Integrating Transcriptomics, Proteomics, and Metabolomics to Investigate the Mechanism of Fetal Placental Overgrowth in Somatic Cell Nuclear Transfer Cattle
Abstract
:1. Introduction
2. Results
2.1. RNA Sequencing and Identification of Differential Expression Genes (DEGs)
2.2. DEGs Function Enrichment Analysis
2.3. Proteomics Detection and Identification of Different Expression of Proteins (DEPs)
2.4. Functional Enrichment Analysis of Differentially Expressed Proteins
2.5. Metabolomic Analysis of SCNT and CON Bovine Placenta Tissues
2.6. Correlation Analysis of Transcriptomics and Metabolomics Data
3. Discussion
4. Materials and Methods
4.1. Ethics Approval and Consent to Participate
4.2. SCNT Animals and Sample Collection
4.3. Transcriptomic Sequencing and Analysis
4.4. Protein Sequencing and Analysis
4.5. Metabolomics Analysis
4.6. Combined Metabolome and Transcriptome Analysis
4.7. Data Availability Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matob, S.; Zhang, Y. Somatic cell nuclear transfer reprogramming: Mechanisms and applications. Cell Stem Cell 2018, 23, 471–485. [Google Scholar] [CrossRef]
- Vajta, G. Somatic cell nuclear transfer in its first and second decades: Successes, setbacks, paradoxes and perspectives. Reprod. Biomed. Online 2007, 15, 582–590. [Google Scholar] [CrossRef]
- Oback, B. Climbing Mount Efficiency—Small steps, not giant leaps towards higher cloning success in farm animals. Reprod. Domest. Anim. 2008, 2, 407–416. [Google Scholar] [CrossRef]
- Loi, P.; Ptak, G.; Barboni, B.; Fulka, J.J.; Cappai, P.; Clinton, M. Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat. Biotechnol. 2001, 19, 962–964. [Google Scholar] [CrossRef]
- Wilmut, I.; Schnieke, A.E.; McWhir, J.; Kind, A.J.; Campbell, K.H. Viable offspring derived from fetal and adult mammalian cells. Cloning Stem Cells 2007, 9, 3–7. [Google Scholar] [CrossRef]
- Wakayama, T.; Perry, A.C.; Zuccotti, M.; Johnson, K.R.; Yanagimachi, R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998, 394, 369–374. [Google Scholar] [CrossRef]
- Zhou, Q.; Renard, J.P.; Friec, G.L.; Brochard, V.; Beaujean, N.; Cherifi, Y.; Fraichard, A.; Cozzi, J. Generation of fertile cloned rats by regulating oocyte activation. Science 2003, 302, 1179. [Google Scholar] [CrossRef]
- Kato, Y.; Tani, T.; Sotomaru, Y.; Kurokawa, K.; Kato, J.; Doguchi, H.; Yasue, H.; Tsunoda, Y. Eight calves cloned from somatic cells of a single adult. Science 1998, 282, 2095–2098. [Google Scholar] [CrossRef]
- Polejaeva, I.A.; Chen, S.H.; Vaught, T.D.; Page, R.L.; Mullins, J.; Ball, S.; Dai, Y.; Boone, J.; Walker, S.; Ayares, D.L.; et al. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature 2000, 407, 86–90. [Google Scholar] [CrossRef]
- Shin, T.; Kraemer, D.; Pryor, J.; Liu, L.; Rugila, J.; Howe, L.; Buck, S.; Murphy, K.; Lyons, L. A cat cloned by nuclear transplantation. Nature 2002, 415, 859. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kim, M.K.; Jang, G.; Oh, H.J.; Yuda, F.; Kim, H.J.; Hossein, M.S.; Kim, J.J.; Kang, S.K.; Schatten, G.; et al. Dogs cloned from adult somatic cells. Nature 2005, 436, 641. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cai, Y.; Wang, Y.; Nie, Y.; Zhang, C.; Xu, Y.; Zhang, X.; Lu, Y.; Wang, Z.; Poo, M.; et al. Cloning of Macaque Monkeys by somatic cell nuclear transfer. Cell 2018, 172, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Inoue, K.; Wakayama, T. Recent advancements in cloning by somatic cell nuclear transfer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20110329. [Google Scholar] [CrossRef] [PubMed]
- Loi, P.; Luso, D.; Czernik, M.; Ogura, A. A new, dynamic era for somatic cell nuclear transfer? Trends Biotechnol. 2016, 34, 791–797. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Oh, J.N.; Park, C.H.; Lee, D.K.; Lee, C.K. Dosage compensation of X-chromosome inactivation center-linked genes in porcine preimplantation embryos: Non-chromosome-wide initiation of X-chromosome inactivation in blastocysts. Mech. Dev. 2015, 3, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, L.S.; Liu, X.F.; Xia, Q.; Bai, L.G.; Gao, L.; Gao, G.Q.; Wang, Y.; Wei, Z.Y.; Bai, C.L.; et al. The maternal effect genes UTX and JMJD3 play contrasting roles in Mus musculus preimplantation embryo development. Sci. Rep. 2016, 6, 26711. [Google Scholar] [CrossRef]
- Yu, M.; Hu, X.; Pan, Z.; Du, C.; Jiang, J.; Zheng, W.; Cai, H.; Wang, Y.; Deng, W.; Wang, H.; et al. Endogenous retrovirus-derived enhancers confer the transcriptional regulation of human trophoblast syncytialization. Nucleic Acids Res. 2023, 51, 4745–4759. [Google Scholar] [CrossRef]
- Guttmacher, A.E.; Maddox, Y.T.; Spong, C.Y. The human placenta project: Placental structure, development, and function in real time. Placenta 2014, 35, 303–304. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Rozanova, S.; Barkovits, K.; Nikolov, M.; Schmidt, C.; Urlaub, H.; Marcus, K. Quantitative mass spectrometry-based proteomics: An overview. Methods Mol. Biol. 2021, 2228, 85–116. [Google Scholar]
- Bauermeister, A.; Mannochio-Russo, H.; Costa-Lotufo, L.V.; Jarmusch, A.K.; Dorrestein, P.C. Mass spectrometry-based metabolomics in microbiome investigations. Nat. Rev. Microbiol. 2022, 20, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Chavatte-Palmer, P.; Camous, S.; Jammes, H.; Cleac’h, N.; Guillomot, M.; Lee, R.S. Review: Placental perturbations induce the developmental abnormalities often observed in bovine somatic cell nuclear transfer. Placenta 2012, 33, S99–S104. [Google Scholar] [CrossRef] [PubMed]
- Stice, S.L.; Strelchenko, N.S.; Keefer, C.L.; Matthews, L. Pluripotent bovine embryonic cell lines direct embryonic development following nuclear transfer. Biol. Reprod. 1996, 54, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.I.; Lee, H.S.; Deb, G.K.; Ha, A.N.; Kwon, Y.S.; Cho, S.K.; Kim, B.W.; Kong, I.K. Proteomic identification of abnormally expressed proteins in early-stage placenta derived from cloned cat embryos. Theriogenology 2013, 79, 358–366.e351. [Google Scholar] [CrossRef]
- Kim, H.R.; Han, R.X.; Yoon, J.T.; Park, C.S.; Jin, D.I. A two-dimensional electrophoresis reference map for the bovine placenta during late pregnancy. Proteomics 2010, 10, 564–573. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, J.Y.; Choi, Y.J.; Cho, S.K.; Ahn, J.D.; Kwon, D.N.; Hwang, K.C.; Kang, S.J.; Paik, S.S.; Seo, H.G.; et al. Comparative proteomic analysis associated with term placental insufficiency in cloned pig. Proteomics 2007, 7, 1303–1315. [Google Scholar] [CrossRef]
- Gao, G.; Wang, S.; Zhang, J.; Su, G.; Zheng, Z.; Bai, C.; Yang, L.; Wei, Z.; Wang, X.; Liu, X.; et al. Transcriptome-wide analysis of the SCNT bovine abnormal placenta during mid- to late gestation. Sci. Rep. 2019, 9, 20035. [Google Scholar] [CrossRef]
- Su, J.; Liu, X.; Sun, H.; Wang, Y.; Wu, Y.; Guo, Z.; Zhang, Y. Identification of differentially expressed microRNAs in placentas of cloned and normally produced calves by Solexa sequencing. Anim. Reprod. Sci. 2015, 155, 64–74. [Google Scholar] [CrossRef]
- Okae, H.; Matoba, S.; Nagashima, T.; Mizutani, E.; Inoue, K.; Ogonuki, N.; Chiba, H.; Funayama, R.; Tanaka, S.; Yaegashi, N.; et al. RNA sequencing-based identification of aberrant imprinting in cloned mice. Hum. Mol. Genet. 2014, 23, 992–1001. [Google Scholar] [CrossRef]
- Farin, P.W.; Farin, C.E. Transfer of bovine embryos produced in vivo or in vitro: Survival and fetal development. Biol. Reprod. 1995, 52, 676–682. [Google Scholar] [CrossRef]
- Hill, J.R.; Burghardt, R.C.; Jones, K.; Long, C.R.; Looney, C.R.; Shin, T.; Spencer, T.E.; Thompson, J.A.; Winger, Q.A.; Westhusin, M.E. Evidence for placental abnormality as the major cause of mortality in first-trimester somatic cell cloned bovine fetuses. Biol. Reprod. 2000, 63, 1787–1794. [Google Scholar] [CrossRef]
- Hoffmann, B.; Schuler, G. The bovine placenta; a source and target of steroid hormones: Observations during the second half of gestation. Domest. Anim. Endocrinol. 2002, 23, 309–320. [Google Scholar] [CrossRef]
- Sousa, N.M.; Ayad, A.; Beckers, J.F.; Gajewski, Z. Pregnancy-associated glycoproteins (PAG) as pregnancy markers in the ruminants. J. Physiol. Pharmacol. 2006, 8, 153–171. [Google Scholar]
- Ba, Y.; Yang, S.; Yu, S.; Hou, X.; Du, Y.; Gao, M.; Zuo, J.; Sun, L.; Fu, X.; Li, Z.; et al. Role of glycolysis/gluconeogenesis and HIF-1 signaling pathways in rats with dental fluorosis integrated proteomics and metabolomics analysis. Int. J. Mol. Sci. 2022, 23, 8266. [Google Scholar] [CrossRef]
- Prentki, M.; Madiraju, S.R. Glycerolipid metabolism and signaling in health and disease. Endocr. Rev. 2008, 29, 647–676. [Google Scholar] [CrossRef]
- Han, T.L.; Cannon, R.D.; Gallo, S.M.; Villas-Bôas, S.G. A metabolomic study of the effect of Candida albicans glutamate dehydrogenase deletion on growth and morphogenesis. NPJ Biofilms Microbiomes 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Gumy, C.; Blatter, E.; Boeffel, S.; Fricke, W.; Rentsch, D. In planta function of compatible solute transporters of the AtProT family. J. Exp. Bot. 2011, 62, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M., Jr. Arginine metabolism revisited. J. Nutr. 2016, 146, 2579s–2586s. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Roberts, M.R. Ets-2 and C/EBP-beta are important mediators of ovine trophoblast Kunitz domain protein-1 gene expression in trophoblast. BMC Mol. Biol. 2007, 8, 14. [Google Scholar] [CrossRef]
- Cheng, D.; Jiang, S.; Chen, J.; Li, J.; Ao, L.; Zhang, Y. Upregulated long noncoding RNA Linc00261 in pre-eclampsia and its effect on trophoblast invasion and migration via regulating miR-558/TIMP4 signaling pathway. J. Cell. Biochem. 2019, 120, 13243–13253. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Fan, Y.; Peng, Y.; Song, D.; Fu, J.; Wang, X. Human placenta-based genome-wide mRNA sequencing to identify TEK/IGF1/CSF1/ANGPT2 as crucial segments in the pathogenesis of pre-eclampsia. Front. Genet. 2022, 13, 944932. [Google Scholar] [CrossRef]
- Pollard, J.W.; Bartocci, A.; Arceci, R.; Orlofsky, A.; Ladner, M.B.; Stanley, E.R. Apparent role of the macrophage growth factor, CSF-1, in placental development. Nature 1987, 330, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Hoffert-Goeres, K.A.; Batchelder, C.A.; Bertolini, M.; Moyer, A.L.; Famula, T.R.; Anderson, G.B. Angiogenesis in day-30 bovine pregnancies derived from nuclear transfer. Cloning Stem Cells 2007, 9, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Naruse, K.; Lee, H.R.; Han, R.X.; Park, C.S.; Jin, D.I. Abnormal expression of TIMP-2, SOD, vimentin and PAI proteins in cloned bovine placentae. Reprod. Domest. Anim. 2009, 44, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, D.; Romero, R.; Kim, S.S.; Tarca, A.L.; Draghici, S.; Kusanovic, J.P.; Kim, J.S.; Lee, D.C.; Eraz, O.; Gotsch, F.; et al. Expression patterns of microRNAs in the chorioamniotic membranes: A role for microRNAs in human pregnancy and parturition. J. Pathol. 2009, 217, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Sferruzzi-Perri, A.N.; López-Tello, J.; Fowden, A.L.; Constancia, M. Maternal and fetal genomes interplay through phosphoinositol 3-kinase (PI3K)-p110α signaling to modify placental resource allocation. Proc. Natl. Acad. Sci. USA 2016, 113, 11255–11260. [Google Scholar] [CrossRef]
- Qian, X.; Esteban, L.; Vass, W.C.; Upadhyaya, C.; Papageorge, A.G. The Sos1 and Sos2 Ras-specific exchange factors: Differences in placental expression and signaling properties. EMBO J. 2000, 19, 642–654. [Google Scholar] [CrossRef]
- Scagliotti, V.; Esse, R.; Willis, T.L.; Howard, M.; Carrus, I.; Lodge, E.; Andoniadou, C.L.; Charalambous, M. Dynamic expression of imprinted genes in the developing and postnatal pituitary gland. Genes 2021, 12, 509. [Google Scholar] [CrossRef]
- Ishihara, T.; Griffith, O.W.; Suzuki, S.; Renfree, M.B. Placental imprinting of SLC22A3 in the IGF2R imprinted domain is conserved in therian mammals. Epigenetics Chromatin 2022, 15, 32. [Google Scholar] [CrossRef]
- Guillomot, M.; Taghouti, G.; Constant, F.; Degrelle, S.; Hue, I.; Chavatte-Palmer, P.; Jammes, H. Abnormal expression of the imprinted gene Phlda2 in cloned bovine placenta. Placenta 2010, 1, 482–490. [Google Scholar] [CrossRef]
- Gardner, R.L.; Squire, S.; Zaina, S.; Hills, S.; Graham, C.F. Insulin-like growth factor-2 regulation of conceptus composition: Effects of the trophectoderm and inner cell mass genotypes in the mouse. Biol. Reprod. 1999, 60, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Kohda, T.; Lee, J.; Ogonuki, N.; Mochida, K.; Noguchi, Y.; Tanemura, K.; Kaneko-Ishino, T.; Ishino, F.; Ogura, A. Faithful expression of imprinted genes in cloned mice. Science 2002, 295, 297. [Google Scholar] [CrossRef] [PubMed]
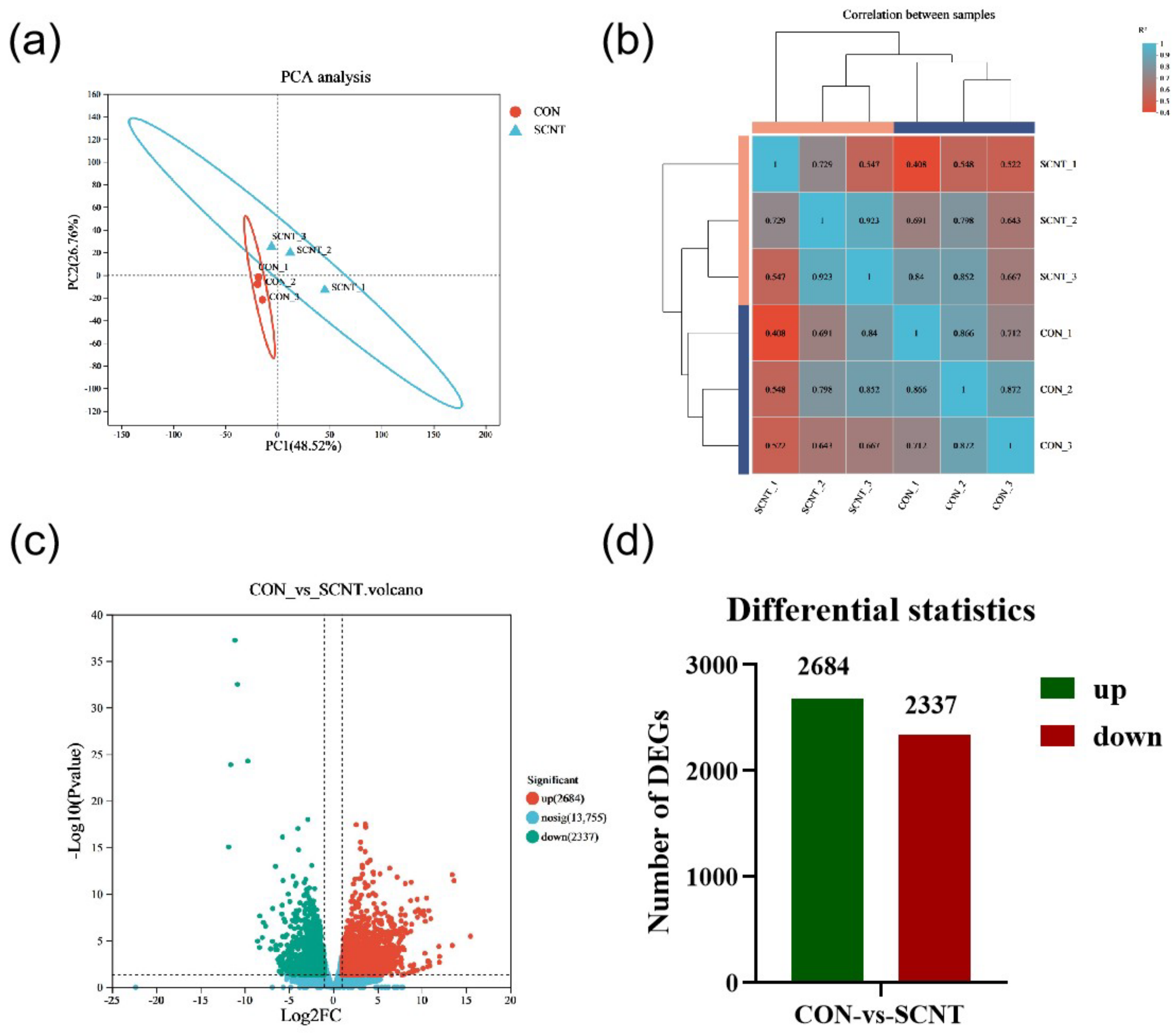

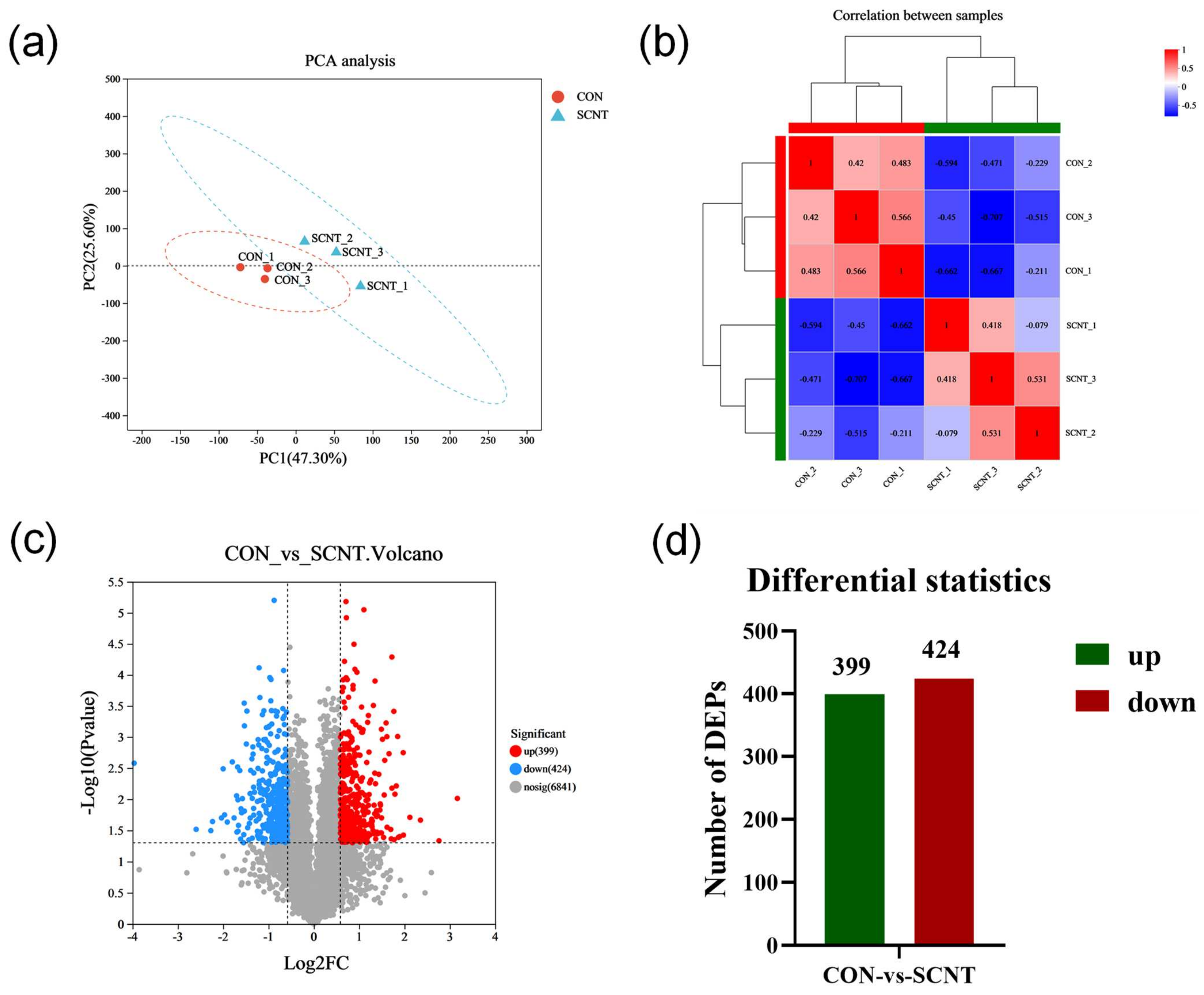
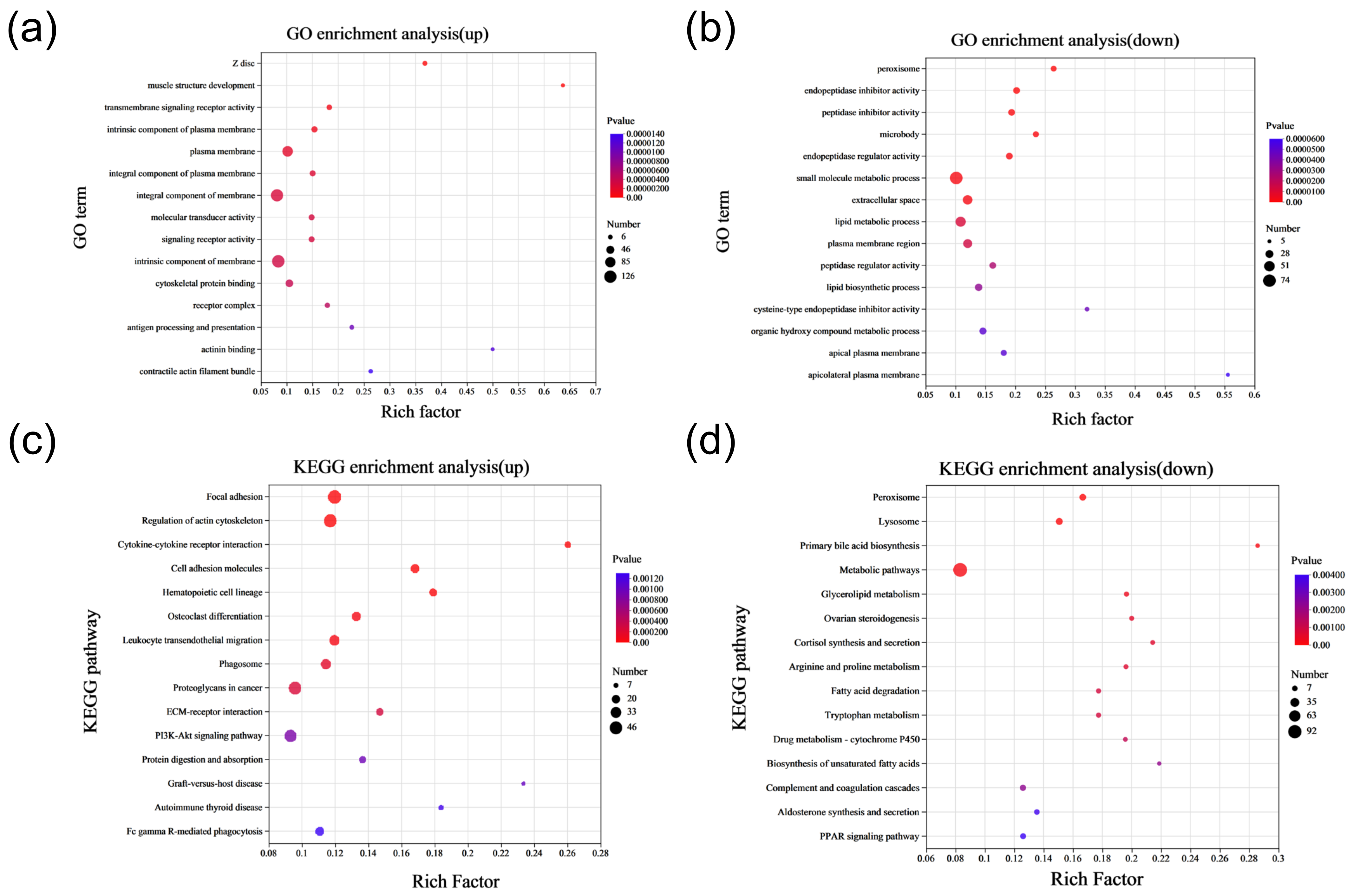


| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Error Rate (%) | Q20 (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|---|---|---|---|
| CON_1 | 47,653,834 | 7,195,728,934 | 47,052,430 | 6,941,489,461 | 0.0246 | 98.11 | 94.58 | 51.13 |
| CON_2 | 49,275,204 | 7,440,555,804 | 48,669,080 | 7,107,312,975 | 0.0244 | 98.19 | 94.81 | 52.5 |
| CON_3 | 41,920,862 | 6,330,050,162 | 41,293,700 | 6,084,502,265 | 0.0247 | 98.05 | 94.49 | 52.49 |
| SCNT_1 | 44,027,118 | 6,648,094,818 | 43,098,966 | 6,291,349,718 | 0.0243 | 98.21 | 94.95 | 57.15 |
| SCNT_2 | 42,114,108 | 6,359,230,308 | 41,502,758 | 6,139,152,712 | 0.0243 | 98.25 | 94.97 | 55.06 |
| SCNT_3 | 49,399,126 | 7,459,268,026 | 48,727,536 | 7,177,370,735 | 0.0248 | 98.05 | 94.45 | 53.86 |
| Gene Name | p-Value | Log2 FC | Regulation |
|---|---|---|---|
| ENSBTAG00000054274 | 5.93 × 10−38 | −11.10993504 | Down |
| TKDP4 | 3.15 × 10−33 | −10.8104713 | Down |
| CYP4B1 | 5.47 × 10−25 | −9.657055193 | Down |
| TKDP1 | 1.35 × 10−24 | −11.57468493 | Down |
| TIMP4 | 1.01 × 10−18 | −2.87067796 | Down |
| MKI67 | 3.15 × 10−18 | 3.621668856 | Up |
| IMPDH1 | 3.61 × 10−18 | 2.597485953 | Up |
| EPB41L1 | 6.47 × 10−18 | 3.666560722 | Up |
| ENSBTAG00000037799 | 9.37 × 10−18 | −3.982927754 | Down |
| ENSBTAG00000053827 | 7.48 × 10−17 | −5.731321654 | Down |
| Protein Name | p-Value | Log2 FC | Regulation |
|---|---|---|---|
| ITGB4 | 6.00 × 10−6 | −0.875523 | Down |
| ABCC1 | 7.00 × 10−6 | 0.709874 | Up |
| PDLIM7 | 9.00 × 10−6 | 1.105236 | Up |
| GARRE1 | 1.20 × 10−5 | 0.718408 | Up |
| MICAL1 | 3.20 × 10−5 | 0.886415 | Up |
| CSF1 | 5.20 × 10−5 | 1.722665 | Up |
| OLFML3 | 6.10 × 10−5 | 0.676249 | Up |
| OR10AG63 | 7.70 × 10−5 | −1.206875 | Down |
| ITGA2 | 8.10 × 10−5 | 0.907248 | Up |
| NAD3 | 8.50 × 10−5 | −0.665457 | Down |
| Metabolite | p-Value | FC | VIP | Regulate |
|---|---|---|---|---|
| Hoduloside VII | 1.88 × 10−6 | 2.5188 | 1.5456 | Up |
| PC(18:2(9Z,12Z)/20:4(5Z,8Z,11Z,14Z)) | 6.90 × 10−6 | 0.2083 | 1.691 | Down |
| Nomilinic acid | 9.91 × 10−6 | 101.0917 | 1.5009 | Up |
| Yucalexin P21 | 1.11 × 10−5 | 7.3884 | 1.7241 | Up |
| 13,14-dihydro-15-keto-PGA2 | 1.25 × 10−5 | 1.3036 | 1.0464 | Up |
| 1-hexadecyl-glycero-3-phosphate | 1.45 × 10−5 | 0.4739 | 1.3535 | Down |
| (3S,7E,9R)-4,7-Megastigmadiene-3,9-diol 9-[apiosyl-(1- > 6)-glucoside] | 3.26 × 10−5 | 3.6881 | 1.5094 | Up |
| 2-Methyl-3-(2-pentenyl)-2-cyclopenten-1-one | 3.27 × 10−5 | 8.5901 | 1.5401 | Up |
| Prostaglandin F3a | 4.22 × 10−5 | 1.3644 | 1.1154 | Up |
| 11Z-Eicosenoic acid | 5.18 × 10−5 | 1.9694 | 1.4041 | Up |
| Pathway ID | Pathway Name | p-Value |
|---|---|---|
| map04976 | Bile secretion | 0.1458 |
| map02010 | ABC transporters | 0.2184 |
| map00230 | Purine metabolism | 0.2937 |
| map05230 | Central carbon metabolism in cancer | 0.3569 |
| map04080 | Neuroactive ligand-receptor interaction | 0.3569 |
| map00970 | Aminoacyl-tRNA biosynthesis | 0.393 |
| map04974 | Protein digestion and absorption | 0.393 |
| map00360 | Phenylalanine metabolism | 0.4326 |
| map00240 | Pyrimidine metabolism | 0.4758 |
| map00480 | Glutathione metabolism | 0.4758 |
| KEGG Pathway | KEGG ID | Metabolites | Genes |
|---|---|---|---|
| Choline metabolism in cancer | bta05231 | PC(18:2(9Z, 12Z)/20:4(5Z, 8Z, 11Z, 14Z)), PC(14:0/20:0), PC(18:3(6Z, 9Z, 12Z)/P-16:0), LysoPC(20:4(5Z, 8Z, 11Z, 14Z)), LysoPC(18:3(6Z, 9Z, 12Z)), LysoPC(22:4(7Z, 10Z, 13Z, 16Z)), LysoPC(22:5(4Z, 7Z, 10Z, 13Z, 16Z)), LysoPC(P-18:1(9Z)), LysoPC(17:0) | RPS6KB1, MAPK8, RALGDS, PLA2G4B, NRAS, PIK3CA, PIK3R2, AKT3, SLC22A2, HIF1A, SLC44A4, EGFR, PIK3CD, PDGFRA, PLA2G4E, RHEB, GPCPD1, LYPLA1, SOS2, TSC2, DGKG, SLC44A3, PDGFD, SLC22A3, DGKI, PLPP1, KRAS, WASF2, WAS, PIP5K1B |
| Biosynthesis of unsaturated fatty acids | bta01040 | 11Z-Eicosenoic acid, Alpha-Linolenic acid, 11,14,17-Eicosatrienoic acid | ELOVL1, HACD2, HACD1, SCP2, ACOT7, ACOX1, SCD, ELOVL5, ELOVL4, FADS2, ENSBTAG00000054697, TECR, FADS1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Wu, S.; Yun, Y.; Du, Z.; Liu, S.; Bo, C.; Gao, Y.; Yang, L.; Song, L.; Bai, C.; et al. Integrating Transcriptomics, Proteomics, and Metabolomics to Investigate the Mechanism of Fetal Placental Overgrowth in Somatic Cell Nuclear Transfer Cattle. Int. J. Mol. Sci. 2024, 25, 9388. https://doi.org/10.3390/ijms25179388
Zhao X, Wu S, Yun Y, Du Z, Liu S, Bo C, Gao Y, Yang L, Song L, Bai C, et al. Integrating Transcriptomics, Proteomics, and Metabolomics to Investigate the Mechanism of Fetal Placental Overgrowth in Somatic Cell Nuclear Transfer Cattle. International Journal of Molecular Sciences. 2024; 25(17):9388. https://doi.org/10.3390/ijms25179388
Chicago/Turabian StyleZhao, Xiaoyu, Shanshan Wu, Yuan Yun, Zhiwen Du, Shuqin Liu, Chunjie Bo, Yuxin Gao, Lei Yang, Lishuang Song, Chunling Bai, and et al. 2024. "Integrating Transcriptomics, Proteomics, and Metabolomics to Investigate the Mechanism of Fetal Placental Overgrowth in Somatic Cell Nuclear Transfer Cattle" International Journal of Molecular Sciences 25, no. 17: 9388. https://doi.org/10.3390/ijms25179388






