Overexpression of SlALC Increases Drought and Salt Tolerance and Affects Fruit Dehiscence in Tomatoes
Abstract
:1. Introduction
2. Results
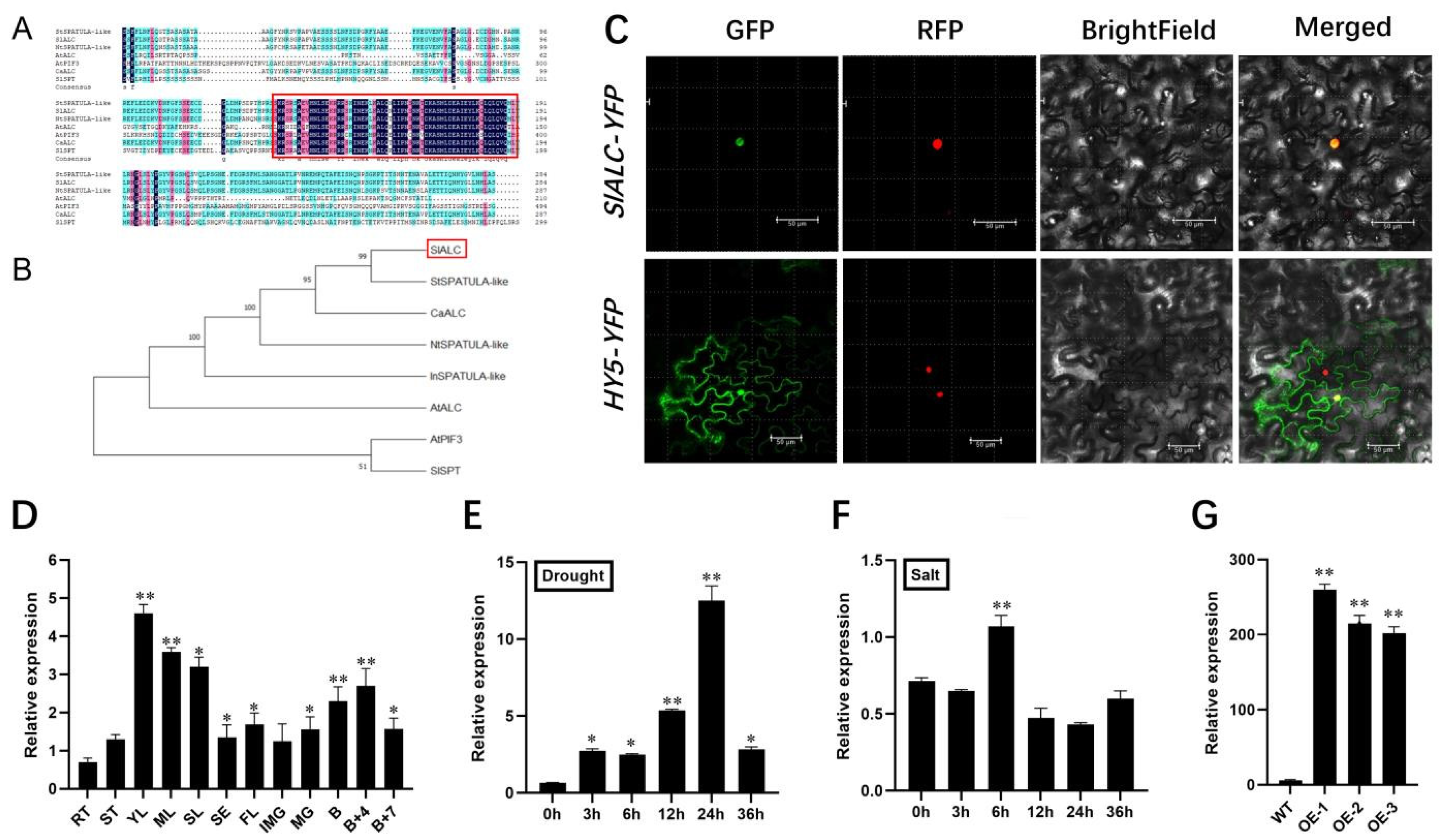
2.1. Bioinformatics Analysis, Subcellular Localization, and Expression Pattern of SlALC
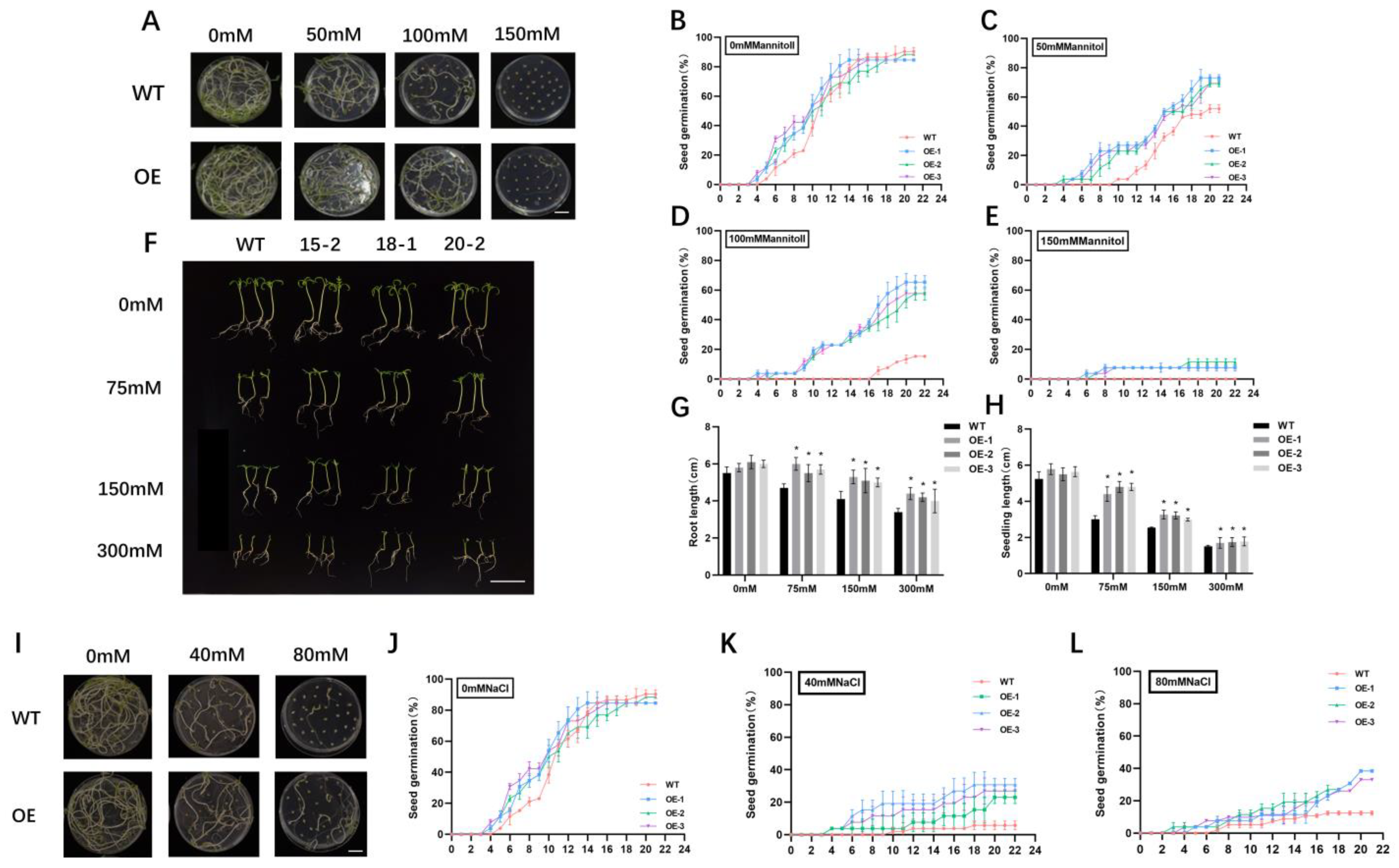
2.2. Overexpression of SlALC Confers Tolerance to Mannitol and Salt at the Stages of Seed Germination and Seedling Growth
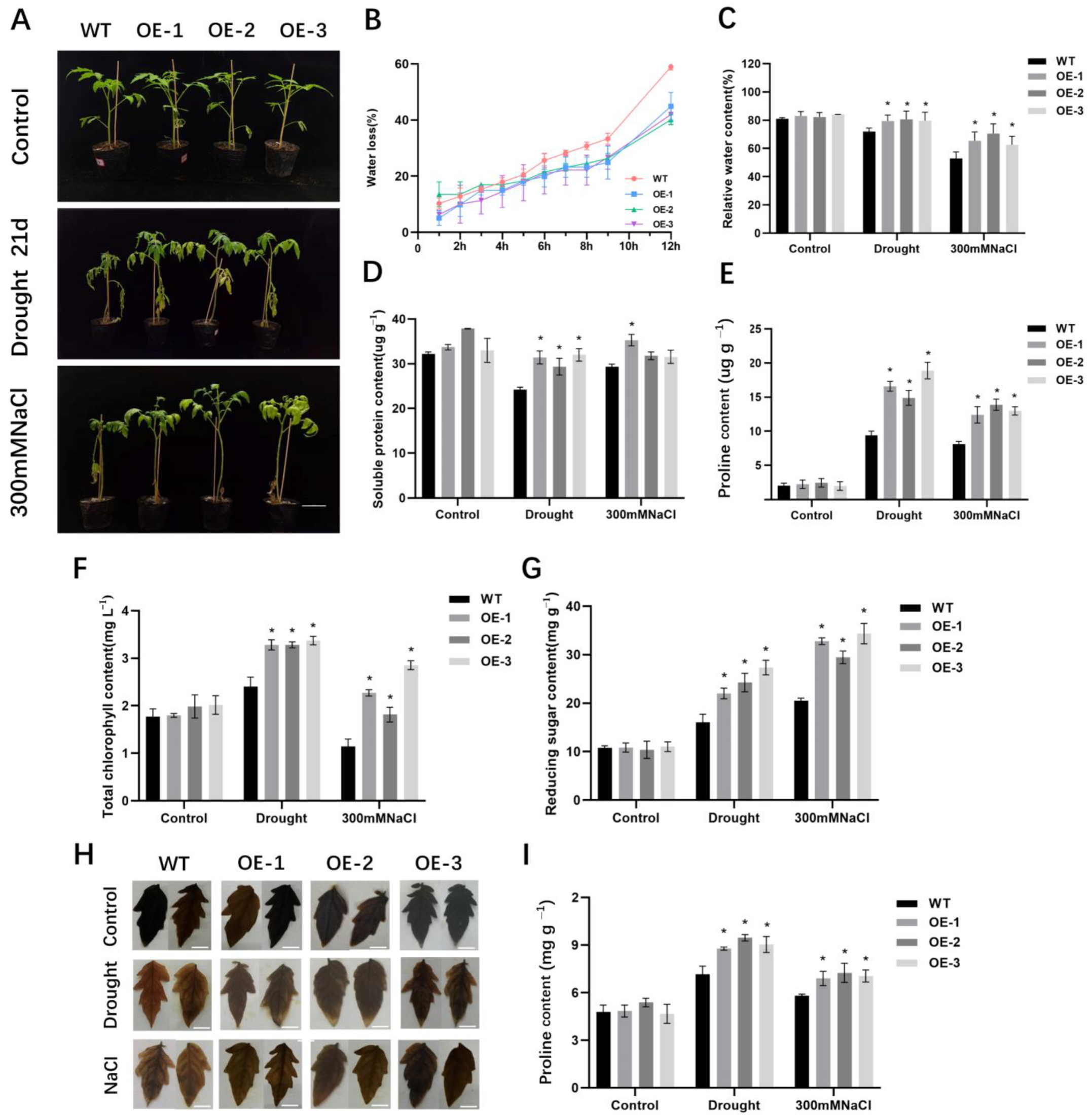
2.3. Overexpression of SlALC Increases Drought and Salt Tolerance of Tomato Plant
2.4. Expression Levels of Stress-Related Genes in SlALC-OE Lines under Drought and Salt Stress
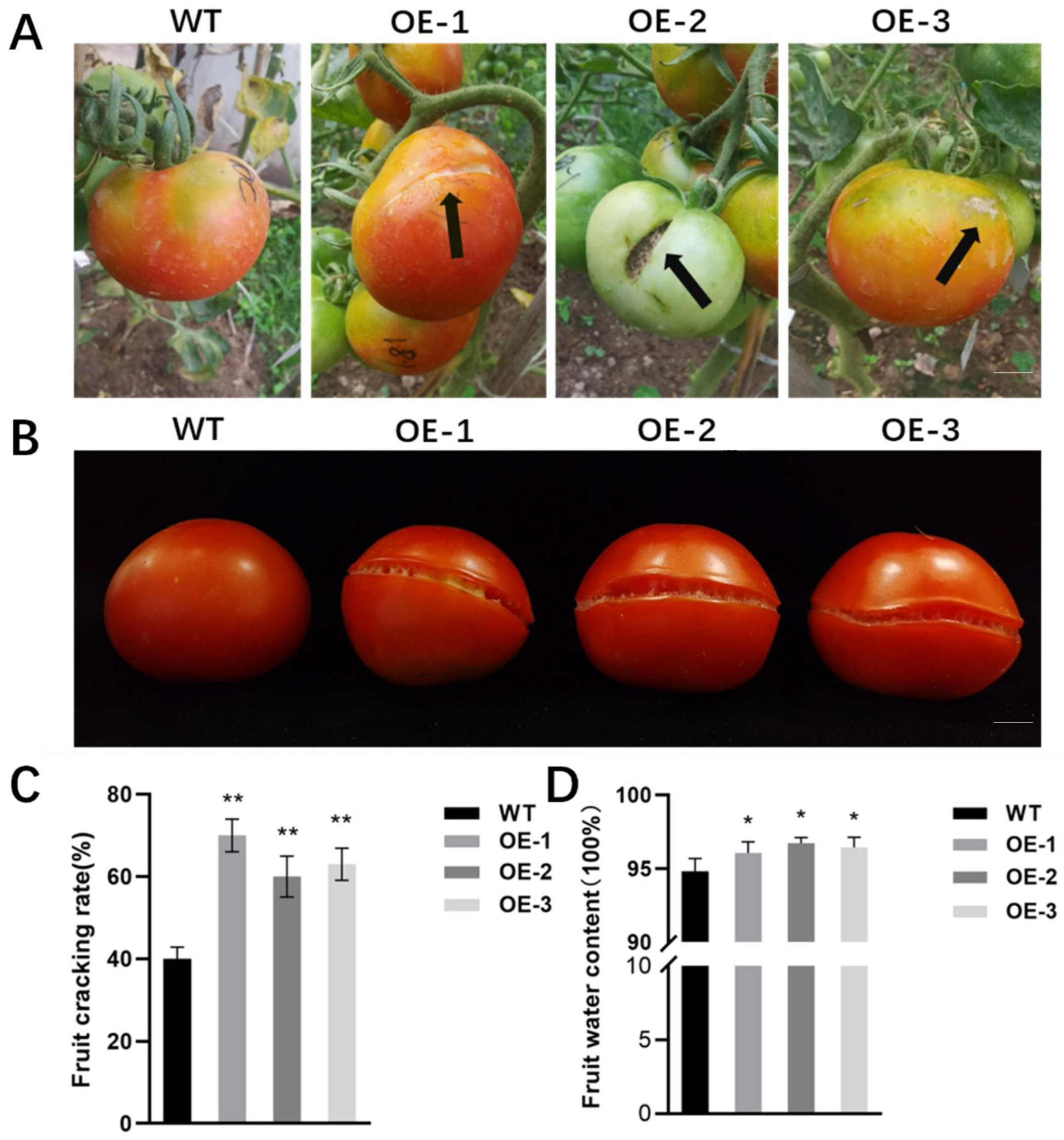
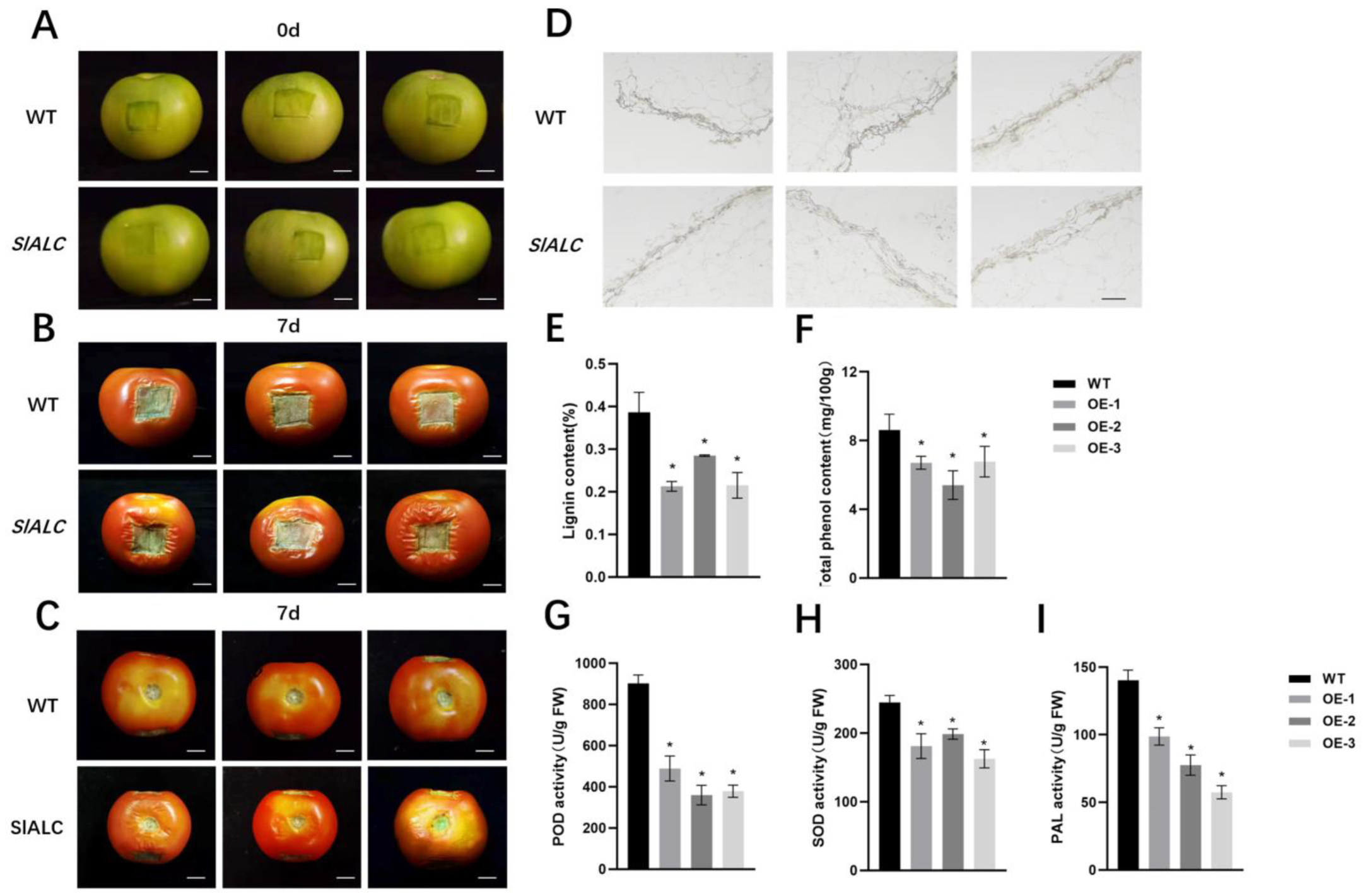
2.5. Overexpression of SlALC Gene Affects Fruit Dehiscence
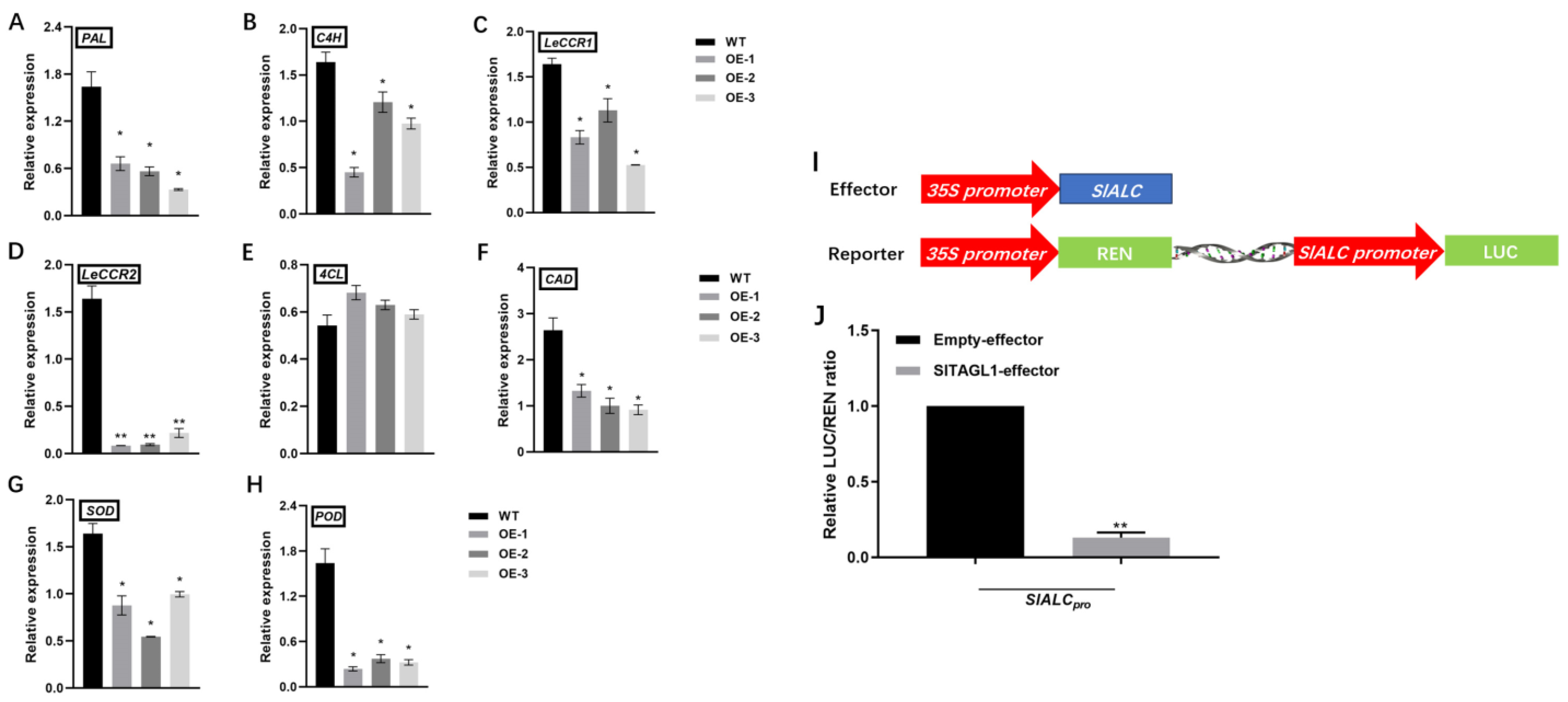
2.6. Overexpression of SlALC Affects the Expression of Genes Related to Lignin Synthesis in Fruits
2.7. Tomato SlTAGL1 Represses the Activity of the Promoter of SlALC Gene
3. Discussion
3.1. Tomato SlALC Gene Regulates Plant Drought and Salt Tolerance
3.2. SlALC Affects Fruit Lignification, Thereby Influencing Fruit Dehiscence
4. Materials and Methods
4.1. Plant Material Stress Treatments
4.2. Sequence Analysis and Phylogenetic Tree Construction of SlALC
4.3. Subcellular Localization Experiment
4.4. Seed Germination and Seedling Growth Assay under D-Mannitol and Salt Treatment
4.5. Drought and Salinity Stress Tolerance Experiment
4.6. Measurement of Physiological Parameters
4.7. DAB, NBT, and Trypan Blue Staining Methods
4.8. Construction of SlALC Overexpression Vector and Plant Transformation
4.9. RNA Extraction and Quantitative Real-Time PCR Analysis
4.10. Dual-Luciferase Assay
4.11. Fruit Dehiscence Rate and FY Staining Method
4.12. Determination of Lignin and Phenolics Content
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ledent, V.; Vervoort, M. The basic helix-loop-helix protein family: Comparative genomics and phylogenetic analysis. Genome Res. 2001, 11, 754–770. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Sun, B.; He, H.; Zhang, Y.; Tian, H.; Wang, B. Current Understanding of bHLH Transcription Factors in Plant Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 4921. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, W.; Dai, Y.; Xiao, N.; Zhang, C.; Li, H.; Lu, Y.; Wu, M.; Tao, X.; Deng, D.; et al. A maize phytochrome-interacting factor 3 improves drought and salt stress tolerance in rice. Plant Mol. Biol. 2015, 87, 413–428. [Google Scholar] [CrossRef]
- Qiu, J.R.; Xiang, X.Y.; Wang, J.T.; Xu, W.X.; Huang, Z. MfPIF1 of Resurrection Plant Myrothamnus flabellifolia Plays a Positive Regulatory Role in Responding to Drought and Salinity Stresses in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 3011. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, C.; Han, X.; Tang, S.; Liu, S.; Xia, X.; Yin, W. A novel bHLH transcription factor PebHLH35 from Populus euphratica confers drought tolerance through regulating stomatal development, photosynthesis and growth in Arabidopsis. Biochem. Biophys. Res. Commun. 2014, 450, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Joo, J.; Kim, M.J.; Kim, Y.K.; Nahm, B.H.; Song, S.I.; Cheong, J.J.; Lee, J.S.; Kim, J.K.; Choi, Y.D. OsbHLH148, a basic helix-loop-helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant J. 2011, 65, 907–921. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, L.; Xia, C.; Fu, S.; Zhao, G.; Jia, J.; Kong, X. The wheat transcription factor, TabHLH39, improves tolerance to multiple abiotic stressors in transgenic plants. Biochem. Biophys. Res. Commun. 2016, 473, 1321–1327. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, J.; Chen, X.; Zhou, Y.; Pei, Y.; Chen, L.; Ul, H.S.; Lu, M.; Gong, H.; Chen, R. Pepper bHLH transcription factor CabHLH035 contributes to salt tolerance by modulating ion homeostasis and proline biosynthesis. Hortic. Res. 2022, 9, uhac203. [Google Scholar] [CrossRef]
- Qiu, J.R.; Huang, Z.; Xiang, X.Y.; Xu, W.X.; Wang, J.T.; Chen, J.; Song, L.; Xiao, Y.; Li, X.; Ma, J.; et al. MfbHLH38, a Myrothamnus flabellifolia bHLH transcription factor, confers tolerance to drought and salinity stresses in Arabidopsis. BMC Plant Biol. 2020, 20, 542. [Google Scholar] [CrossRef]
- Babitha, K.C.; Vemanna, R.S.; Nataraja, K.N.; Udayakumar, M. Overexpression of EcbHLH57 Transcription Factor from Eleusine coracana L. in Tobacco Confers Tolerance to Salt, Oxidative and Drought Stress. PLoS ONE 2015, 10, e137098. [Google Scholar] [CrossRef]
- Considine, J.; Brown, K. Physical Aspects of Fruit Growth: Theoretical analysis of distribution of surface growth forces in fruit in relation to cracking and splitting. Plant Physiol. 1981, 68, 371–376. [Google Scholar] [CrossRef]
- Gine-Bordonaba, J.; Echeverria, G.; Ubach, D.; Aguilo-Aguayo, I.; Lopez, M.L.; Larrigaudiere, C. Biochemical and physiological changes during fruit development and ripening of two sweet cherry varieties with different levels of cracking tolerance. Plant Physiol. Biochem. 2017, 111, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Mucha-Pelzer, T.; Muller, S.; Rohr, F.; Mewis, I. Cracking susceptibility of sweet cherries (Prunus avium L.) under different conditions. Commun. Agric. Appl. Biol. Sci. 2006, 71, 215–223. [Google Scholar] [PubMed]
- Hendrik, B.; Christoph, N. Tomato (Lycopersicon esculentum Mill.) fruit growth and ripening as related to the biomechanical properties of fruit skin and isolated cuticle. J. Exp. Bot. 2005, 56, 1049–1060. [Google Scholar]
- Liu, Y.; Zhang, P.; Geng, Y.; Xie, X.; Wen, P. Cracking of jujube fruits is associated with differential expression of metabolic genes. FEBS Open Bio 2020, 10, 1765–1773. [Google Scholar] [CrossRef]
- Liao, N.; Hu, Z.; Li, Y.; Hao, J.; Chen, S.; Xue, Q.; Ma, Y.; Zhang, K.; Mahmoud, A.; Ali, A.; et al. Ethylene-responsive factor 4 is associated with the desirable rind hardness trait conferring cracking resistance in fresh fruits of watermelon. Plant Biotechnol. J. 2020, 18, 1066–1077. [Google Scholar] [CrossRef]
- Wang, J.; Gao, X.; Ma, Z.; Chen, J.; Liu, Y. Analysis of the molecular basis of fruit cracking susceptibility in Litchi chinensis cv. Baitangying by transcriptome and quantitative proteome profiling. J. Plant Physiol. 2019, 234–235, 106–116. [Google Scholar] [CrossRef]
- Emmons, C.L.W.; Scott, J.W. Environmental and Physiological Effects on Cuticle Cracking in Tomato. Am. Soc. Hortic. Sci. 1997, 122, 797–801. [Google Scholar] [CrossRef]
- Tani, E.; Tsaballa, A.; Stedel, C.; Kalloniati, C.; Papaefthimiou, D.; Polidoros, A.; Darzentas, N.; Ganopoulos, I.; Flemetakis, E.; Katinakis, P. The study of a SPATULA-like bHLH transcription factor expressed during peach (Prunus persica) fruit development. Plant Physiol. Biochem. 2011, 49, 654–663. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Roeder, A.H.; Kempin, S.A.; Gremski, K.; Ostergaard, L.; Guimil, S.; Reyes, D.K.; Yanofsky, M.F. Control of fruit patterning in Arabidopsis by INDEHISCENT. Cell 2004, 116, 843–853. [Google Scholar] [CrossRef]
- Zumajo-Cardona, C.; Ambrose, B.A.; Pabon-Mora, N. Evolution of the SPATULA/ALCATRAZ gene lineage and expression analyses in the basal eudicot, Bocconia frutescens L. (Papaveraceae). EvoDevo 2017, 8, 5. [Google Scholar] [CrossRef]
- Liu, T.; Du, Q.; Li, S.; Yang, J.; Li, X.; Xu, J.; Chen, P.; Li, J.; Hu, X. GSTU43 gene involved in ALA-regulated redox homeostasis, to maintain coordinated chlorophyll synthesis of tomato at low temperature. BMC Plant Biol. 2019, 19, 323. [Google Scholar] [CrossRef]
- Lavrova, V.V.; Zinovieva, S.V.; Udalova, Z.V.; Matveeva, E.M. Expression of PR genes in tomato tissues infected by nematode Meloidogyne incognita (Kofoid et White, 1919) Chitwood, 1949. Dokl. Biochem. Biophys. 2017, 476, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Ibanez, S.; Boter, M.; Ortigosa, A.; Garcia-Casado, G.; Chini, A.; Lewsey, M.G.; Ecker, J.R.; Ntoukakis, V.; Solano, R. JAZ2 controls stomata dynamics during bacterial invasion. New Phytol. 2017, 213, 1378–1392. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chong, P.; Zhao, M.; Liu, H. Physiological response and proteomics analysis of Reaumuria soongorica under salt stress. Sci. Rep. 2022, 12, 2539. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Postel, S.; Kemmerling, B.; Ludewig, U. Altered growth and improved resistance of Arabidopsis against Pseudomonas syringae by overexpression of the basic amino acid transporter AtCAT1. Plant Cell Environ. 2014, 37, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Damari-Weissler, H.; Rachamilevitch, S.; Aloni, R.; German, M.A.; Cohen, S.; Zwieniecki, M.A.; Michele, H.N.; Granot, D. LeFRK2 is required for phloem and xylem differentiation and the transport of both sugar and water. Planta 2009, 230, 795–805. [Google Scholar] [CrossRef]
- Li, F.; Chen, X.; Zhou, S.; Xie, Q.; Wang, Y.; Xiang, X.; Hu, Z.; Chen, G. Overexpression of SlMBP22 in Tomato Affects Plant Growth and Enhances Tolerance to Drought Stress. Plant Sci. 2020, 301, 110672. [Google Scholar]
- Tokumaru, M.; Adachi, F.; Toda, M.; Ito-Inaba, Y.; Yazu, F.; Hirosawa, Y.; Sakakibara, Y.; Suiko, M.; Kakizaki, T.; Inaba, T. Ubiquitin-Proteasome Dependent Regulation of the GOLDEN2-LIKE 1 Transcription Factor in Response to Plastid Signals. Plant Physiol. 2017, 173, 524–535. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, S.; Chen, J.; Zhu, X.; Zhou, X.; Hortensteiner, S.; Ren, G.; Kuai, B. The C-terminal cysteine-rich motif of NYE1/SGR1 is indispensable for its function in chlorophyll degradation in Arabidopsis. Plant Mol. Biol. 2019, 101, 257–268. [Google Scholar] [CrossRef]
- Rajani, S.; Sundaresan, V. The Arabidopsis myc/bHLH gene ALCATRAZ enables cell separation in fruit dehiscence. Curr. Biol. 2001, 11, 1914–1922. [Google Scholar] [CrossRef]
- Quiroga, M.; Guerrero, C.; Botella, M.A.; Barcelo, A.; Amaya, I.; Medina, M.I.; Alonso, F.J.; de Forchetti, S.M.; Tigier, H.; Valpuesta, V. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol. 2000, 122, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- van der Rest, B.; Danoun, S.; Boudet, A.M.; Rochange, S.F. Down-regulation of cinnamoyl-CoA reductase in tomato (Solanum lycopersicum L.) induces dramatic changes in soluble phenolic pools. J. Exp. Bot. 2006, 57, 1399–1411. [Google Scholar] [CrossRef]
- Lulai, E.C.; Suttle, J.C. Signals involved in tuber wound-healing. Plant Signal. Behav. 2009, 4, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Leide, J.; Hildebrandt, U.; Hartung, W.; Riederer, M.; Vogg, G. Abscisic acid mediates the formation of a suberized stem scar tissue in tomato fruits. New Phytol. 2012, 194, 402–415. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, F.; Li, B.; Guo, C.; Hu, T.; Zhang, M.; Liang, Y.; Zhu, J.; Zhan, X. A bHLH transcription factor, SlbHLH96, promotes drought tolerance in tomato. Hortic. Res.-Engl. 2022, 9, uhac198. [Google Scholar]
- Cordeiro, A.M.; Andrade, L.; Monteiro, C.C.; Leitao, G.; Wigge, P.A.; Saibo, N. PHYTOCHROME-INTERACTING FACTORS: A promising tool to improve crop productivity. J. Exp. Bot. 2022, 73, 3881–3897. [Google Scholar] [PubMed]
- Vaistij, F.E.; Gan, Y.; Penfield, S.; Gilday, A.D.; Dave, A.; He, Z.; Josse, E.M.; Choi, G.; Halliday, K.J.; Graham, I.A. Differential control of seed primary dormancy in Arabidopsis ecotypes by the transcription factor SPATULA. Proc. Natl. Acad. Sci. USA 2013, 110, 10866–10871. [Google Scholar] [CrossRef]
- Jian, W.; Zheng, Y.; Yu, T.; Cao, H.; Chen, Y.; Cui, Q.; Xu, C.; Li, Z. SlNAC6, A NAC transcription factor, is involved in drought stress response and reproductive process in tomato. J. Plant Physiol. 2021, 264, 153483. [Google Scholar] [CrossRef]
- Mega, R.; Abe, F.; Kim, J.S.; Tsuboi, Y.; Tanaka, K.; Kobayashi, H.; Sakata, Y.; Hanada, K.; Tsujimoto, H.; Kikuchi, J.; et al. Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors. Nat. Plants 2019, 5, 153–159. [Google Scholar] [CrossRef]
- Shinde, S.; Villamor, J.G.; Lin, W.; Sharma, S.; Verslues, P.E. Proline Coordination with Fatty Acid Synthesis and Redox Metabolism of Chloroplast and Mitochondria. Plant Physiol. 2016, 172, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- Zarattini, M.; Forlani, G. Toward Unveiling the Mechanisms for Transcriptional Regulation of Proline Biosynthesis in the Plant Cell Response to Biotic and Abiotic Stress Conditions. Front. Plant Sci. 2017, 8, 927. [Google Scholar]
- Lopez-Delacalle, M.; Camejo, D.M.; Garcia-Marti, M.; Nortes, P.A.; Nieves-Cordones, M.; Martinez, V.; Rubio, F.; Mittler, R.; Rivero, R.M. Using Tomato Recombinant Lines to Improve Plant Tolerance to Stress Combination through a More Efficient Nitrogen Metabolism. Front. Plant Sci. 2019, 10, 1702. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, S.; Liu, K.; Wang, S.; Huang, L.; Guo, L. Proteomics: A powerful tool to study plant responses to biotic stress. Plant Methods 2019, 15, 135. [Google Scholar] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Ortiz-Ramirez, C.I.; Giraldo, M.A.; Ferrandiz, C.; Pabon-Mora, N. Expression and function of the bHLH genes ALCATRAZ and SPATULA in selected Solanaceae species. Plant J. 2019, 99, 686–702. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Ditta, G.S.; Eshed, Y.; Savidge, B.; Bowman, J.L.; Yanofsky, M.F. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 2000, 404, 766–770. [Google Scholar] [CrossRef]
- Gimenez, E.; Pineda, B.; Capel, J.; Anton, M.T.; Atares, A.; Perez-Martin, F.; Garcia-Sogo, B.; Angosto, T.; Moreno, V.; Lozano, R. Functional analysis of the Arlequin mutant corroborates the essential role of the Arlequin/TAGL1 gene during reproductive development of tomato. PLoS ONE 2010, 5, e14427. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Tang, B.; Wu, T.; Chen, G.; Xie, Q.; Hu, Z. Silencing of SlMYB55 affects plant flowering and enhances tolerance to drought and salt stress in tomato. Plant Sci. 2022, 316, 111166. [Google Scholar]
- Khare, T.; Srivastava, A.K.; Suprasanna, P.; Kumar, V. Individual and additive stress impacts of Na+ and Cl− on proline metabolism and nitrosative responses in rice. Plant Physiol. Biochem. 2020, 152, 44–52. [Google Scholar] [CrossRef]
- Sedmak, J.J.; Grossberg, S.E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal. Biochem. 1977, 79, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Matvieieva, N.; Shakhovsky, A.; Tashyreva, H.; Ratushnyak, Y.; Duplij, V.; Bohdanovych, T.; Kuchuk, M. Study of Superoxide Dismutase Activity in Long-Term Cultivated Artemisia and Althaea “hairy” Roots. Curr. Microbiol. 2021, 79, 14. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Ma, X.; Shah, S.; Wu, X.; Shaheen, A.; Xiao, L.; Wu, Y.; Wang, S. Drought-hardening improves drought tolerance in Nicotiana tabacum at physiological, biochemical, and molecular levels. BMC Plant Biol. 2020, 20, 486. [Google Scholar] [CrossRef]
- Zhou, S.; Cheng, X.; Li, F.; Feng, P.; Hu, G.; Chen, G.; Xie, Q.; Hu, Z. Overexpression of SlOFP20 in Tomato Affects Plant Growth, Chlorophyll Accumulation, and Leaf Senescence. Front. Plant Sci. 2019, 10, 1510. [Google Scholar]
- Shao, G.C.; Wang, M.H.; Liu, N.; Yuan, M.; Kumar, P.; She, D.L. Growth and comprehensive quality index of tomato under rain shelters in response to different irrigation and drainage treatments. Sci. World J. 2014, 2014, 457937. [Google Scholar] [CrossRef]
- Fryer, M.J.; Oxborough, K.; Mullineaux, P.M.; Baker, N.R. Imaging of photo-oxidative stress responses in leaves. J. Exp. Bot. 2002, 53, 1249–1254. [Google Scholar]
- Bournonville, C.F.; Diaz-Ricci, J.C. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem. Anal. 2011, 22, 268–271. [Google Scholar] [CrossRef]
- Tan, T.; Li, Y.; Tang, B.; Chen, Y.; Chen, X.; Xie, Q.; Hu, Z.; Chen, G. Knockout of SlALKBH2 weakens the DNA damage repair ability of tomato. Plant Sci. 2022, 319, 111266. [Google Scholar]
- Chen, G.; Hackett, R.; Walker, D.; Taylor, A.; Lin, Z.; Grierson, D. Identification of a specific isoform of tomato lipoxygenase (TomloxC) involved in the generation of fatty acid-derived flavor compounds. Plant Physiol. 2004, 136, 2641–2651. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, J.; Xie, Q.; Gong, J.; Shen, H.; Chen, Y.; Chen, G.; Hu, Z. The basic helix-loop-helix transcription factor bHLH95 affects fruit ripening and multiple metabolisms in tomato. J. Exp. Bot. 2020, 71, 6311–6327. [Google Scholar] [CrossRef]
- Exposito-Rodriguez, M.; Borges, A.A.; Borges-Perez, A.; Perez, J.A. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol. 2008, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, M.; Ren, L.; Li, A.; Chen, G.; Hu, Z. The SlFSR gene controls fruit shelf-life in tomato. J. Exp. Bot. 2018, 69, 2897–2909. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Gerova, D.; Chervenkov, T.; Yankova, T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J. Ethnopharmacol. 2005, 96, 145–150. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Tu, Y.; Liao, C.; Guo, P.; Tian, Y.; Zhou, Y.; Xie, Q.; Chen, G.; Hu, Z. Overexpression of SlALC Increases Drought and Salt Tolerance and Affects Fruit Dehiscence in Tomatoes. Int. J. Mol. Sci. 2024, 25, 9433. https://doi.org/10.3390/ijms25179433
Gao Z, Tu Y, Liao C, Guo P, Tian Y, Zhou Y, Xie Q, Chen G, Hu Z. Overexpression of SlALC Increases Drought and Salt Tolerance and Affects Fruit Dehiscence in Tomatoes. International Journal of Molecular Sciences. 2024; 25(17):9433. https://doi.org/10.3390/ijms25179433
Chicago/Turabian StyleGao, Zihan, Yuqing Tu, Changguang Liao, Pengyu Guo, Yanling Tian, Ying Zhou, Qiaoli Xie, Guoping Chen, and Zongli Hu. 2024. "Overexpression of SlALC Increases Drought and Salt Tolerance and Affects Fruit Dehiscence in Tomatoes" International Journal of Molecular Sciences 25, no. 17: 9433. https://doi.org/10.3390/ijms25179433





