillary Acidic Protein: From Intermediate Filament Assembly and Gliosis to Neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Werkman, I.L.; Dubbelaar, M.L.; van der Vlies, P.; de Boer-Bergsma, J.J.; Eggen, B.J.L.; Baron, W. Transcriptional Heterogeneity between Primary Adult Grey and White Matter Astrocytes Underlie Differences in Modulation of In Vitro Myelination. J. Neuroinflammation 2020, 17, 373. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Huo, J. A1/A2 Astrocytes in Central Nervous System Injuries and Diseases: Angels or Devils? Neurochem. Int. 2021, 148, 105080. [Google Scholar] [CrossRef]
- El Fatimi, H.; Khalki, L. Involvement of Glial Cells in the Pathophysiology and Treatment of Depression. In Physiology and Function of Glial Cells in Health and Disease; IGI Global: Hershey, PA, USA, 2023; pp. 331–361. [Google Scholar]
- Sanacora, G.; Banasr, M. From Pathophysiology to Novel Antidepressant Drugs: Glial Contributions to the Pathology and Treatment of Mood Disorders. Biol. Psychiatry 2013, 73, 1172–1179. [Google Scholar] [CrossRef]
- Banasr, M.; Sanacora, G.; Esterlis, I. Macro- and Microscale Stress–Associated Alterations in Brain Structure: Translational Link with Depression. Biol. Psychiatry 2021, 90, 118–127. [Google Scholar] [CrossRef]
- Li, B.; Zhang, D.; Verkhratsky, A. Astrocytes in Post-Traumatic Stress Disorder. Neurosci. Bull. 2022, 38, 953–965. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, Q.; Xie, L.; Yang, F.; Wang, L.; Tu, J. Astrocyte, a Promising Target for Mood Disorder Interventions. Front. Mol. Neurosci. 2019, 12, 136. [Google Scholar] [CrossRef]
- Zhou, B.; Zuo, Y.X.; Jiang, R.T. Astrocyte Morphology: Diversity, Plasticity, and Role in Neurological Diseases. CNS Neurosci. Ther. 2019, 25, 665–673. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Juster, R.P.; McEwen, B.S.; Lupien, S.J. Allostatic Load Biomarkers of Chronic Stress and Impact on Health and Cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Matos, T.M.; Souza-Talarico, J.N. How Stress Mediators Can Cumulatively Contribute to Alzheimer’s Disease an Allostatic Load Approach. Dement. Neuropsychol. 2019, 13, 11–21. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress 2017, 1, 2470547017692328. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Hidalgo, J.J. Astroglia in the Vulnerability to and Maintenance of Stress-Mediated Neuropathology and Depression. Front. Cell Neurosci. 2022, 16, 869779. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Royal, C.; Johnston, A.D.; Boyce, A.K.J.; Diaz-Castro, B.; Institoris, A.; Peringod, G.; Zhang, O.; Stout, R.F.; Spray, D.C.; Thompson, R.J.; et al. Stress Gates an Astrocytic Energy Reservoir to Impair Synaptic Plasticity. Nat. Commun. 2020, 11, 2014. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid Regulation of Inflammation and Its Functional Correlates: From HPA Axis to Glucocorticoid Receptor Dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The Role of the Hypothalamic-Pituitary-Adrenal Axis in Neuroendocrine Responses to Stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Tertil, M.; Skupio, U.; Barut, J.; Dubovyk, V.; Wawrzczak-Bargiela, A.; Soltys, Z.; Golda, S.; Kudla, L.; Wiktorowska, L.; Szklarczyk, K.; et al. Glucocorticoid Receptor Signaling in Astrocytes Is Required for Aversive Memory Formation. Transl. Psychiatry 2018, 8, 255. [Google Scholar] [CrossRef]
- Lu, C.L.; Ren, J.; Mo, J.W.; Fan, J.; Guo, F.; Chen, L.Y.; Wen, Y.L.; Li, S.J.; Fang, Y.Y.; Wu, Z.F.; et al. Glucocorticoid Receptor-Dependent Astrocytes Mediate Stress Vulnerability. Biol. Psychiatry 2022, 92, 204–215. [Google Scholar] [CrossRef]
- Murphy-Royal, C.; Gordon, G.R.; Bains, J.S. Stress-Induced Structural and Functional Modifications of Astrocytes-Further Implicating Glia in the Central Response to Stress. Glia 2019, 67, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Luarte, A.; Cisternas, P.; Caviedes, A.; Batiz, L.F.; Lafourcade, C.; Wyneken, U.; Henzi, R. Astrocytes at the Hub of the Stress Response: Potential Modulation of Neurogenesis by MiRNAs in Astrocyte-Derived Exosomes. Stem Cells Int. 2017, 2017, 1719050. [Google Scholar] [CrossRef]
- Olejniczak, M.; Kotowska-Zimmer, A.; Krzyzosiak, W. Stress-Induced Changes in MiRNA Biogenesis and Functioning. Cell Mol. Life Sci. 2018, 75, 177–191. [Google Scholar] [CrossRef]
- Ding, R.; Su, D.; Zhao, Q.; Wang, Y.; Wang, J.Y.; Lv, S.; Ji, X. The Role of MicroRNAs in Depression. Front. Pharmacol. 2023, 14, 1129186. [Google Scholar] [CrossRef]
- Luarte, A.; Henzi, R.; Fernández, A.; Gaete, D.; Cisternas, P.; Pizarro, M.; Batiz, L.F.; Villalobos, I.; Masalleras, M.; Vergara, R.; et al. Astrocyte-Derived Small Extracellular Vesicles Regulate Dendritic Complexity through MiR-26a-5p Activity. Cells 2020, 9, 930. [Google Scholar] [CrossRef]
- Du, L.; Jiang, Y.; Sun, Y. Astrocyte-Derived Exosomes Carry MicroRNA-17-5p to Protect Neonatal Rats from Hypoxic-Ischemic Brain Damage via Inhibiting BNIP-2 Expression. Neurotoxicology 2021, 83, 28–39. [Google Scholar] [CrossRef]
- Leggio, L.; L’Episcopo, F.; Magrì, A.; Ulloa-Navas, M.J.; Paternò, G.; Vivarelli, S.; Bastos, C.A.P.; Tirolo, C.; Testa, N.; Caniglia, S.; et al. Small Extracellular Vesicles Secreted by Nigrostriatal Astrocytes Rescue Cell Death and Preserve Mitochondrial Function in Parkinson’s Disease. Adv. Healthc. Mater. 2022, 11, e2201203. [Google Scholar] [CrossRef]
- Ranjit, S.; Patters, B.J.; Gerth, K.A.; Haque, S.; Choudhary, S.; Kumar, S. Potential Neuroprotective Role of Astroglial Exosomes against Smoking-Induced Oxidative Stress and HIV-1 Replication in the Central Nervous System. Expert. Opin. Ther. Targets 2018, 22, 703–714. [Google Scholar] [CrossRef]
- Weiss Roberts, L. Textbook of Psychiatry, 7th ed.; The American Psychiatric Association Publishing: Washington, DC, USA, 2019. [Google Scholar]
- Wu, Z.; Fang, Y. Comorbidity of Depressive and Anxiety Disorders: Challenges in Diagnosis and Assessment. Shanghai Arch. Psychiatry 2014, 26, 227–231. [Google Scholar] [CrossRef]
- Hirschfeld, R.M. The Comorbidity of Major Depression and Anxiety Disorders: Recognition and Management in Primary Care. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Groen, R.N.; Ryan, O.; Wigman, J.T.W.; Riese, H.; Penninx, B.W.J.H.; Giltay, E.J.; Wichers, M.; Hartman, C.A. Comorbidity between Depression and Anxiety: Assessing the Role of Bridge Mental States in Dynamic Psychological Networks. BMC Med. 2020, 18, 308. [Google Scholar] [CrossRef]
- Lamers, F.; van Oppen, P.; Comijs, H.C.; Smit, J.H.; Spinhoven, P.; van Balkom, A.J.; Nolen, W.A.; Zitman, F.G.; Beekman, A.T.; Penninx, B.W. Comorbidity Patterns of Anxiety and Depressive Disorders in a Large Cohort Study: The Netherlands Study of Depression and Anxiety (NESDA). J. Clin. Psychiatry 2011, 72, 341–348. [Google Scholar] [CrossRef]
- Rajkowska, G.; Stockmeier, C.A. Astrocyte Pathology in Major Depressive Disorder: Insights from Human Postmortem Brain Tissue. Curr. Drug Targets 2013, 14, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Cotter, D.R.; Pariante, C.M.; Everall, I.P. Glial Cell Abnormalities in Major Psychiatric Disorders: The Evidence and Implications. Brain Res. Bull. 2001, 55, 585–595. [Google Scholar] [CrossRef]
- Cotter, D.; Mackay, D.; Chana, G.; Beasley, C.; Landau, S.; Everall, I.P. Reduced Neuronal Size and Glial Cell Density in Area 9 of the Dorsolateral Prefrontal Cortex in Subjects with Major Depressive Disorder. Cereb. Cortex 2002, 12, 386–394. [Google Scholar] [CrossRef]
- Ongür, D.; Drevets, W.C.; Price, J.L. Glial Reduction in the Subgenual Prefrontal Cortex in Mood Disorders. Proc. Natl. Acad. Sci. USA 1998, 95, 13290–13295. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J.J.; Wei, J.; Dilley, G.; Pittman, S.D.; Meltzer, H.Y.; Overholser, J.C.; Roth, B.L.; Stockmeier, C.A. Morphometric Evidence for Neuronal and Glial Prefrontal Cell Pathology in Major Depression. Biol. Psychiatry 1999, 45, 1085–1098. [Google Scholar] [CrossRef]
- Gittins, R.A.; Harrison, P.J. A Morphometric Study of Glia and Neurons in the Anterior Cingulate Cortex in Mood Disorder. J. Affect. Disord. 2011, 133, 328–332. [Google Scholar] [CrossRef]
- Cobb, J.A.; O’Neill, K.; Milner, J.; Mahajan, G.J.; Lawrence, T.J.; May, W.L.; Miguel-Hidalgo, J.; Rajkowska, G.; Stockmeier, C.A. Density of GFAP-Immunoreactive Astrocytes Is Decreased in Left Hippocampi in Major Depressive Disorder. Neuroscience 2016, 316, 209–220. [Google Scholar] [CrossRef]
- O’Leary, L.A.; Belliveau, C.; Davoli, M.A.; Ma, J.C.; Tanti, A.; Turecki, G.; Mechawar, N. Widespread Decrease of Cerebral Vimentin-Immunoreactive Astrocytes in Depressed Suicides. Front. Psychiatry 2021, 12, 640963. [Google Scholar] [CrossRef] [PubMed]
- Khundakar, A.; Morris, C.; Oakley, A.; Thomas, A.J. A Morphometric Examination of Neuronal and Glial Cell Pathology in the Orbitofrontal Cortex in Late-Life Depression. Int. Psychogeriatr. 2011, 23, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Khundakar, A.A.; Morris, C.M.; Oakley, A.E.; Thomas, A.J. Cellular Pathology within the Anterior Cingulate Cortex of Patients with Late-Life Depression: A Morphometric Study. Psychiatry Res. Neuroimaging 2011, 194, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.A.; Simpson, J.; Mahajan, G.J.; Overholser, J.C.; Jurjus, G.J.; Dieter, L.; Herbst, N.; May, W.; Rajkowska, G.; Stockmeier, C.A. Hippocampal Volume and Total Cell Numbers in Major Depressive Disorder. J. Psychiatr. Res. 2013, 47, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Hidalgo, J.J.; Waltzer, R.; Whittom, A.A.; Austin, M.C.; Rajkowska, G.; Stockmeier, C.A. Glial and Glutamatergic Markers in Depression, Alcoholism, and Their Comorbidity. J. Affect. Disord. 2010, 127, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Suderman, M.; Yang, J.; Szyf, M.; Mechawar, N.; Ernst, C.; Turecki, G. Astrocytic Abnormalities and Global DNA Methylation Patterns in Depression and Suicide. Mol. Psychiatry 2015, 20, 320–328. [Google Scholar] [CrossRef]
- Si, X.; Miguel-Hidalgo, J.J.; O’Dwyer, G.; Stockmeier, C.A.; Rajkowska, G. Age-Dependent Reductions in the Level of Glial Fibrillary Acidic Protein in the Prefrontal Cortex in Major Depression. Neuropsychopharmacology 2004, 29, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; Legutko, B.; Moulana, M.; Syed, M.; Romero, D.G.; Stockmeier, C.A.; Miguel-Hidalgo, J.J. Astrocyte Pathology in the Ventral Prefrontal White Matter in Depression. J. Psychiatr. Res. 2018, 102, 150–158. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Wei, J.; Andrew, M.; Overholser, J.C.; Jurjus, G.; Stockmeier, C.A.; Rajkowska, G. Glia Pathology in the Prefrontal Cortex in Alcohol Dependence with and without Depressive Symptoms. Biol. Psychiatry 2002, 52, 1121–1133. [Google Scholar] [CrossRef]
- Rajkowska, G.; Hughes, J.; Stockmeier, C.A.; Javier Miguel-Hidalgo, J.; Maciag, D. Coverage of Blood Vessels by Astrocytic Endfeet Is Reduced in Major Depressive Disorder. Biol. Psychiatry 2013, 73, 613–621. [Google Scholar] [CrossRef]
- Michel, M.; Fiebich, B.L.; Kuzior, H.; Meixensberger, S.; Berger, B.; Maier, S.; Nickel, K.; Runge, K.; Denzel, D.; Pankratz, B.; et al. Increased GFAP Concentrations in the Cerebrospinal Fluid of Patients with Unipolar Depression. Transl. Psychiatry 2021, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Klempan, T.A.; Sequeira, A.; Canetti, L.; Lalovic, A.; Ernst, C.; ffrench-Mullen, J.; Turecki, G. Altered Expression of Genes Involved in ATP Biosynthesis and GABAergic Neurotransmission in the Ventral Prefrontal Cortex of Suicides with and without Major Depression. Mol. Psychiatry 2009, 14, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Gos, T.; Schroeter, M.L.; Lessel, W.; Bernstein, H.G.; Dobrowolny, H.; Schiltz, K.; Bogerts, B.; Steiner, J. S100B-Immunopositive Astrocytes and Oligodendrocytes in the Hippocampus Are Differentially Afflicted in Unipolar and Bipolar Depression: A Postmortem Study. J. Psychiatr. Res. 2013, 47, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, M.L.; Abdul-Khaliq, H.; Diefenbacher, A.; Blasig, I.E. S100B Is Increased in Mood Disorders and May Be Reduced by Antidepressive Treatment. Neuroreport 2002, 13, 1675–1678. [Google Scholar] [CrossRef] [PubMed]
- Tural, U.; Irvin, M.K.; Iosifescu, D. V Correlation between S100B and Severity of Depression in MDD: A Meta-Analysis. World J. Biol. Psychiatry 2022, 23, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T.; Bargiel, W.; Grabarczyk, M.; Skibinska, M. Peripheral S100B Protein Levels in Five Major Psychiatric Disorders: A Systematic Review. Brain Sci. 2023, 13, 1334. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xiao, S.F.; Zhang, S.Y.; Qiu, Q.; Wang, T.; Li, X. Increased Plasma S100β Level in Patients with Major Depressive Disorder. CNS Neurosci. Ther. 2016, 22, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Rothermundt, M.; Arolt, V.; Wiesmann, M.; Missler, U.; Peters, M.; Rudolf, S.; Kirchner, H. S-100B Is Increased in Melancholic but Not in Non-Melancholic Major Depression. J. Affect. Disord. 2001, 66, 89–93. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Mergl, R.; Stach, B.; Jahn, I.; Schönknecht, P. Elevated Levels of Cerebrospinal Fluid Neuron-Specific Enolase (NSE), but Not S100B in Major Depressive Disorder. World J. Biol. Psychiatry 2015, 16, 106–113. [Google Scholar] [CrossRef]
- Navinés, R.; Oriolo, G.; Horrillo, I.; Cavero, M.; Aouizerate, B.; Schaefer, M.; Capuron, L.; Meana, J.J.; Martin-Santos, R. High S100B Levels Predict Antidepressant Response in Patients with Major Depression even When Considering Inflammatory and Metabolic Markers. Int. J. Neuropsychopharmacol. 2022, 25, 468–478. [Google Scholar] [CrossRef]
- Levchuk, L.A.; Roschina, O.V.; Mikhalitskaya, E.V.; Epimakhova, E.V.; Simutkin, G.G.; Bokhan, N.A.; Ivanova, S.A. Serum Levels of S100B Protein and Myelin Basic Protein as a Potential Biomarkers of Recurrent Depressive Disorders. J. Pers. Med. 2023, 13, 1423. [Google Scholar] [CrossRef]
- Takebayashi, M.; Hisaoka, K.; Nishida, A.; Tsuchioka, M.; Miyoshi, I.; Kozuru, T.; Hikasa, S.; Okamoto, Y.; Shinno, H.; Morinobu, S.; et al. Decreased Levels of Whole Blood Glial Cell Line-Derived Neurotrophic Factor (GDNF) in Remitted Patients with Mood Disorders. Int. J. Neuropsychopharmacol. 2006, 9, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Duarte Azevedo, M.; Sander, S.; Tenenbaum, L. GDNF, A Neuron-Derived Factor Upregulated in Glial Cells during Disease. J. Clin. Med. 2020, 9, 456. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, G.Y.; Ding, S. Glial Cell Line-Derived Neurotrophic Factor and Focal Ischemic Stroke. Neurochem. Res. 2021, 46, 2638–2650. [Google Scholar] [CrossRef]
- Ernst, C.; Nagy, C.; Kim, S.; Yang, J.P.; Deng, X.; Hellstrom, I.C.; Choi, K.H.; Gershenfeld, H.; Meaney, M.J.; Turecki, G. Dysfunction of Astrocyte Connexins 30 and 43 in Dorsal Lateral Prefrontal Cortex of Suicide Completers. Biol. Psychiatry 2011, 70, 312–319. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Wilson, B.A.; Hussain, S.; Meshram, A.; Rajkowska, G.; Stockmeier, C.A. Reduced Connexin 43 Immunolabeling in the Orbitofrontal Cortex in Alcohol Dependence and Depression. J. Psychiatr. Res. 2014, 55, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Theis, M.; Giaume, C. Connexin-Based Intercellular Communication and Astrocyte Heterogeneity. Brain Res. 2012, 1487, 88–98. [Google Scholar] [CrossRef]
- Semyanov, A.; Henneberger, C.; Agarwal, A. Making Sense of Astrocytic Calcium Signals—from Acquisition to Interpretation. Nat. Rev. Neurosci. 2020, 21, 551–564. [Google Scholar] [CrossRef]
- Halassa, M.M.; Haydon, P.G. Integrated Brain Circuits: Astrocytic Networks Modulate Neuronal Activity and Behavior. Annu. Rev. Physiol. 2010, 72, 335–355. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Braga, A.; Yu, Y.; Esteras, N.; Korsak, A.; Theparambil, S.M.; Hadjihambi, A.; Hosford, P.S.; Teschemacher, A.G.; Marina, N.; et al. Mechanosensory Signaling in Astrocytes. J. Neurosci. 2020, 40, 9364–9371. [Google Scholar] [CrossRef]
- Newman, E.A. New Roles for Astrocytes: Regulation of Synaptic Transmission. Trends Neurosci. 2003, 26, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Chen, S.; Liu, Y.; Han, P.; Ma, T.; Zeng, L.H. Chemogenetic Manipulation of Astrocytic Activity: Is It Possible to Reveal the Roles of Astrocytes? Biochem. Pharmacol. 2021, 186, 114457. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef]
- Hayashi, M.K. Structure-Function Relationship of Transporters in the Glutamate-Glutamine Cycle of the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 1177. [Google Scholar] [CrossRef]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Lee, E. The Role of Astrocytic Glutamate Transporters GLT-1 and GLAST in Neurological Disorders: Potential Targets for Neurotherapeutics. Neuropharmacology 2019, 161, 107559. [Google Scholar] [CrossRef] [PubMed]
- Choudary, P.V.; Molnar, M.; Evans, S.J.; Tomita, H.; Li, J.Z.; Vawter, M.P.; Myers, R.M.; Bunney, W.E.; Akil, H.; Watson, S.J.; et al. Altered Cortical Glutamatergic and GABAergic Signal Transmission with Glial Involvement in Depression. Proc. Natl. Acad. Sci. USA 2005, 102, 15653–15658. [Google Scholar] [CrossRef]
- Hashimoto, K.; Sawa, A.; Iyo, M. Increased Levels of Glutamate in Brains from Patients with Mood Disorders. Biol. Psychiatry 2007, 62, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The Stressed Synapse: The Impact of Stress and Glucocorticoids on Glutamate Transmission. Nat. Rev. Neurosci. 2011, 13, 22–37. [Google Scholar] [CrossRef]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a Glutamate Hypothesis of Depression: An Emerging Frontier of Neuropsychopharmacology for Mood Disorders. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef]
- Feyissa, A.M.; Chandran, A.; Stockmeier, C.A.; Karolewicz, B. Reduced Levels of NR2A and NR2B Subunits of NMDA Receptor and PSD-95 in the Prefrontal Cortex in Major Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 70–75. [Google Scholar] [CrossRef]
- Esterlis, I.; DellaGioia, N.; Pietrzak, R.H.; Matuskey, D.; Nabulsi, N.; Abdallah, C.G.; Yang, J.; Pittenger, C.; Sanacora, G.; Krystal, J.H.; et al. Ketamine-Induced Reduction in MGluR5 Availability Is Associated with an Antidepressant Response: An [11C] ABP688 and PET imaging study in depression. Mol. Psychiatry 2018, 23, 824–832. [Google Scholar] [CrossRef]
- Andersen, J.V.; Schousboe, A.; Wellendorph, P. Astrocytes Regulate Inhibitory Neurotransmission through GABA Uptake, Metabolism, and Recycling. Essays Biochem. 2023, 67, 77–91. [Google Scholar] [CrossRef]
- Hertz, L. The Glutamate-Glutamine (GABA) Cycle: Importance of Late Postnatal Development and Potential Reciprocal Interactions between Biosynthesis and Degradation. Front. Endocrinol. 2013, 4, 59. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Jackowski, A.; Sato, J.R.; Mao, X.; Kang, G.; Cheema, R.; Coplan, J.D.; Mathew, S.J.; Shungu, D.C. Prefrontal Cortical GABA Abnormalities Are Associated with Reduced Hippocampal Volume in Major Depressive Disorder. Eur. Neuropsychopharmacol. 2015, 25, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Fee, C.; Banasr, M.; Sibille, E. Somatostatin-Positive Gamma-Aminobutyric Acid Interneuron Deficits in Depression: Cortical Microcircuit and Therapeutic Perspectives. Biol. Psychiatry 2017, 82, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Prévot, T.; Sibille, E. Altered GABA-Mediated Information Processing and Cognitive Dysfunctions in Depression and Other Brain Disorders. Mol. Psychiatry 2021, 26, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Petty, F.; Schlesser, M.A. Plasma GABA in Affective Illness. J. Affect. Disord. 2002, 3, 339–343. [Google Scholar] [CrossRef]
- Gold, B.I.; Bowers, M.B.; Roth, R.H.; Sweeney, D.W. GABA Levels in CSF of Patients with Psychiatric Disorders. Am. J. Psychiatry 1980, 137, 362–364. [Google Scholar] [CrossRef]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef]
- Gerner, R.H.; Hare, T.A. CSF GABA in Normal Subjects and Patients with Depression, Schizophrenia, Mania, and Anorexia Nervosa. Am. J. Psychiatry 1981, 138, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bao, A.M.; Qi, X.R.; Kamphuis, W.; Luchetti, S.; Lou, J.S.; Swaab, D.F. Gene Expression of GABA and Glutamate Pathway Markers in the Prefrontal Cortex of Non-Suicidal Elderly Depressed Patients. J. Affect. Disord. 2012, 138, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Barres, B.A. Neuroscience: Glia—More than Just Brain Glue. Nature 2009, 457, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Somjen, G.G. Nervenkitt: Notes on the History of the Concept of Neuroglia. Glia 1988, 1, 2–9. [Google Scholar] [CrossRef]
- Takahashi, S. Neuroprotective Function of High Glycolytic Activity in Astrocytes: Common Roles in Stroke and Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 6568. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Pellerin, L. Cellular Mechanisms of Brain Energy Metabolism and Their Relevance to Functional Brain Imaging. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Martinet, J.L.; Hardy, P.; Feline, A.; Huret, J.D.; Mazoyer, B.; Attar-Levy, D.; Pappata, S.; Syrota, A. Left Prefrontal Glucose Hypometabolism in the Depressed State: A Confirmation. Am. J. Psychiatry 1990, 147, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Nofzinger, E.A.; Nichols, T.E.; Meltzer, C.C.; Price, J.; Steppe, D.A.; Miewald, J.M.; Kupfer, D.J.; Moore, R.Y. Changes in Forebrain Function from Waking to REM Sleep in Depression: Preliminary Analyses [of 18F]FDG PET Studies. Psychiatry Res. Neuroimaging 1999, 91, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Hundal, Ø. Major Depressive Disorder Viewed as a Dysfunction in Astroglial Bioenergetics. Med. Hypotheses 2007, 68, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.; Watling, S.E.; Richardson, J.D.; McCluskey, T.; Tong, J.; Meyer, J.H.; Warsh, J.; Jetly, R.; Hutchison, M.G.; Rhind, S.G.; et al. Imaging of Astrocytes in Posttraumatic Stress Disorder: A PET Study with the Monoamine Oxidase B Radioligand [11C] SL25. 1188. Eur. Neuropsychopharmacol. 2022, 54, 54–61. [Google Scholar] [CrossRef]
- Wingo, T.S.; Gerasimov, E.S.; Liu, Y.; Duong, D.M.; Vattathil, S.M.; Lori, A.; Gockley, J.; Breen, M.S.; Maihofer, A.X.; Nievergelt, C.M.; et al. Integrating Human Brain Proteomes with Genome-Wide Association Data Implicates Novel Proteins in Post-Traumatic Stress Disorder. Mol. Psychiatry 2022, 27, 3075–3084. [Google Scholar] [CrossRef]
- Chatzinakos, C.; Pernia, C.D.; Morrison, F.G.; Iatrou, A.; McCullough, K.M.; Schuler, H.; Snijders, C.; Bajaj, T.; DiPietro, C.P.; Soliva Estruch, M.; et al. Single-Nucleus Transcriptome Profiling of Dorsolateral Prefrontal Cortex: Mechanistic Roles for Neuronal Gene Expression, Including the 17q21.31 Locus, in PTSD Stress Response. Am. J. Psychiatry 2023, 180, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Atrooz, F.; Alkadhi, K.A.; Salim, S. Understanding Stress: Insights from Rodent Models. Curr. Res. Neurobiol. 2021, 2, 100013. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Aguiar, D.C.; Guimarães, F.S. Animal Models of Anxiety Disorders and Stress. Braz. J. Psychiatry 2013, 35 (Suppl. S2), S101–S111. [Google Scholar] [CrossRef]
- Planchez, B.; Surget, A.; Belzung, C. Animal Models of Major Depression: Drawbacks and Challenges. J. Neural. Transm. 2019, 126, 1383–1408. [Google Scholar] [CrossRef]
- Banasr, M.; Duman, R.S. Glial Loss in the Prefrontal Cortex Is Sufficient to Induce Depressive-like Behaviors. Biol. Psychiatry 2008, 64, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.D.; Gibney, S.; O’Malley, D.; Dinan, T.G.; Cryan, J.F. Region Specific Decrease in Glial Fibrillary Acidic Protein Immunoreactivity in the Brain of a Rat Model of Depression. Neuroscience 2009, 159, 915–925. [Google Scholar] [CrossRef]
- Shilpa, B.M.; Bhagya, V.; Harish, G.; Srinivas Bharath, M.M.; Shankaranarayana Rao, B.S. Environmental Enrichment Ameliorates Chronic Immobilisation Stress-Induced Spatial Learning Deficits and Restores the Expression of BDNF, VEGF, GFAP and Glucocorticoid Receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 76, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Sántha, P.; Veszelka, S.; Hoyk, Z.; Mészáros, M.; Walter, F.R.; Tóth, A.E.; Kiss, L.; Kincses, A.; Oláh, Z.; Seprényi, G.; et al. Restraint Stress-Induced Morphological Changes at the Blood-Brain Barrier in Adult Rats. Front. Mol. Neurosci. 2016, 8, 88. [Google Scholar] [CrossRef]
- Tynan, R.J.; Beynon, S.B.; Hinwood, M.; Johnson, S.J.; Nilsson, M.; Woods, J.J.; Walker, F.R. Chronic Stress-Induced Disruption of the Astrocyte Network Is Driven by Structural Atrophy and Not Loss of Astrocytes. Acta Neuropathol. 2013, 126, 75–91. [Google Scholar] [CrossRef]
- Codeluppi, S.A.; Chatterjee, D.; Prevot, T.D.; Bansal, Y.; Misquitta, K.A.; Sibille, E.; Banasr, M. Chronic Stress Alters Astrocyte Morphology in Mouse Prefrontal Cortex. Int. J. Neuropsychopharmacol. 2021, 24, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Czéh, B.; Simon, M.; Schmelting, B.; Hiemke, C.; Fuchs, E. Astroglial Plasticity in the Hippocampus Is Affected by Chronic Psychosocial Stress and Concomitant Fluoxetine Treatment. Neuropsychopharmacology 2006, 31, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.X.; Li, J.; Wang, Z.Z.; Xia, C.Y.; Chen, N.H. Glucocorticoid Receptor Activation Induces Decrease of Hippocampal Astrocyte Number in Rats. Psychopharmacology 2018, 235, 2529–2540. [Google Scholar] [CrossRef] [PubMed]
- Araya-Callís, C.; Hiemke, C.; Abumaria, N.; Flugge, G. Chronic Psychosocial Stress and Citalopram Modulate the Expression of the Glial Proteins GFAP and NDRG2 in the Hippocampus. Psychopharmacology 2012, 224, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; Wang, J.; Xie, Z.M.; Xu, N.; Zhang, G.F.; Jia, M.; Zhou, Z.Q.; Hashimoto, K.; Yang, J.J. Regulation of Glutamate Transporter 1 via BDNF-TrkB Signaling Plays a Role in the Anti-Apoptotic and Antidepressant Effects of Ketamine in Chronic Unpredictable Stress Model of Depression. Psychopharmacology 2016, 233, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.R.; Zhang, Z.G.; Bai, Y.Y.; Zhou, H.F.; Zhou, L.; Ruan, C.S.; Li, F.; Li, C.Q.; Zheng, H.Y.; Shen, L.J.; et al. Foraging Activity Is Reduced in a Mouse Model of Depression. Neurotox. Res. 2014, 25, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, G.; Wang, H.; Wang, X. Brain-Derived Neurotrophic Factor (BDNF) Infusion Restored Astrocytic Plasticity in the Hippocampus of a Rat Model of Depression. Neurosci. Lett. 2011, 503, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Naskar, S.; Chattarji, S. Stress Elicits Contrasting Effects on the Structure and Number of Astrocytes in the Amygdala versus Hippocampus. eNeuro 2019, 6, ENEURO.0338-18.2019. [Google Scholar] [CrossRef] [PubMed]
- Naskar, S.; Datta, S.; Chattarji, S. Riluzole Prevents Stress-Induced Spine Plasticity in the Hippocampus but Mimics It in the Amygdala. Neurobiol. Stress. 2022, 18, 100442. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Seo, Y.J.; Lee, J.K.; Lee, H.K.; Jung, J.S.; Jang, J.E.; Park, S.H.; Suh, H.W. The Repeated Immobilization Stress Increases IL-1beta Immunoreactivities in Only Neuron, but Not Astrocyte or Microglia in Hippocampal CA1 Region, Striatum and Paraventricular Nucleus. Neurosci. Lett. 2008, 430, 258–263. [Google Scholar] [CrossRef]
- Dong, L.; Wang, S.; Li, Y.; Zhao, Z.; Shen, Y.; Liu, L.; Xu, G.; Ma, C.; Li, S.; Zhang, X.; et al. RU486 Reverses Emotional Disorders by Influencing Astrocytes and Endoplasmic Reticulum Stress in Chronic Restraint Stress Challenged Rats. Cell Physiol. Biochem. 2017, 42, 1098–1108. [Google Scholar] [CrossRef]
- Du Preez, A.; Onorato, D.; Eiben, I.; Musaelyan, K.; Egeland, M.; Zunszain, P.A.; Fernandes, C.; Thuret, S.; Pariante, C.M. Chronic Stress Followed by Social Isolation Promotes Depressive-like Behaviour, Alters Microglial and Astrocyte Biology and Reduces Hippocampal Neurogenesis in Male Mice. Brain Behav. Immun. 2021, 91, 24–47. [Google Scholar] [CrossRef]
- Simard, S.; Coppola, G.; Rudyk, C.A.; Hayley, S.; McQuaid, R.J.; Salmaso, N. Profiling Changes in Cortical Astroglial Cells Following Chronic Stress. Neuropsychopharmacology 2018, 43, 1961–1971. [Google Scholar] [CrossRef]
- Imbe, H.; Kimura, A.; Donishi, T.; Kaneoke, Y. Chronic Restraint Stress Decreases Glial Fibrillary Acidic Protein and Glutamate Transporter in the Periaqueductal Gray Matter. Neuroscience 2012, 223, 209–218. [Google Scholar] [CrossRef]
- Baek, J.H.; Vignesh, A.; Son, H.; Lee, D.H.; Roh, G.S.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Kim, H.J. Glutamine Supplementation Ameliorates Chronic Stress-Induced Reductions in Glutamate and Glutamine Transporters in the Mouse Prefrontal Cortex. Exp. Neurobiol. 2019, 28, 270–278. [Google Scholar] [CrossRef]
- Veeraiah, P.; Noronha, J.M.; Maitra, S.; Bagga, P.; Khandelwal, N.; Chakravarty, S.; Kumar, A.; Patel, A.B. Dysfunctional Glutamatergic and γ-Aminobutyric Acidergic Activities in Prefrontal Cortex of Mice in Social Defeat Model of Depression. Biol. Psychiatry 2014, 76, 231–238. [Google Scholar] [CrossRef]
- Chen, J.X.; Yao, L.H.; Xu, B.B.; Qian, K.; Wang, H.L.; Liu, Z.C.; Wang, X.P.; Wang, G.H. Glutamate Transporter 1-Mediated Antidepressant-like Effect in a Rat Model of Chronic Unpredictable Stress. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2014, 34, 838–844. [Google Scholar] [CrossRef]
- Zink, M.; Vollmayr, B.; Gebicke-Haerter, P.J.; Henn, F.A. Reduced Expression of Glutamate Transporters VGluT1, EAAT2 and EAAT4 in Learned Helpless Rats, an Animal Model of Depression. Neuropharmacology 2010, 58, 465–473. [Google Scholar] [CrossRef]
- Reagan, L.P.; Rosell, D.R.; Wood, G.E.; Spedding, M.; Muñoz, C.; Rothstein, J.; McEwen, B.S. Chronic Restraint Stress Up-Regulates GLT-1 MRNA and Protein Expression in the Rat Hippocampus: Reversal by Tianeptine. Proc. Natl. Acad. Sci. USA 2004, 101, 2179–2184. [Google Scholar] [CrossRef]
- Raudensky, J.; Yamamoto, B.K. Effects of Chronic Unpredictable Stress and Methamphetamine on Hippocampal Glutamate Function. Brain Res. 2007, 1135, 129–135. [Google Scholar] [CrossRef]
- Liu, F.; Wu, J.; Gong, Y.; Wang, P.; Zhu, L.; Tong, L.; Chen, X.; Ling, Y.; Huang, C. Harmine Produces Antidepressant-like Effects via Restoration of Astrocytic Functions. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 258–267. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Moulana, M.; Deloach, P.H.; Rajkowska, G. Chronic Unpredictable Stress Reduces Immunostaining for Connexins 43 and 30 and Myelin Basic Protein in the Rat Prelimbic and Orbitofrontal Cortices. Chronic Stress 2018, 2, 2470547018814186. [Google Scholar] [CrossRef]
- Sun, J.D.; Liu, Y.; Yuan, Y.H.; Li, J.; Chen, N.H. Gap Junction Dysfunction in the Prefrontal Cortex Induces Depressive-like Behaviors in Rats. Neuropsychopharmacology 2012, 37, 1305–1320. [Google Scholar] [CrossRef]
- Cao, X.; Li, L.P.; Wang, Q.; Wu, Q.; Hu, H.H.; Zhang, M.; Fang, Y.Y.; Zhang, J.; Li, S.J.; Xiong, W.C.; et al. Astrocyte-Derived ATP Modulates Depressive-like Behaviors. Nat. Med. 2013, 19, 773–777. [Google Scholar] [CrossRef]
- Srivastava, I.; Vazquez-Juarez, E.; Henning, L.; Gómez-Galán, M.; Lindskog, M. Blocking Astrocytic GABA Restores Synaptic Plasticity in Prefrontal Cortex of Rat Model of Depression. Cells 2020, 9, 1705. [Google Scholar] [CrossRef]
- Flandreau, E.I.; Toth, M. Animal Models of PTSD: A Critical Review. Curr. Top. Behav. Neurosci. 2018, 38, 47–68. [Google Scholar] [CrossRef]
- Perez-Urrutia, N.; Mendoza, C.; Alvarez-Ricartes, N.; Oliveros-Matus, P.; Echeverria, F.; Grizzell, J.A.; Barreto, G.E.; Iarkov, A.; Echeverria, V. Intranasal Cotinine Improves Memory, and Reduces Depressive-like Behavior, and GFAP+ Cells Loss Induced by Restraint Stress in Mice. Exp. Neurol. 2017, 295, 211–221. [Google Scholar] [CrossRef]
- Feng, D.; Guo, B.; Liu, G.; Wang, B.; Wang, W.; Gao, G.; Qin, H.; Wu, S. FGF2 Alleviates PTSD Symptoms in Rats by Restoring GLAST Function in Astrocytes via the JAK/STAT Pathway. Eur. Neuropsychopharmacol. 2015, 25, 1287–1299. [Google Scholar] [CrossRef]
- Saur, L.; Baptista, P.P.A.; Bagatini, P.B.; Neves, L.T.; de Oliveira, R.M.; Vaz, S.P.; Ferreira, K.; Machado, S.A.; Mestriner, R.G.; Xavier, L.L. Experimental Post-Traumatic Stress Disorder Decreases Astrocyte Density and Changes Astrocytic Polarity in the CA1 Hippocampus of Male Rats. Neurochem. Res. 2016, 41, 892–904. [Google Scholar] [CrossRef]
- Han, F.; Xiao, B.; Wen, L. Loss of Glial Cells of the Hippocampus in a Rat Model of Post-Traumatic Stress Disorder. Neurochem. Res. 2015, 40, 942–951. [Google Scholar] [CrossRef]
- Li, H.; Tofigh, A.M.; Amirfakhraei, A.; Chen, X.; Tajik, M.; Xu, D.; Motevalli, S. Modulation of Astrocyte Activity and Improvement of Oxidative Stress through Blockage of NO/NMDAR Pathway Improve Posttraumatic Stress Disorder (PTSD)-like Behavior Induced by Social Isolation Stress. Brain Behav. 2022, 12, e2620. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhai, M.; Wang, L.; Miao, D.; Zhu, X.; Wang, W. FGF2 Blocks PTSD Symptoms via an Astrocyte-Based Mechanism. Behav. Brain Res. 2013, 256, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Shirayama, Y.; Ishida, H.; Hazama, G.I.; Nakagome, K. Hippocampal Astrocytes Are Necessary for Antidepressant Treatment of Learned Helplessness Rats. Hippocampus 2011, 21, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Brockett, A.T.; Kane, G.A.; Monari, P.K.; Briones, B.A.; Vigneron, P.A.; Barber, G.A.; Bermudez, A.; Dieffenbach, U.; Kloth, A.D.; Buschman, T.J.; et al. Evidence Supporting a Role for Astrocytes in the Regulation of Cognitive Flexibility and Neuronal Oscillations through the Ca2+ Binding Protein S100β. PLoS ONE 2018, 13, e0195726. [Google Scholar] [CrossRef] [PubMed]
- Etiévant, A.; Oosterhof, C.; Bétry, C.; Abrial, E.; Novo-Perez, M.; Rovera, R.; Scarna, H.; Devader, C.; Mazella, J.; Wegener, G.; et al. Astroglial Control of the Antidepressant-Like Effects of Prefrontal Cortex Deep Brain Stimulation. EBioMedicine 2015, 2, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Domin, H.; Szewczyk, B.; Woźniak, M.; Wawrzak-Wleciał, A.; Śmiałowska, M. Antidepressant-like Effect of the MGluR5 Antagonist MTEP in an Astroglial Degeneration Model of Depression. Behav. Brain Res. 2014, 273, 23–33. [Google Scholar] [CrossRef] [PubMed]
- David, J.; Gormley, S.; McIntosh, A.L.; Kebede, V.; Thuery, G.; Varidaki, A.; Coffey, E.T.; Harkin, A. L-Alpha-Amino Adipic Acid Provokes Depression-like Behaviour and a Stress Related Increase in Dendritic Spine Density in the Pre-Limbic Cortex and Hippocampus in Rodents. Behav. Brain Res. 2019, 362, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, H.-Y.; Choi, Y.-J.; Cho, S.-H. Antidepressant Effects of Ginsenoside Rf on Behavioral Change in the Glial Degeneration Model of Depression by Reversing Glial Loss. J. Ginseng Res. 2020, 44, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, Y.; Lee, H.Y.; Cho, S.H. Tetragonia Tetragonioides Relieves Depressive-Like Behavior through the Restoration of Glial Loss in the Prefrontal Cortex. Evid. Based Complement. Alternat Med. 2021, 2021, 8888841. [Google Scholar] [CrossRef]
- Codeluppi, S.A.; Xu, M.; Bansal, Y.; Lepack, A.E.; Duric, V.; Chow, M.; Muir, J.; Bagot, R.C.; Licznerski, P.; Wilber, S.L.; et al. Prefrontal Cortex Astroglia Modulate Anhedonia-like Behavior. Mol. Psychiatry 2023, 28, 4632–4641. [Google Scholar] [CrossRef]
- Bechtholt-Gompf, A.J.; Walther, H.V.; Adams, M.A.; Carlezon, W.A.; Ngür, D.; Cohen, B.M. Blockade of Astrocytic Glutamate Uptake in Rats Induces Signs of Anhedonia and Impaired Spatial Memory. Neuropsychopharmacology 2010, 35, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.W.; Yu, X.D.; Cen, L.; Xiao, Z.Y. Glutamate Transporter GLT1 Inhibitor Dihydrokainic Acid Impairs Novel Object Recognition Memory Performance in Mice. Physiol. Behav. 2019, 199, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, A.; Soda, A.; Kayashima, S.; Yoshizawa, K.; Oka, J.I.; Nagase, H.; Yamada, M. A Delta Opioid Receptor Agonist, KNT-127, in the Prelimbic Medial Prefrontal Cortex Attenuates Glial Glutamate Transporter Blocker-Induced Anxiety-like Behavior in Mice. J. Pharmacol. Sci. 2018, 138, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Fullana, M.N.; Ruiz-Bronchal, E.; Ferrés-Coy, A.; Juárez-Escoto, E.; Artigas, F.; Bortolozzi, A. Regionally Selective Knockdown of Astroglial Glutamate Transporters in Infralimbic Cortex Induces a Depressive Phenotype in Mice. Glia 2019, 67, 1122–1137. [Google Scholar] [CrossRef]
- Gasull-Camós, J.; Arrés-Gatius, M.T.; Artigas, F.; Castañé, A. Glial GLT-1 Blockade in Infralimbic Cortex as a New Strategy to Evoke Rapid Antidepressant-like Effects in Rats. Transl. Psychiatry 2017, 7, e1038. [Google Scholar] [CrossRef]
- Gasull-Camós, J.; Martínez-Torres, S.; Tarrés-Gatius, M.; Ozaita, A.; Artigas, F.; Castañé, A. Serotonergic Mechanisms Involved in Antidepressant-like Responses Evoked by GLT-1 Blockade in Rat Infralimbic Cortex. Neuropharmacology 2018, 139, 41–51. [Google Scholar] [CrossRef]
- Liu, X.; Guo, H.; Sayed, M.D.S.; Lu, Y.; Yang, T.; Zhou, D.; Chen, Z.; Wang, H.; Wang, C.; Xu, J. CAMP/PKA/CREB/GLT1 Signaling Involved in the Antidepressant-like Effects of Phosphodiesterase 4D Inhibitor (GEBR-7b) in Rats. Neuropsychiatr. Dis. Treat. 2016, 12, 219–227. [Google Scholar] [CrossRef]
- Gasull-Camós, J.; Soto-Montenegro, M.L.; Casquero-Veiga, M.; Desco, M.; Artigas, F.; Castañé, A. Differential Patterns of Subcortical Activity Evoked by Glial GLT-1 Blockade in Prelimbic and Infralimbic Cortex: Relationship to Antidepressant-Like Effects in Rats. Int. J. Neuropsychopharmacol. 2017, 20, 988–993. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, Y.; Ni, Z.; Dong, Y.; Cai, G.; Foncelle, A.; Ma, S.; Sang, K.; Tang, S.; Li, Y.; et al. Astroglial Kir4.1 in the Lateral Habenula Drives Neuronal Bursts in Depression. Nature 2018, 554, 323–327. [Google Scholar] [CrossRef]
- Wu, X.; Li, L.; Zhou, B.; Wang, J.; Shao, W. Connexin 43 Regulates Astrocyte Dysfunction and Cognitive Deficits in Early Life Stress-Treated Mice. Exp. Brain Res. 2023, 241, 1207–1214. [Google Scholar] [CrossRef]
- Papouin, T.; Dunphy, J.; Tolman, M.; Foley, J.C.; Haydon, P.G. Astrocytic Control of Synaptic Function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160154. [Google Scholar] [CrossRef] [PubMed]
- Araque, A. Astrocytes Process Synaptic Information. Neuron Glia Biol. 2008, 4, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.H.; Noh, K.; Lee, B.H.; Barcelon, E.; Jun, S.B.; Park, H.Y.; Lee, S.J. Hippocampal Astrocytes Modulate Anxiety-like Behavior. Nat. Commun. 2022, 13, 6536. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Paniccia, J.E.; Lebonville, C.L.; Reissner, K.J.; Lysle, D.T. Chemogenetic Manipulation of Dorsal Hippocampal Astrocytes Protects Against the Development of Stress-Enhanced Fear Learning. Neuroscience 2018, 388, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.M.; Korshunov, K.S.; Grant, R.A.; Martin, M.E.; Valencia, H.A.; Budinger, G.R.S.; Radulovic, J.; Prakriya, M. Astrocyte Reactivity and Inflammation-Induced Depression-like Behaviors Are Regulated by Orai1 Calcium Channels. Nat. Commun. 2023, 14, 5500. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Huang, B.S.; Venugopal, S.; Johnston, A.D.; Chai, H.; Zeng, H.; Golshani, P.; Khakh, B.S. Ca(2+) Signaling in Astrocytes from Ip3r2(−/−) Mice in Brain Slices and during Startle Responses In Vivo. Nat. Neurosci. 2015, 18, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Petravicz, J.; Boyt, K.M.; McCarthy, K.D. Astrocyte IP3R2-Dependent Ca(2+) Signaling Is Not a Major Modulator of Neuronal Pathways Governing Behavior. Front. Behav. Neurosci. 2014, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Delcourte, S.; Bouloufa, A.; Rovera, R.; Bétry, C.; Abrial, E.; Dkhissi-Benyahya, O.; Heinrich, C.; Marcy, G.; Raineteau, O.; Haddjeri, N.; et al. Chemogenetic Activation of Prefrontal Astroglia Enhances Recognition Memory Performance in Rat. Biomed. Pharmacother. 2023, 166, 115384. [Google Scholar] [CrossRef] [PubMed]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef] [PubMed]
- Adamsky, A.; Goshen, I. Astrocytes in Memory Function: Pioneering Findings and Future Directions. Neuroscience 2018, 370, 14–26. [Google Scholar] [CrossRef]
- Kol, A.; Adamsky, A.; Groysman, M.; Kreisel, T.; London, M.; Goshen, I. Astrocytes Contribute to Remote Memory Formation by Modulating Hippocampal-Cortical Communication during Learning. Nat. Neurosci. 2020, 23, 1229. [Google Scholar] [CrossRef] [PubMed]
- Mederos, S.; Hernández-Vivanco, A.; Ramírez-Franco, J.; Martín-Fernández, M.; Navarrete, M.; Yang, A.; Boyden, E.S.; Perea, G. Melanopsin for Precise Optogenetic Activation of Astrocyte-Neuron Networks. Glia 2019, 67, 915–934. [Google Scholar] [CrossRef] [PubMed]
- Suthard, R.L.; Senne, R.A.; Buzharsky, M.D.; Diep, A.H.; Pyo, A.Y.; Ramirez, S. Engram Reactivation Mimics Cellular Signatures of Fear. Cell Rep. 2024, 43, 113850. [Google Scholar] [CrossRef] [PubMed]
- Suthard, R.L.; Senne, R.A.; Buzharsky, M.D.; Pyo, A.Y.; Dorst, K.E.; Diep, A.H.; Cole, R.H.; Ramirez, S. Basolateral Amygdala Astrocytes Are Engaged by the Acquisition and Expression of a Contextual Fear Memory. J. Neurosci. 2023, 43, 4997–5013. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Xie, L.; Li, C.H.; Lam, Y.Y.; Ramkrishnan, A.S.; Fu, Z.; Zeng, X.; Liu, S.; Iqbal, Z.; Li, Y. Chemogenetic Activation of Astrocytes in the Basolateral Amygdala Contributes to Fear Memory Formation by Modulating the Amygdala-Prefrontal Cortex Communication. Int. J. Mol. Sci. 2022, 23, 6092. [Google Scholar] [CrossRef] [PubMed]
- González-Arias, C.; Sánchez-Ruiz, A.; Esparza, J.; Sánchez-Puelles, C.; Arancibia, L.; Ramírez-Franco, J.; Gobbo, D.; Kirchhoff, F.; Perea, G. Dysfunctional Serotonergic Neuron-Astrocyte Signaling in Depressive-like States. Mol. Psychiatry 2023, 28, 3856–3873. [Google Scholar] [CrossRef] [PubMed]
- Erickson, E.K.; DaCosta, A.J.; Mason, S.C.; Blednov, Y.A.; Mayfield, R.D.; Harris, R.A. Cortical Astrocytes Regulate Ethanol Consumption and Intoxication in Mice. Neuropsychopharmacology 2021, 46, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Boden, J.M.; Fergusson, D.M. Alcohol and Depression. Addiction 2011, 106, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.F.; Goldstein, R.B.; Saha, T.D.; Patricia Chou, S.; Jung, J.; Zhang, H.; Pickering, R.P.; June Ruan, W.; Smith, S.M.; Huang, B.; et al. Epidemiology of DSM-5 Alcohol Use Disorder Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015, 72, 757–766. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.; Shoyama, Y.; Wanzo, V. Infusion of Gliotoxins or a Gap Junction Blocker in the Prelimbic Cortex Increases Alcohol Preference in Wistar Rats. J. Psychopharmacol. 2009, 23, 550–557. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Lin, L.; Xu, C.; Xiong, Y.; Qiu, H.; Li, X.; Li, S.; Cao, H. Unveiling the Hidden Pathways: Exploring Astrocytes as a Key Target for Depression Therapy. J. Psychiatr. Res. 2024, 174, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Verkhratsky, A.; Gu, L.; Li, B. Targeting Astrocytes in Major Depression. Expert. Rev. Neurother. 2015, 15, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Manev, H.; Uz, T.; Manev, R. Glia as a Putative Target for Antidepressant Treatments. J. Affect. Disord. 2003, 75, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Quesseveur, G.; Gardier, A.; Guiard, B. The Monoaminergic Tripartite Synapse: A Putative Target for Currently Available Antidepressant Drugs. Curr. Drug Targets 2013, 14, 1277–1294. [Google Scholar] [CrossRef] [PubMed]
- Frizzo, M.E.; Ohno, Y. Perisynaptic Astrocytes as a Potential Target for Novel Antidepressant Drugs. J. Pharmacol. Sci. 2021, 145, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.V.; D’almeida, P.L.; Virmani, G.; Bathini, P.; Alberi, L. Effects of Monoamines and Antidepressants on Astrocyte Physiology: Implications for Monoamine Hypothesis of Depression. J. Exp. Neurosci. 2018, 12, 1179069518789149. [Google Scholar] [CrossRef] [PubMed]
- Banasr, M.; Dwyer, J.M.; Duman, R.S. Cell Atrophy and Loss in Depression: Reversal by Antidepressant Treatment. Curr. Opin. Cell Biol. 2011, 23, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Duman, C.H.; Duman, R.S. Spine Synapse Remodeling in the Pathophysiology and Treatment of Depression. Neurosci. Lett. 2015, 601, 20–29. [Google Scholar] [CrossRef]
- Harmer, C.J.; Duman, R.S.; Cowen, P.J. How Do Antidepressants Work? New Perspectives for Refining Future Treatment Approaches. Lancet Psychiatry 2017, 4, 409–418. [Google Scholar] [CrossRef]
- Allaman, I.; Fiumelli, H.; Magistretti, P.J.; Martin, J.L. Fluoxetine Regulates the Expression of Neurotrophic/Growth Factors and Glucose Metabolism in Astrocytes. Psychopharmacology 2011, 216, 75–84. [Google Scholar] [CrossRef]
- Hisaoka-Nakashima, K.; Kajitani, N.; Kaneko, M.; Shigetou, T.; Kasai, M.; Matsumoto, C.; Yokoe, T.; Azuma, H.; Takebayashi, M.; Morioka, N.; et al. Amitriptyline Induces Brain-Derived Neurotrophic Factor (BDNF) MRNA Expression through ERK-Dependent Modulation of Multiple BDNF MRNA Variants in Primary Cultured Rat Cortical Astrocytes and Microglia. Brain Res. 2016, 1634, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Kittel-Schneider, S.; Kenis, G.; Schek, J.; van den Hove, D.; Prickaerts, J.; Lesch, K.P.; Steinbusch, H.; Reif, A. Expression of Monoamine Transporters, Nitric Oxide Synthase 3, and Neurotrophin Genes in Antidepressant-Stimulated Astrocytes. Front. Psychiatry 2012, 3, 33. [Google Scholar] [CrossRef]
- Jeanson, T.; Pondaven, A.; Ezan, P.; Mouthon, F.; Charvériat, M.; Giaume, C. Antidepressants Impact Connexin 43 Channel Functions in Astrocytes. Front. Cell Neurosci. 2016, 9, 495. [Google Scholar] [CrossRef]
- Kinoshita, M.; Hirayama, Y.; Fujishita, K.; Shibata, K.; Shinozaki, Y.; Shigetomi, E.; Takeda, A.; Le, H.P.N.; Hayashi, H.; Hiasa, M.; et al. Anti-Depressant Fluoxetine Reveals Its Therapeutic Effect Via Astrocytes. EBioMedicine 2018, 32, 72–83. [Google Scholar] [CrossRef]
- Kong, H.; Sha, L.L.; Fan, Y.; Xiao, M.; Ding, J.H.; Wu, J.; Hu, G. Requirement of AQP4 for Antidepressive Efficiency of Fluoxetine: Implication in Adult Hippocampal Neurogenesis. Neuropsychopharmacology 2009, 34, 1263–1276. [Google Scholar] [CrossRef]
- Xia, C.Y.; Zhang, N.N.; Jiang, H.; Lou, Y.X.; Ren, Q.; Zhang, X.L.; Yang, P.F.; Shao, Q.H.; Zhu, H.Y.; Wan, J.F.; et al. Gap Junction Is Essential for the Antidepressant Effects of Fluoxetine. J. Pharm. Pharmacol. 2023, 75, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Fullana, M.N.; Covelo, A.; Bortolozzi, A.; Araque, A.; Artigas, F. In Vivo Knockdown of Astroglial Glutamate Transporters GLT-1 and GLAST Increases Excitatory Neurotransmission in Mouse Infralimbic Cortex: Relevance for Depressive-like Phenotypes. Eur. Neuropsychopharmacol. 2019, 29, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Fullana, M.N.; Paz, V.; Artigas, F.; Bortolozzi, A. Ketamine Triggers Rapid Antidepressant Effects by Modulating Synaptic Plasticity in a New Depressive-like Mouse Model Based on Astrocyte Glutamate Transporter GLT-1 Knockdown in Infralimbic Cortex. Rev. Psiquiatr. Salud. Ment. (Engl. Ed.) 2022, 15, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Stenovec, M. Ketamine Alters Functional Plasticity of Astroglia: An Implication for Antidepressant Effect. Life 2021, 11, 573. [Google Scholar] [CrossRef]
- Stenovec, M.; Li, B.; Verkhratsky, A.; Zorec, R. Astrocytes in Rapid Ketamine Antidepressant Action. Neuropharmacology 2020, 173, 108158. [Google Scholar] [CrossRef]
- Pham, T.H.; Defaix, C.; Nguyen, T.M.L.; Mendez-David, I.; Tritschler, L.; David, D.J.; Gardier, A.M. Cortical and Raphe GABAA, AMPA Receptors and Glial GLT-1 Glutamate Transporter Contribute to the Sustained Antidepressant Activity of Ketamine. Pharmacol. Biochem. Behav. 2020, 192, 172913. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Hu, W.Y.; Chang, H.X.; Bao, J.H.; Kong, X.X.; Ma, H.; Li, Y.F. Astrocytes Underlie a Faster-Onset Antidepressant Effect of Hypidone Hydrochloride (YL-0919). Front. Pharmacol. 2023, 14, 1175938. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, S.; Zhang, Z.; Liu, L.; Shi, W.; Yang, S.; Li, S.; Cai, X.; Zhou, Q. Rapid and Sustained Restoration of Astrocytic Functions by Ketamine in Depression Model Mice. Biochem. Biophys. Res. Commun. 2022, 616, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Mineur, Y.S.; Picciotto, M.R.; Sanacora, G. Antidepressant-like Effects of Ceftriaxone in Male C57BL/6J Mice. Biol. Psychiatry 2007, 61, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Banasr, M.; Chowdhury, G.M.I.; Terwilliger, R.; Newton, S.S.; Duman, R.S.; Behar, K.L.; Sanacora, G. Glial Pathology in an Animal Model of Depression: Reversal of Stress-Induced Cellular, Metabolic and Behavioral Deficits by the Glutamate-Modulating Drug Riluzole. Mol. Psychiatry 2010, 15, 501–511. [Google Scholar] [CrossRef]
- Bansal, Y.; Fee, C.; Misquitta, K.A.; Codeluppi, S.A.; Sibille, E.; Berman, R.M.; Coric, V.; Sanacora, G.; Banasr, M. Prophylactic Efficacy of Riluzole against Anxiety- and Depressive-Like Behaviors in Two Rodent Stress Models. Complex. Psychiatry 2023, 9, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.F.; Nonose, Y.; Ganzella, M.; Loureiro, S.O.; Rocha, A.; Machado, D.G.; Bellaver, B.; Fontella, F.U.; Leffa, D.T.; Pettenuzzo, L.F.; et al. Antidepressant-Like Effects of Chronic Guanosine in the Olfactory Bulbectomy Mouse Model. Front. Psychiatry 2021, 12, 701408. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.B.; Bettio, L.E.B.; Neis, V.B.; Moretti, M.; Kaufmann, F.N.; Tavares, M.K.; Werle, I.; Dalsenter, Y.; Platt, N.; Rosado, A.F.; et al. Antidepressant-like Effect of Guanosine Involves Activation of AMPA Receptor and BDNF/TrkB Signaling. Purinergic Signal 2021, 17, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, J.J.; Réus, G.Z.; Kirsch, T.R.; Stringari, R.B.; Fries, G.R.; Kapczinski, F.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Quevedo, J. Effects of Beta-Carboline Harmine on Behavioral and Physiological Parameters Observed in the Chronic Mild Stress Model: Further Evidence of Antidepressant Properties. Brain Res. Bull. 2010, 81, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, J.J.; Réus, G.Z.; Kirsch, T.R.; Stringari, R.B.; Fries, G.R.; Kapczinski, F.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Quevedo, J. Chronic Administration of Harmine Elicits Antidepressant-like Effects and Increases BDNF Levels in Rat Hippocampus. J. Neural. Transm. 2010, 117, 1131–1137. [Google Scholar] [CrossRef]
- Gourley, S.L.; Espitia, J.W.; Sanacora, G.; Taylor, J.R. Antidepressant-like Properties of Oral Riluzole and Utility of Incentive Disengagement Models of Depression in Mice. Psychopharmacology 2012, 219, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Prakash, A. Ceftriaxone Attenuates Glutamate-Mediated Neuro-Inflammation and Restores BDNF in MPTP Model of Parkinson’s Disease in Rats. Pathophysiology 2017, 24, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Katoh-Semba, R.; Asano, T.; Ueda, H.; Morishita, R.; Takeuchi, I.K.; Inaguma, Y.; Kato, K. Riluzole Enhances Expression of Brain-Derived Neurotrophic Factor with Consequent Proliferation of Granule Precursor Cells in the Rat Hippocampus. FASEB J. 2002, 16, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Wang, Y.W.; Qi, S.L.; Zhang, Y.P.; Deng, G.; Ding, W.Z.; Ma, C.; Lin, Q.Y.; Guan, H.D.; Liu, W.; et al. Analogous β-Carboline Alkaloids Harmaline and Harmine Ameliorate Scopolamine-Induced Cognition Dysfunction by Attenuating Acetylcholinesterase Activity, Oxidative Stress, and Inflammation in Mice. Front. Pharmacol. 2018, 9, 351160. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Huang, B.W.; You, W.J.; Hu, P.; Wang, X.H.; Zhang, J.Y.; Xu, X.B.; Zhang, Z.Y.; Pan, B.X.; Zhang, W.H. Harmine Enhances GABAergic Transmission onto Basoamygdala Projection Neurons in Mice. Brain Res. Bull. 2018, 137, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Wang, W.; Dong, Y.; Wu, L.; Huang, J.; Ma, Y.; Zhang, Z.; Wu, S.; Gao, G.; Qin, H. Ceftriaxone Alleviates Early Brain Injury after Subarachnoid Hemorrhage by Increasing Excitatory Amino Acid Transporter 2 Expression via the PI3K/Akt/NF-ΚB Signaling Pathway. Neuroscience 2014, 268, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Dalmagro, A.P.; Delanogare, E.; Fraga, D.B.; Wolin, I.A.V.; Zeni, A.L.B.; Brocardo, P.S.; Rodrigues, A.L.S. Guanosine Boosts the Fast, but Not Sustained, Antidepressant-like and pro-Synaptogenic Effects of Ketamine by Stimulating MTORC1-Driven Signaling Pathway. Eur. Neuropsychopharmacol. 2022, 57, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Salardini, E.; Zeinoddini, A.; Mohammadinejad, P.; Khodaie-Ardakani, M.R.; Zahraei, N.; Zeinoddini, A.; Akhondzadeh, S. Riluzole Combination Therapy for Moderate-to-Severe Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Psychiatr. Res. 2016, 75, 24–30. [Google Scholar] [CrossRef]
- Mathew, S.J.; Gueorguieva, R.; Brandt, C.; Fava, M.; Sanacora, G. A Randomized, Double-Blind, Placebo-Controlled, Sequential Parallel Comparison Design Trial of Adjunctive Riluzole for Treatment-Resistant Major Depressive Disorder. Neuropsychopharmacology 2017, 42, 2567–2574. [Google Scholar] [CrossRef]
- Zarate, C.A.; Payne, J.L.; Quiroz, J.; Sporn, J.; Denicoff, K.K.; Luckenbaugh, D.; Charney, D.S.; Manji, H.K. An Open-Label Trial of Riluzole in Patients with Treatment-Resistant Major Depression. Am. J. Psychiatry 2004, 161, 171–174. [Google Scholar] [CrossRef]
- Sanacora, G.; Kendell, S.F.; Levin, Y.; Simen, A.A.; Fenton, L.R.; Coric, V.; Krystal, J.H. Preliminary Evidence of Riluzole Efficacy in Antidepressant-Treated Patients with Residual Depressive Symptoms. Biol. Psychiatry 2007, 61, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Spangler, P.T.; West, J.C.; Dempsey, C.L.; Possemato, K.; Bartolanzo, D.; Aliaga, P.; Zarate, C.; Vythilingam, M.; Benedek, D.M. Randomized Controlled Trial of Riluzole Augmentation for Posttraumatic Stress Disorder: Efficacy of a Glutamatergic Modulator for Antidepressant-Resistant Symptoms. J. Clin. Psychiatry 2020, 81, 18364. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Li, J.; Bekker, A.; Ye, J.H. Rescue of Glutamate Transport in the Lateral Habenula Alleviates Depression- and Anxiety-like Behaviors in Ethanol-Withdrawn Rats. Neuropharmacology 2018, 129, 47–56. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.F.; Pocharski, C.B.; Rodrigues, A.L.S.; Elisabetsky, E.; Souza, D.O. Guanosine Fast Onset Antidepressant-like Effects in the Olfactory Bulbectomy Mice Model. Sci. Rep. 2020, 10, 8429. [Google Scholar] [CrossRef]
- Fontana, A.C.K. Current Approaches to Enhance Glutamate Transporter Function and Expression. J. Neurochem. 2015, 134, 982–1007. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bansal, Y.; Codeluppi, S.A.; Banasr, M. Astroglial Dysfunctions in Mood Disorders and Rodent Stress Models: Consequences on Behavior and Potential as Treatment Target. Int. J. Mol. Sci. 2024, 25, 6357. https://doi.org/10.3390/ijms25126357
Bansal Y, Codeluppi SA, Banasr M. Astroglial Dysfunctions in Mood Disorders and Rodent Stress Models: Consequences on Behavior and Potential as Treatment Target. International Journal of Molecular Sciences. 2024; 25(12):6357. https://doi.org/10.3390/ijms25126357
Chicago/Turabian StyleBansal, Yashika, Sierra A. Codeluppi, and Mounira Banasr. 2024. "Astroglial Dysfunctions in Mood Disorders and Rodent Stress Models: Consequences on Behavior and Potential as Treatment Target" International Journal of Molecular Sciences 25, no. 12: 6357. https://doi.org/10.3390/ijms25126357



