Systematic Identification and Characterization of O-Methyltransferase Gene Family Members Involved in Flavonoid Biosynthesis in Chrysanthemum indicum L.
Abstract
:1. Introduction
2. Results
2.1. Identification and Sequence Analysis of OMT Genes in C. indicum
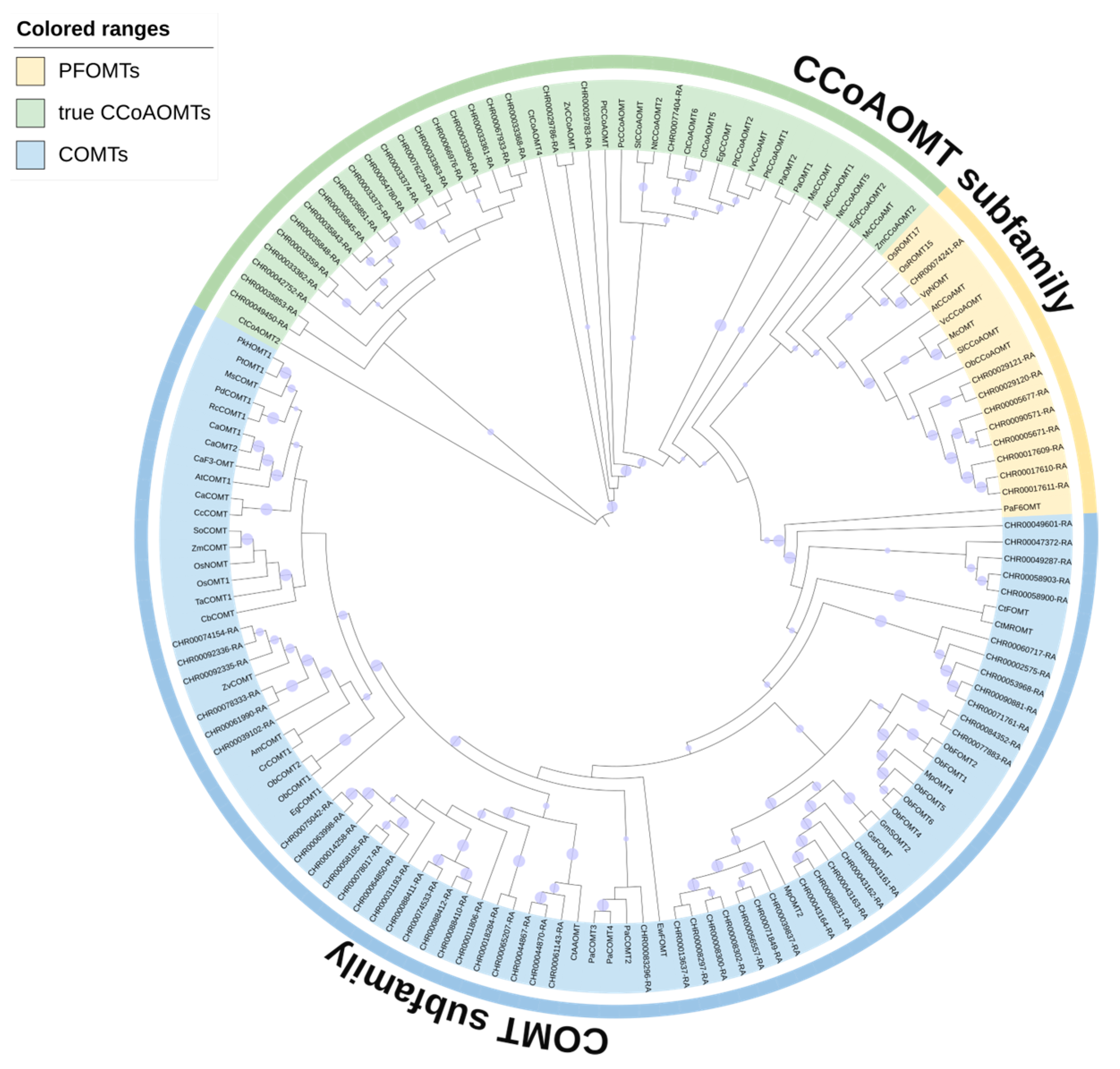
2.2. Phylogenetic Analysis of OMTs
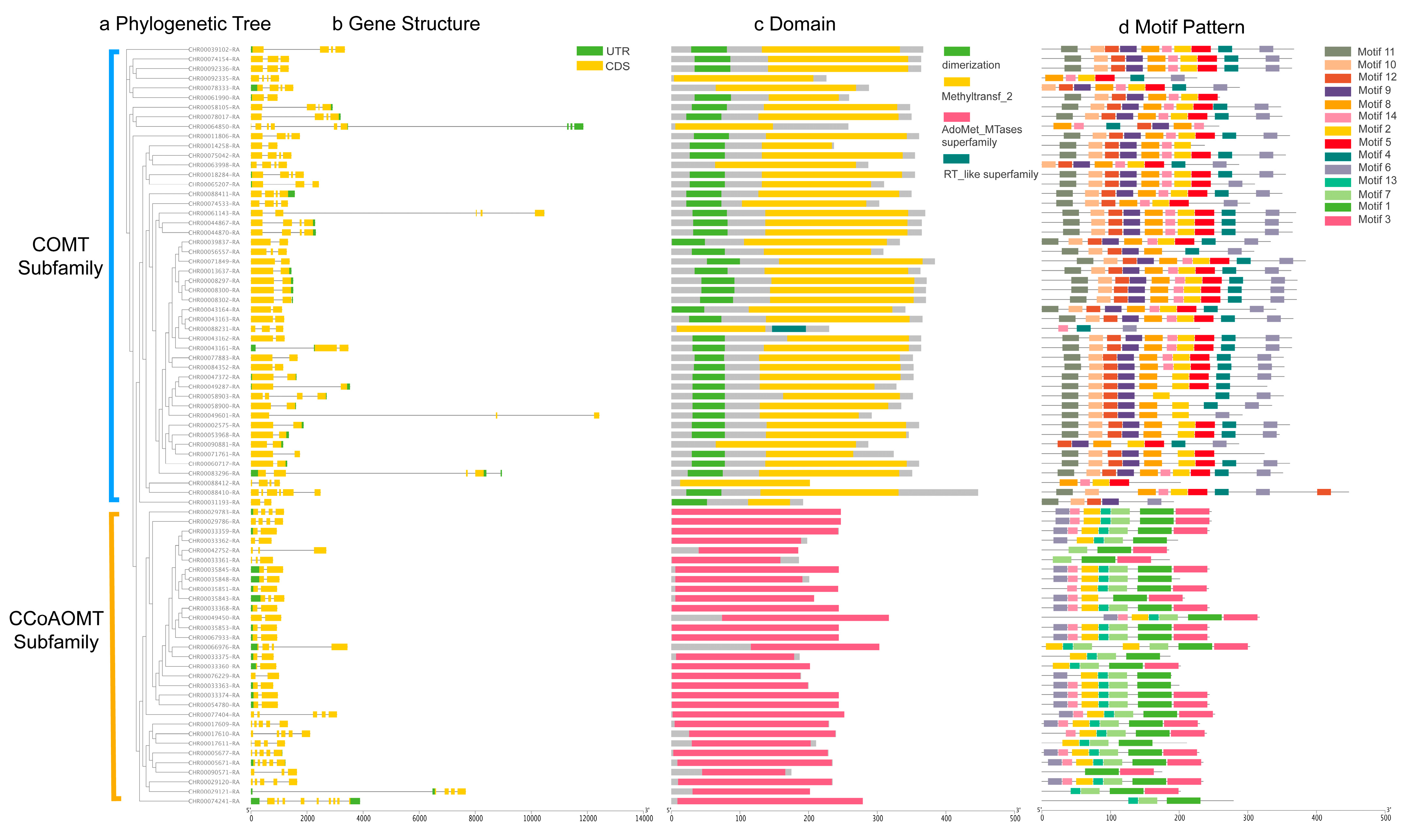
2.3. Gene Structure and Protein Conserved Motifs of C. indicum OMT Genes Family
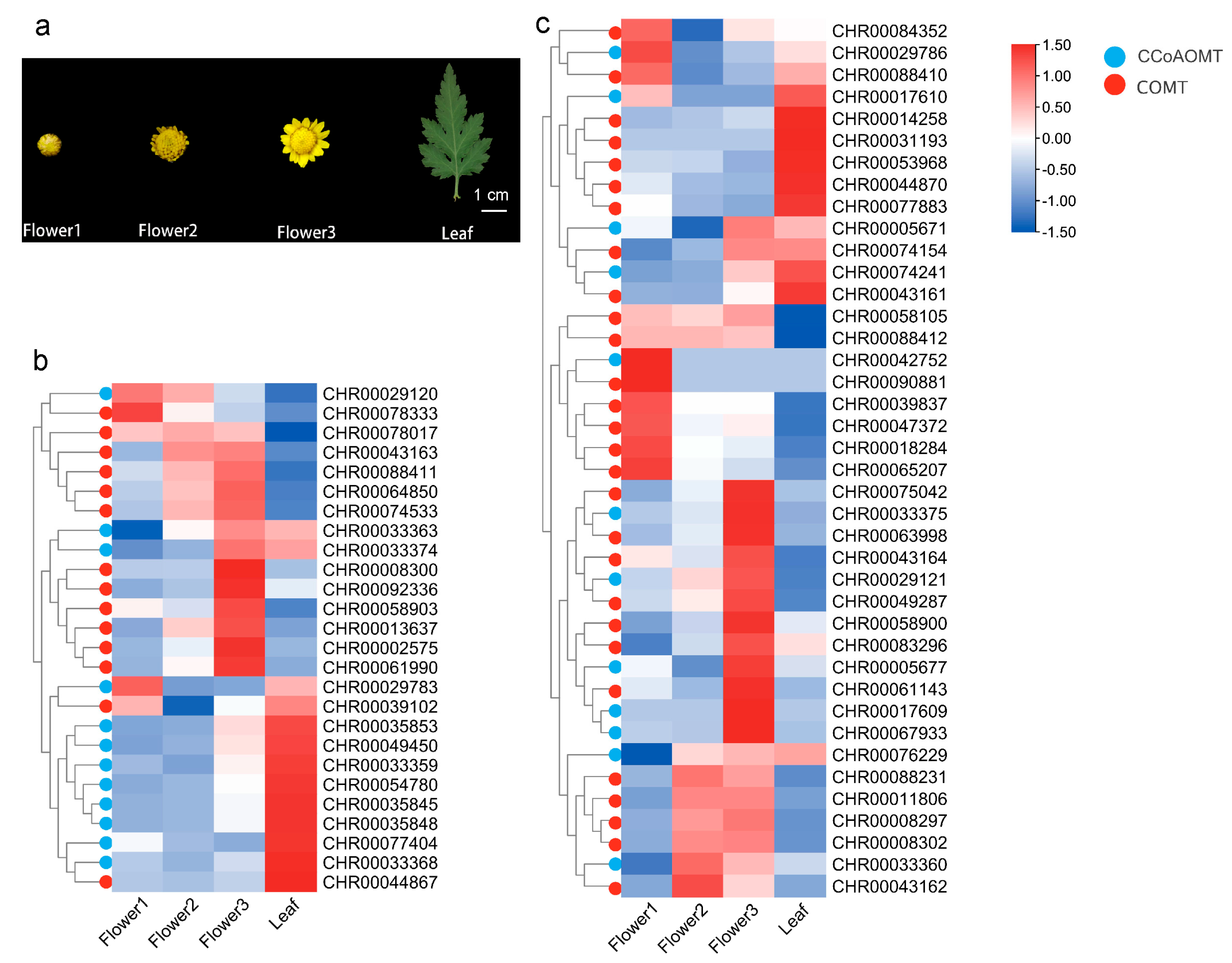
2.4. Expression Patterns of C. indicum OMTs Based on the RNA-seq Data
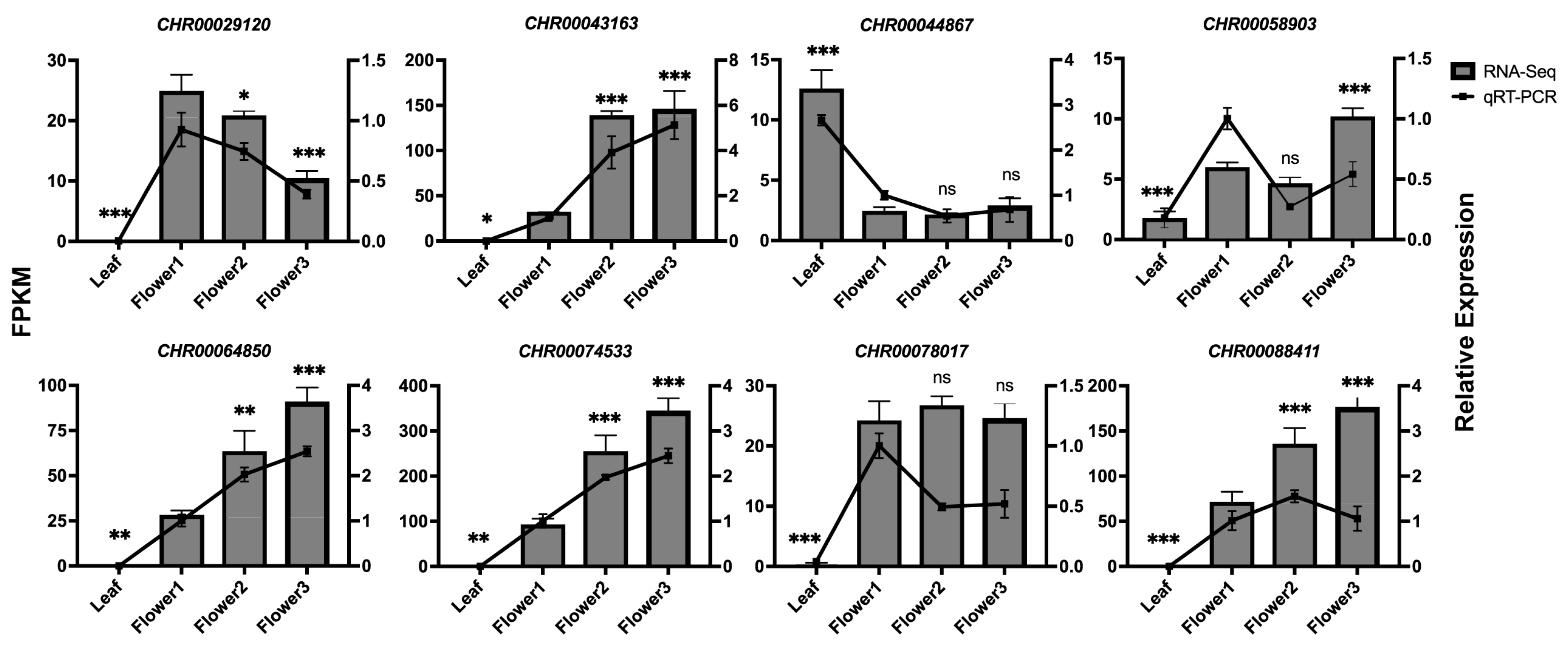
2.5. qRT-PCR Analysis of C. indicum OMTs Expression in Various Tissues
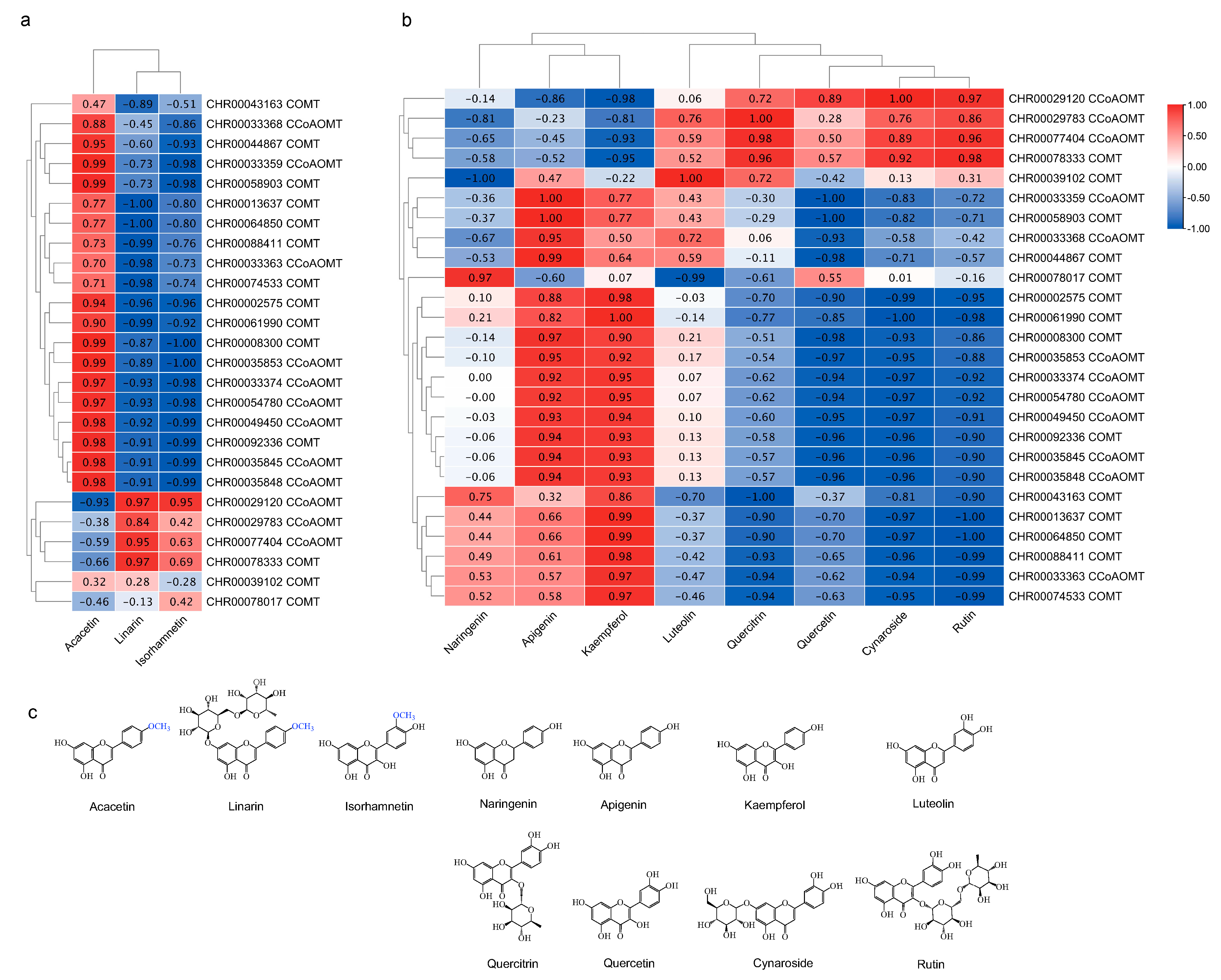
2.6. Identification of OMT Genes Involved in Flavonoids Synthesis during the Development of C. indicum capitulum
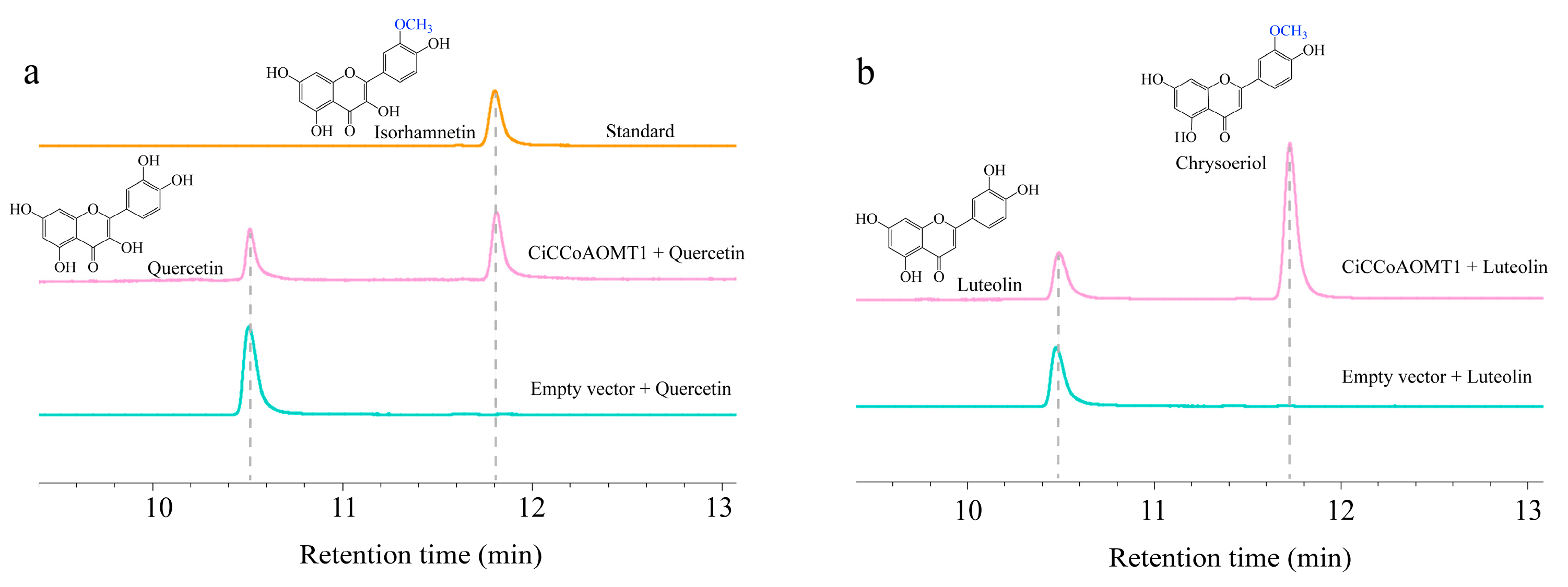
2.7. Heterogeneous Expression of CiCCoAOMT1 in E. coli and In Vitro Enzymatic Activity Assays
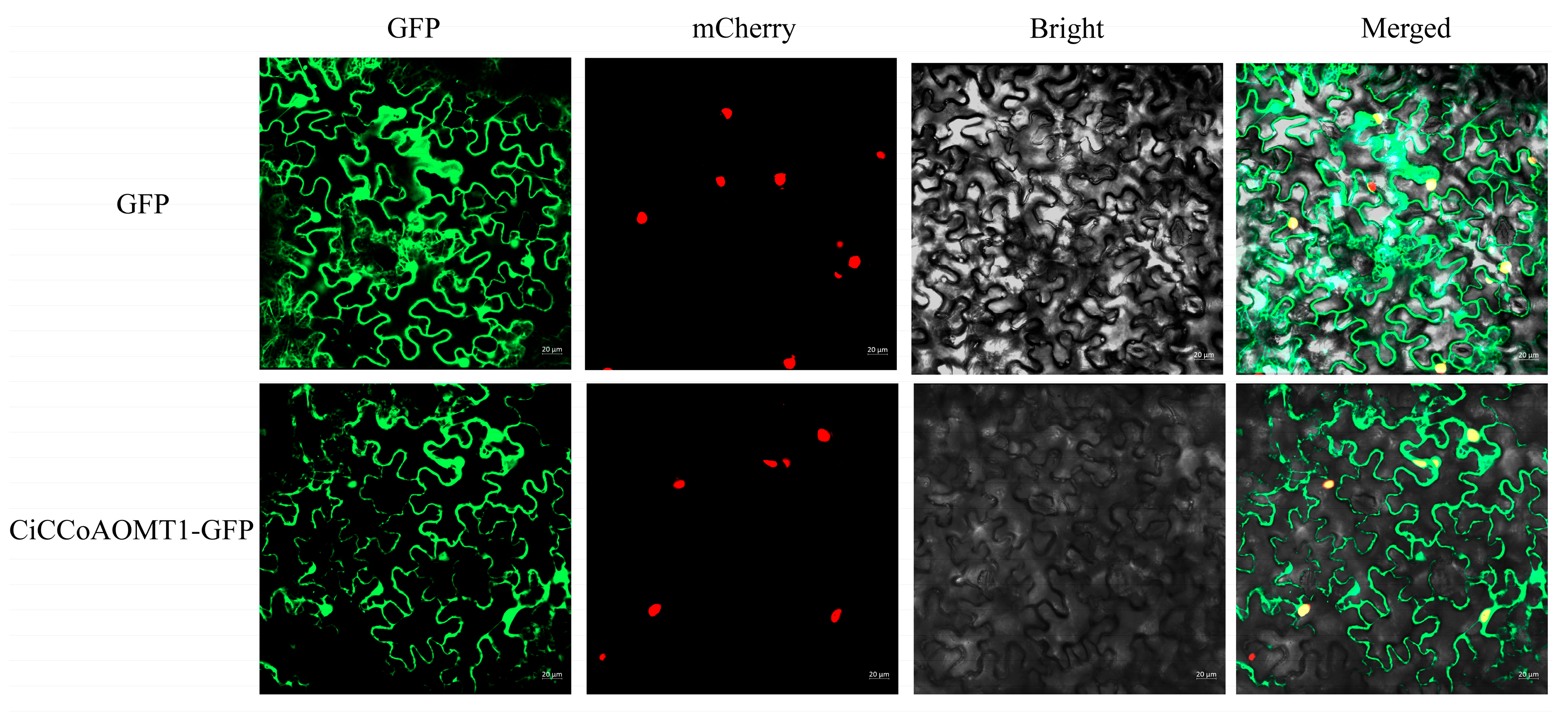
2.8. Subcellular Localization Analysis of CiCCoAOMT1 Protein
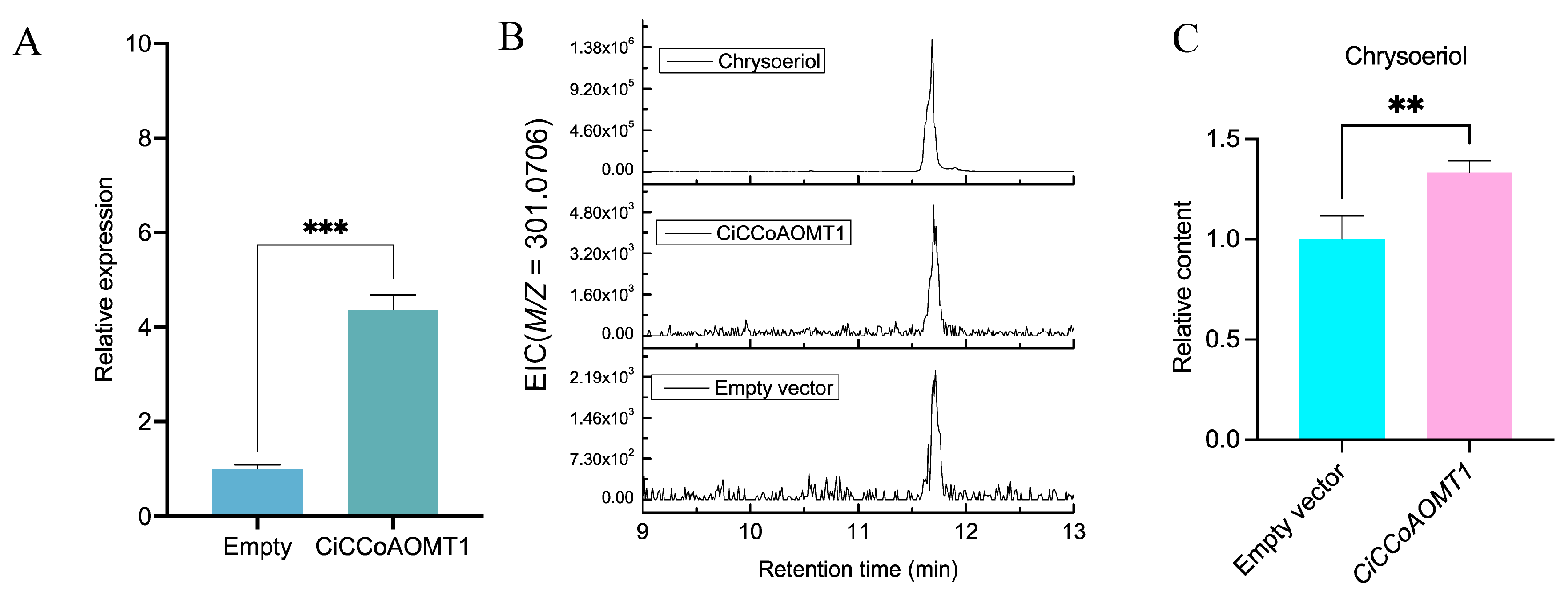
2.9. Overexpression of CiCCoAOMT1 in C. indicum
3. Discussion
3.1. Interspecific Divergence at the Scale of OMTs
3.2. Phylogenetic Relationship and Classification of OMTs
3.3. Tissue-Specificity in OMTs and Flavonoids Accumulation
3.4. CiCCoAOMT1 Is Involved in the Methylation of Flavonoids
4. Materials and Methods
4.1. Plant Materials
4.2. Acquisition and Analysis of Transcriptome Sequencing
4.3. Data Sources and Gene Identification of OMTs
4.4. Sequence Alignment and Phylogenetic Analysis
4.5. Exon–Intron Structure and Conserved Motif Analysis
4.6. Expression Analyses of C. indicum OMT Gene Family Members
4.7. Total RNA Extraction and qRT-PCR Analysis
4.8. Correlation Analysis
4.9. Heterologous Expression, and Purification of Recombinant CiCCoAOMT1 Proteins
4.10. Enzyme Assays and Analysis of C. indicum OMT Reaction Products
4.11. Subcellular Localization
4.12. Overexpression of CiCCoAOMT1 in C. indicum In Vivo
4.13. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Sun, Y.; Li, D.; Chen, Y. Chrysanthemum indicum L.: A Comprehensive Review of its Botany, Phytochemistry and Pharmacology. Am. J. Chin. Med. 2020, 48, 871–897. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jin, C.Y.; Kim, C.H.; Yoo, Y.H.; Choi, S.H.; Kim, G.Y.; Yoon, H.M.; Park, H.T.; Choi, Y.H. Isorhamnetin alleviates lipopolysaccharide-induced inflammatory responses in BV2 microglia by inactivating NF-kappaB, blocking the TLR4 pathway and reducing ROS generation. Int. J. Mol. Med. 2019, 43, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Rasquel-Oliveira, F.S.; Manchope, M.F.; Staurengo-Ferrari, L.; Ferraz, C.R.; Saraiva-Santos, T.; Zaninelli, T.H.; Fattori, V.; Artero, N.A.; Badaro-Garcia, S.; de Freitas, A.; et al. Hesperidin methyl chalcone interacts with NFkappaB Ser276 and inhibits zymosan-induced joint pain and inflammation, and RAW 264.7 macrophage activation. Inflammopharmacology 2020, 28, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Crisante, F.; Ginnasi, M.C. A convenient and safe O-methylation of flavonoids with dimethyl carbonate (DMC). Molecules 2011, 16, 1418–1425. [Google Scholar] [CrossRef]
- Wen, X.; Walle, T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispos. 2006, 34, 1786–1792. [Google Scholar] [CrossRef]
- Joshi, C.P.; Chiang, V.L. Conserved sequence motifs in plant S-adenosyl-L-methionine-dependent methyltransferases. Plant Mol. Biol. 1998, 37, 663–674. [Google Scholar] [CrossRef]
- Zubieta, C.; Kota, P.; Ferrer, J.-L.; Chen, J.; Dixon, R.A.; Noel, J.P. Structural basis for the modulation of lignin monomer methylation by caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase. Plant Cell 2002, 14, 1265–1277. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; Bruneau, A.; Bantignies, B. Plant O-methyltransferases: Molecular analysis, common signature and classification. Plant Mol. Biol. 1998, 36, 1–10. [Google Scholar] [CrossRef]
- Berim, A.; Gang, D.R. Methoxylated flavones: Occurrence, importance, biosynthesis. Phytochem. Rev. 2016, 15, 363–390. [Google Scholar] [CrossRef]
- Guo, D.; Chen, F.; Inoue, K.; Blount, J.W.; Dixon, R.A. Downregulation of caffeic acid 3-O methyltransferase and caffeoyl CoA 3-O-methyltransferase in transgenic alfalfa: Impacts on lignin structure and implications for the biosynthesis of G and S lignin. Plant Cell 2001, 13, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.H.; Kneusel, R.E.; Matern, U.; Varner, J.E. An alternative methylation pathway in lignin biosynthesis in Zinnia. Plant Cell 1994, 6, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Ibdah, M.; Zhang, X.H.; Schmidt, J.; Vogt, T. A novel Mg(2+)-dependent O-methyltransferase in the phenylpropanoid metabolism of Mesembryanthemum crystallinum. J. Biol. Chem. 2003, 278, 43961–43972. [Google Scholar] [CrossRef]
- Liao, Z.; Liu, X.; Zheng, J.; Zhao, C.; Wang, D.; Xu, Y.; Sun, C. A multifunctional true caffeoyl coenzyme A O-methyltransferase enzyme participates in the biosynthesis of polymethoxylated flavones in citrus. Plant Physiol. 2023, 192, 2049–2066. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yang, J.; Jiang, Y.; Sun, S.; Wei, X.; Wang, R.; Bu, J.; Li, D.; Kang, L.; Chen, T.; et al. Identification and characterization of two Isatis indigotica O-methyltransferases methylating C-glycosylflavonoids. Hortic. Res. 2022, 9, uhac140. [Google Scholar] [CrossRef]
- Xu, R.X.; Ni, R.; Gao, S.; Fu, J.; Xiong, R.L.; Zhu, T.T.; Lou, H.X.; Cheng, A.X. Molecular cloning and characterization of two distinct caffeoyl CoA O-methyltransferases (CCoAOMTs) from the liverwort Marchantia paleacea. Plant Sci. 2022, 314, 111102. [Google Scholar] [CrossRef]
- Sung, S.H.; Kim, B.-G.; Chong, Y.; Ahn, J.-H. Characterization of Phenylpropanoid O-Methyltransferase from Rice: Molecular Basis for the Different Reactivity Toward Different Substrates. J. Plant Biol. 2011, 54, 314–320. [Google Scholar] [CrossRef]
- Fellenberg, C.; Milkowski, C.; Hause, B.; Lange, P.R.; Bottcher, C.; Schmidt, J.; Vogt, T. Tapetum-specific location of a cation-dependent O-methyltransferase in Arabidopsis thaliana. Plant J. 2008, 56, 132–145. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Y.; Wu, H.; Xi, W.; Yu, J.; Zhang, Q.; Zhou, Z. Systematic analysis of O-methyltransferase gene family and identification of potential members involved in the formation of O-methylated flavonoids in Citrus. Gene 2016, 575, 458–472. [Google Scholar] [CrossRef]
- Zhao, K.; Yu, K.; Fu, X.; Zhao, X.; Xia, N.; Zhan, Y.; Zhao, X.; Han, Y.; Santalla, M. Genome-wide identification and expression profile analysis of the OMT gene family in response to cyst nematodes and multi-abiotic stresses in soybean. Crop Pasture Sci. 2022, 73, 1279–1290. [Google Scholar] [CrossRef]
- Xu, R.X.; Gao, S.; Zhao, Y.; Lou, H.X.; Cheng, A.X. Functional characterization of a Mg(2+)-dependent O-methyltransferase with coumarin as preferred substrate from the liverwort Plagiochasma appendiculatum. Plant Physiol. Biochem. 2016, 106, 269–277. [Google Scholar] [CrossRef]
- Berim, A.; Hyatt, D.C.; Gang, D.R. A set of regioselective O-methyltransferases gives rise to the complex pattern of methoxylated flavones in sweet basil. Plant Physiol. 2012, 160, 1052–1069. [Google Scholar] [CrossRef]
- Lu, S.; Zhuge, Y.; Hao, T.; Liu, Z.; Zhang, M.; Fang, J. Systematic analysis reveals O-methyltransferase gene family members involved in flavonoid biosynthesis in grape. Plant Physiol. Biochem. 2022, 173, 33–45. [Google Scholar] [CrossRef]
- Cai, T.; Sharif, Y.; Zhuang, Y.; Yang, Q.; Yang, Q.; Chen, X.; Chen, K.; Chen, Y.; Gao, M.; Dang, H.; et al. In-silico identification and characterization of O-methyltransferase gene family in peanut (Arachis hypogaea L.) reveals their putative roles in development and stress tolerance. Front. Plant Sci. 2023, 14, 1145624. [Google Scholar]
- Chen, J.H.; Li, X.M.; Yang, W.Y.; Liu, J. Research progress of plant O-methoxide flavonoids and O-methyltransferases. Nat. Prod. Res. Dev. 2021, 33, 1072–1079. [Google Scholar] [CrossRef]
- Umezawa, T.; Ragamustari, S.K.; Nakatsubo, T.; Wada, S.; Li, L.; Yamamura, M.; Sakakibara, N.; Hattori, T.; Suzuki, S.; Chiang, V.L. A lignan O-methyltransferase catalyzing the regioselective methylation of matairesinol in Carthamus tinctorius. Plant Biotechnol. 2013, 30, 97–109. [Google Scholar] [CrossRef]
- Liu, H.; Xu, R.-X.; Gao, S.; Cheng, A.-X. The Functional Characterization of a Site-Specific Apigenin 4′-O-methyltransferase Synthesized by the Liverwort Species Plagiochasma appendiculatum. Molecules 2017, 22, 759. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, L.; Zhang, H.; Du, X.; An, C.; Xia, X.; Chen, F.; Jiang, J.; Chen, S. CmMYB19 Over-Expression Improves Aphid Tolerance in Chrysanthemum by Promoting Lignin Synthesis. Int. J. Mol. Sci. 2017, 18, 619. [Google Scholar] [CrossRef]
- Lv, G.; Tang, D.; Chen, F.; Sun, Y.; Fang, W.; Guan, Z.; Liu, Z.; Chen, S. The anatomy and physiology of spray cut chrysanthemum pedicels, and expression of a caffeic acid 3-O-methyltransferase homologue. Postharvest. Biol. Technol. 2011, 60, 244–250. [Google Scholar] [CrossRef]
- Jiang, Y.; Ji, X.; Duan, L.; Ye, P.; Yang, J.; Zhan, R.; Chen, W.; Ma, D. Gene mining and identification of a flavone synthase II involved in flavones biosynthesis by transcriptomic analysis and targeted flavonoid profiling in Chrysanthemum indicum L. Ind. Crop. Prod. 2019, 134, 244–256. [Google Scholar] [CrossRef]
- Wu, Q.W.; Wei, M.; Feng, L.F.; Ding, L.; Wei, W.K.; Yang, J.F.; Lin, X.J.; Liang, H.L.; Zhan, R.T.; Ma, D.M. Rhamnosyltransferases involved in the biosynthesis of flavone rutinosides in Chrysanthemum species. Plant Physiol. 2022, 190, 2122–2136. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, Y.; Song, A.; Dong, G.; Zhao, H.; Sun, W.; Ramakrishnan, S.; Wang, Y.; Wang, S.; Li, T.; et al. The Chrysanthemum nankingense Genome Provides Insights into the Evolution and Diversification of Chrysanthemum Flowers and Medicinal Traits. Mol. Plant 2018, 11, 1482–1491. [Google Scholar] [CrossRef]
- Kim, B.G.; Sung, S.H.; Chong, Y.; Lim, Y.; Ahn, J.H. Plant flavonoid O. -methyltransferases: Substrate specificity and application. J. Plant Biol. 2010, 53, 321–329. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile C6-aldehydes and Allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol. 2005, 46, 1093–1102. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Lee, K.; Back, K. Caffeic acid O-methyltransferase is involved in the synthesis of melatonin by methylating N-acetylserotonin in Arabidopsis. J. Pineal Res. 2014, 57, 219–227. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.; Wang, L.; Ali, S.; Guo, Y.; Liu, J.; Wang, J.; Xie, L.; Zhang, Q. Genome-wide identification and characterization of caffeic acid O-Methyltransferase gene family in soybean. Plants 2021, 10, 2816. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Lee, H.J.; Park, Y.; Lim, Y.; Ahn, J.H. Characterization of an O-methyltransferase from soybean. Plant Physiol. Biochem. 2006, 44, 236–241. [Google Scholar] [CrossRef]
- Bhuiyan, N.H.; Selvaraj, G.; Wei, Y.; King, J. Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion. J. Exp. Bot. 2009, 60, 509–521. [Google Scholar] [CrossRef]
- Zou, Q.; Guo, Q.; Wang, T.; Chen, J.; Yang, F.; Yang, C. Comparison of metabolome characteristics and screening of chemical markers in Chrysanthemum indicum from different habitats. Physiol. Mol. Biol. Plants 2022, 28, 65–76. [Google Scholar] [CrossRef]
- Barakat, A.; Choi, A.; Yassin, N.B.M.; Park, J.S.; Sun, Z.; Carlson, J.E. Comparative genomics and evolutionary analyses of the O-methyltransferase gene family in Populus. Gene 2011, 479, 37–46. [Google Scholar] [CrossRef]
- Zhao, D.; Yao, Z.; Zhang, J.; Zhang, R.; Mou, Z.; Zhang, X.; Li, Z.; Feng, X.; Chen, S.; Reiter, R.J. Melatonin synthesis genes N-acetylserotonin methyltransferases evolved into caffeic acid O-methyltransferases and both assisted in plant terrestrialization. J. Pineal Res. 2021, 71, e12737. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.S.; Raes, J. Duplication and divergence: The evolution of new genes and old ideas. Annu. Rev. Genet. 2004, 38, 615–643. [Google Scholar] [CrossRef]
- Tuskan, G.A.; Difazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.C.; Purugganan, M.D. The early stages of duplicate gene evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 15682–15687. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ding, J.; Deng, D.; Tang, W.; Sun, H.; Liu, D.; Zhang, L.; Niu, X.; Zhang, X.; Meng, M.; et al. Draft genome of the kiwifruit Actinidia chinensis. Nat. Commun. 2013, 4, 2640. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, W.; Zhao, Y.; Ren, Y.; Zhao, X.; Yuan, Z. Genome-Wide Identification and Evolutionary Analysis of AOMT Gene Family in Pomegranate (Punica granatum). Agronomy 2021, 11, 318. [Google Scholar] [CrossRef]
- Lam, K.C.; Ibrahim, R.K.; Behdad, B.; Dayanandan, S. Structure, function, and evolution of plant O-methyltransferases. Genome 2007, 50, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Li, C.; Shi, F.; Jones, A.D.; Pichersky, E. Polymethylated myricetin in trichomes of the wild tomato species Solanum habrochaites and characterization of trichome-specific 3’/5’- and 7/4’-myricetin O-methyltransferases. Plant Physiol. 2011, 155, 1999–2009. [Google Scholar] [CrossRef]
- Liu, H.; Xu, R.X.; Zhang, X.S.; Zhu, T.T.; Lou, H.X.; Cheng, A.X. The identification and functional characterization of three liverwort class I O-methyltransferases. Phytochemistry 2019, 159, 190–198. [Google Scholar] [CrossRef]
- Berim, A.; Gang, D.R. Characterization of two candidate flavone 8-O-methyltransferases suggests the existence of two potential routes to nevadensin in sweet basil. Phytochemistry 2013, 92, 33–41. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, 427–432. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, 216–227. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, 585–587. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, 39–49. [Google Scholar] [CrossRef]
- Shen, G.M.; Jiang, H.B.; Wang, X.N.; Wang, J.J. Evaluation of endogenous references for gene expression profiling in different tissues of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). BMC Mol. Biol. 2010, 11, 76. [Google Scholar] [CrossRef]
- Gu, C.; Chen, S.; Liu, Z.; Shan, H.; Luo, H.; Guan, Z.; Chen, F. Reference gene selection for quantitative real-time PCR in Chrysanthemum subjected to biotic and abiotic stress. Mol. Biotechnol. 2011, 49, 192–197. [Google Scholar] [CrossRef]
| No. Motif | Logo | E-Value | Sites | Width |
|---|---|---|---|---|
| 1 |  | 1.3 × 10−1199 | 30 | 50 |
| 2 | 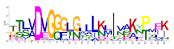 | 2.1 × 10−744 | 70 | 25 |
| 3 |  | 5.5 × 10−851 | 24 | 50 |
| 4 | 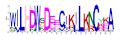 | 1.1 × 10−550 | 40 | 21 |
| 5 | 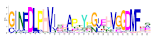 | 1.2 × 10−585 | 39 | 29 |
| 6 |  | 8.4 × 10−545 | 62 | 21 |
| 7 |  | 1.4 × 10−544 | 27 | 28 |
| 8 |  | 1.1 × 10−498 | 42 | 27 |
| 9 | 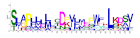 | 1.1 × 10−307 | 40 | 25 |
| 10 |  | 7.3 × 10−297 | 43 | 21 |
| 11 | 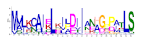 | 1.5 × 10−310 | 32 | 25 |
| 12 | 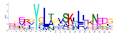 | 3.4 × 10−273 | 45 | 21 |
| 13 |  | 7.0 × 10−261 | 27 | 15 |
| 14 |  | 1.8 × 10−226 | 56 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, T.; Guo, Q.; Su, Y.; Yang, F. Systematic Identification and Characterization of O-Methyltransferase Gene Family Members Involved in Flavonoid Biosynthesis in Chrysanthemum indicum L. Int. J. Mol. Sci. 2024, 25, 10037. https://doi.org/10.3390/ijms251810037
Zhang M, Wang T, Guo Q, Su Y, Yang F. Systematic Identification and Characterization of O-Methyltransferase Gene Family Members Involved in Flavonoid Biosynthesis in Chrysanthemum indicum L. International Journal of Molecular Sciences. 2024; 25(18):10037. https://doi.org/10.3390/ijms251810037
Chicago/Turabian StyleZhang, Man, Tao Wang, Qiaosheng Guo, Yong Su, and Feng Yang. 2024. "Systematic Identification and Characterization of O-Methyltransferase Gene Family Members Involved in Flavonoid Biosynthesis in Chrysanthemum indicum L." International Journal of Molecular Sciences 25, no. 18: 10037. https://doi.org/10.3390/ijms251810037






