An Ingenane-Type Diterpene from Euphorbia kansui Promoted Cell Apoptosis and Macrophage Polarization via the Regulation of PKC Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. Bioactivity of Compound A from E. kansui
2.2. Inhibition of the Proliferation and Migration of HepG2 Cells via Inhibition of the PKC–ERK Signaling Pathways
2.3. Compound A Inhibited M1 Polarization of Macrophages
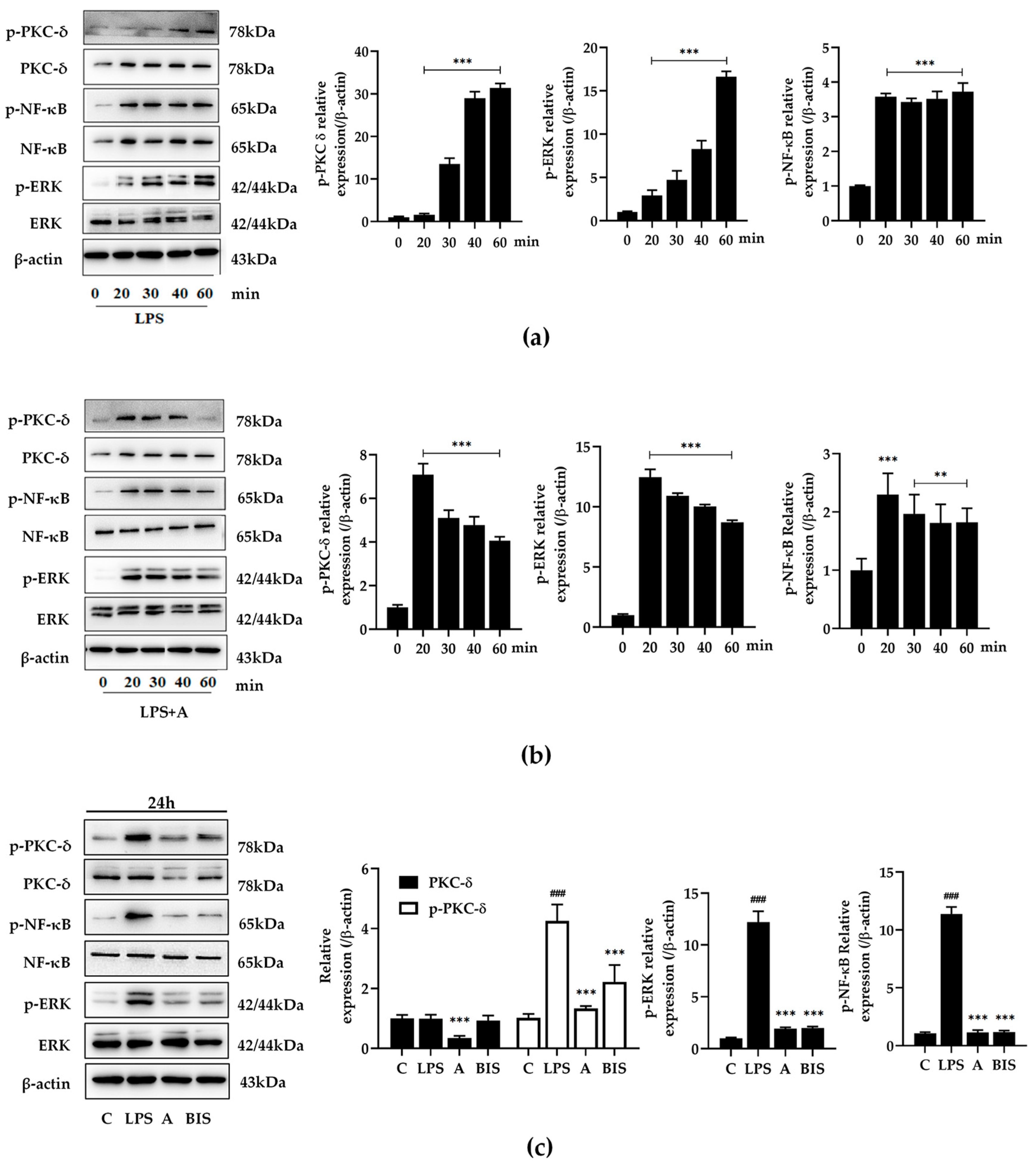
2.3.1. Compound A Inhibited PKC δ, ERK, and NF-κB Activation of M1 Macrophages
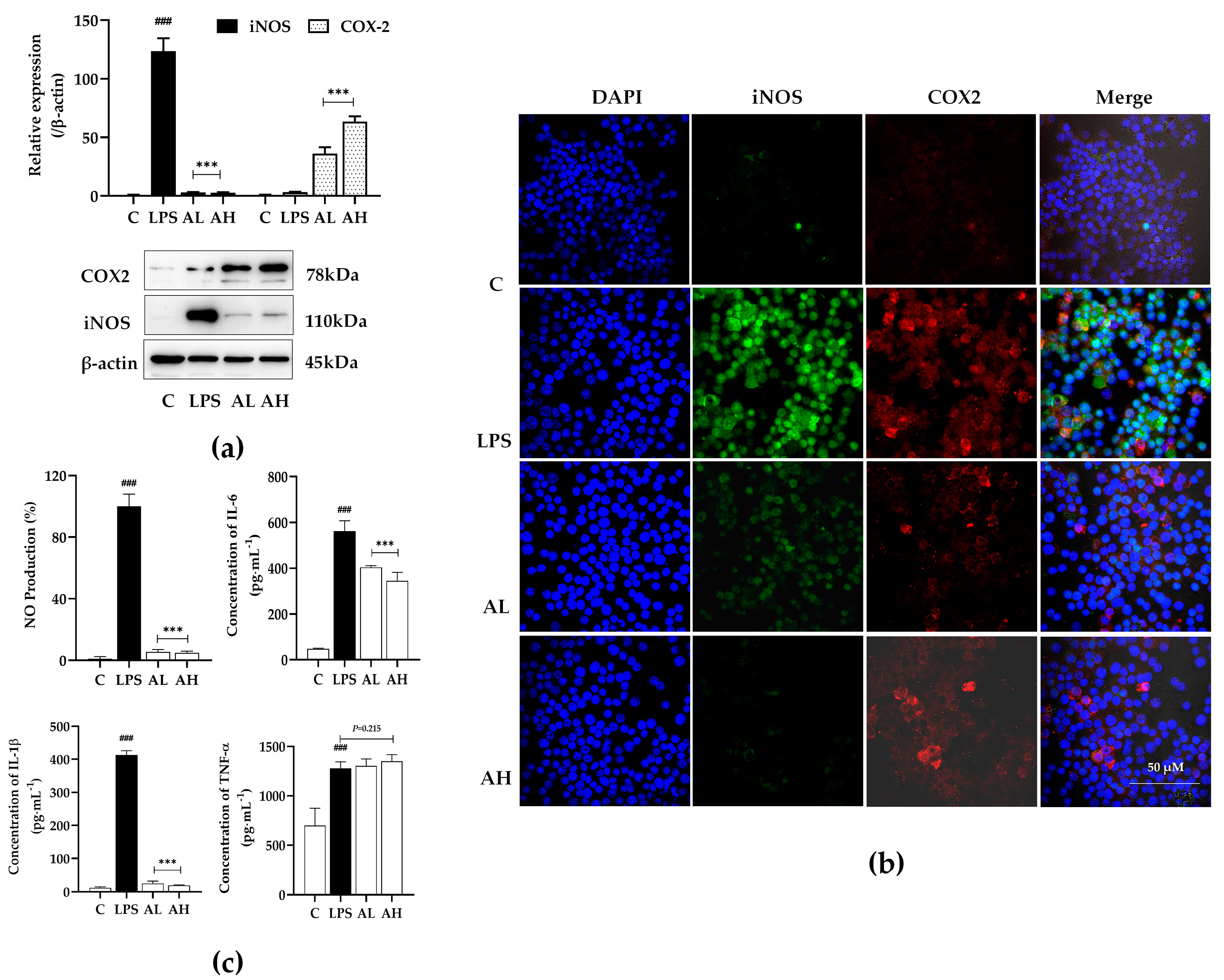
2.3.2. Compound A Showed Significant Anti-Inflammatory Activity on LPS-Induced Macrophages
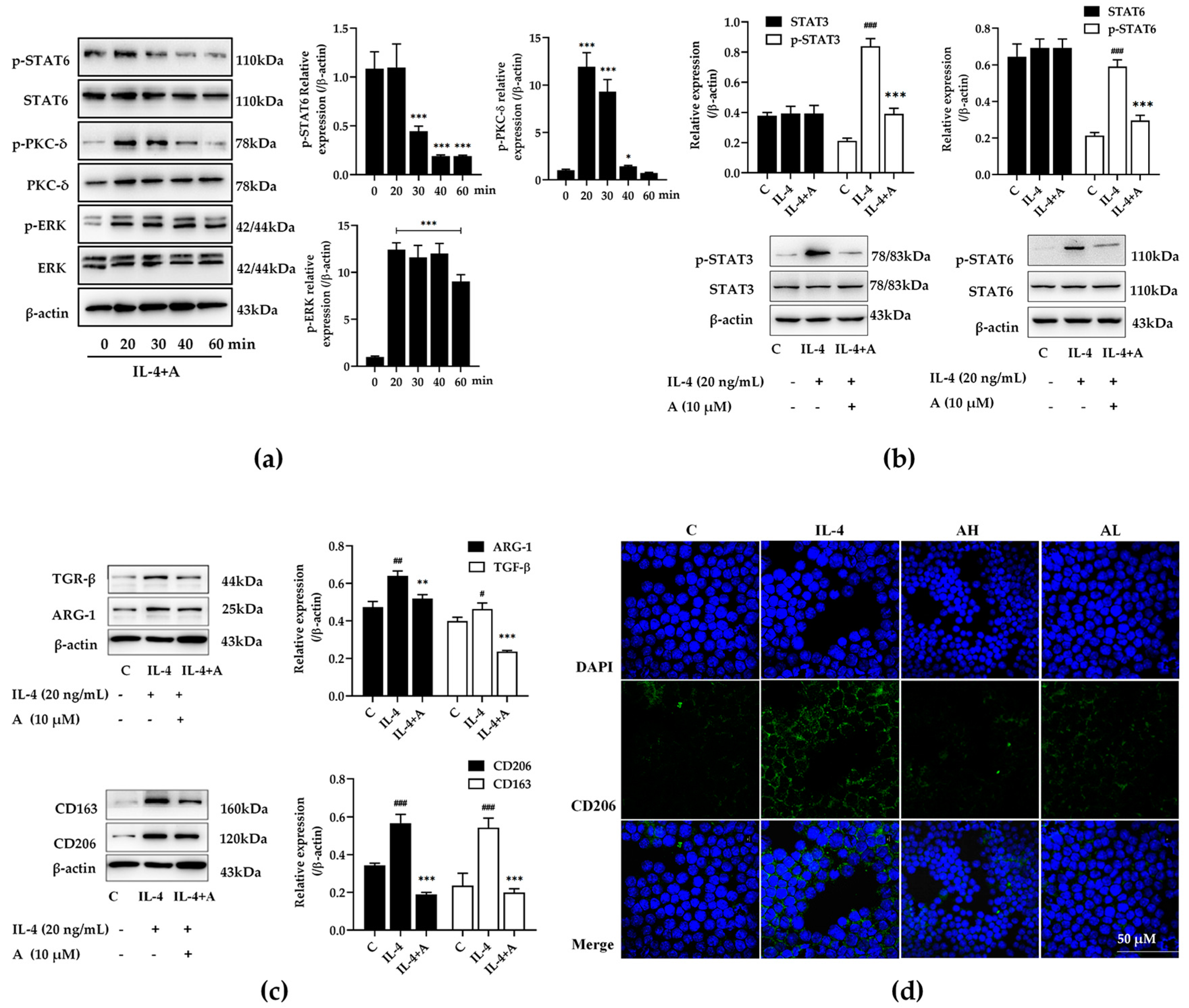
2.4. Compound A Inhibited Protein Expression in IL-4 Stimulated M2-Type Macrophages
2.5. Compound A Inhibited M2 Polarization of Macrophages under HepG2 and RAW264.7 Cells Co-Cultivation
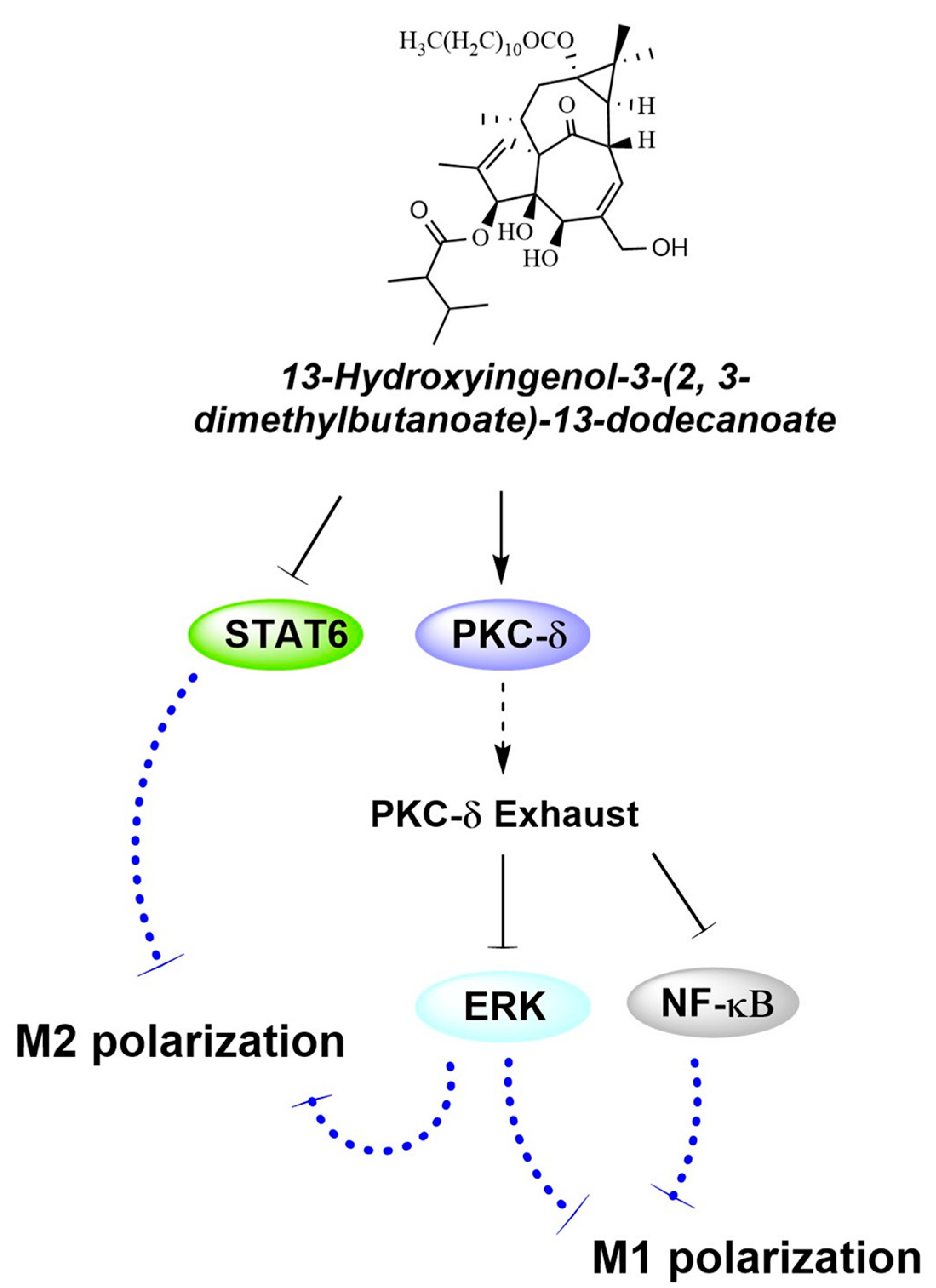
3. Discussion
4. Materials and Methods
4.1. Plant Extraction and Compound Isolation
4.2. Reagents
4.3. Cell Culture and Model Established
4.4. MTT Assay
4.5. Flow Cytometry
4.6. Fluorescence Staining
4.7. Measurement of Nitric Oxide and Cytokines
4.8. Western Blotting Analysis
4.9. Protein Kinase Inhibitor
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, X.Y.; Chen, B.C.; Li, J.C.; Li, J.M.; Li, H.M.; Chen, X.Q.; Liu, D.; Li, R.T. Gansui-Banxia Decoction extraction inhibits MDSCs accumulation via AKT/STAT3/ERK signaling pathways to regulate antitumor immunity in C57bl/6 mice. Phytomedicine 2021, 93, 153779. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Wang, T.; Li, M.; Li, N.; Chen, S.; Xiu, L.; Yu, X.; Liu, H.; Zhong, G. Gansui Banxia decoction modulates immune-inflammatory homeostasis to ameliorate malignant ascites in rats. Phytomedicine 2024, 125, 155246. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.H.; Wang, K.; Chai, T.; Guo, Z.Y.; Zhao, M.; Yang, J.L. Ingenane and jatrophane diterpenoids from Euphorbia kansui and their antiproliferative effects. Phytochemistry 2020, 172, 112257. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, J.; Liu, D.; Yang, T.; Chen, X.; Li, R. Ingenane and jatrophane-type diterpenoids from Euphorbia kansui with multidrug resistance reversal activity. Phytochemistry 2021, 188, 112775. [Google Scholar] [CrossRef]
- Chen, C.S.; Pan, B.Y.; Tsai, P.H.; Chen, F.Y.; Yang, W.C.; Shen, M.Y.; Kansuinine, A. Ameliorates Atherosclerosis and Human Aortic Endothelial Cell Apoptosis by Inhibiting Reactive Oxygen Species Production and Suppressing IKKbeta/IkappaBalpha/NF-kappaB Signaling. Int. J. Mol. Sci. 2021, 22, 10309. [Google Scholar] [CrossRef]
- Silva, V.A.O.; Rosa, M.N.; Miranda-Goncalves, V.; Costa, A.M.; Tansini, A.; Evangelista, A.F.; Martinho, O.; Carloni, A.C.; Jones, C.; Lima, J.P.; et al. Euphol, a tetracyclic triterpene, from Euphorbia tirucalli induces autophagy and sensitizes temozolomide cytotoxicity on glioblastoma cells. Investig. New Drugs 2019, 37, 223–237. [Google Scholar] [CrossRef]
- Wang, P.; Lu, P.; Qu, X.; Shen, Y.; Zeng, H.; Zhu, X.; Zhu, Y.; Li, X.; Wu, H.; Xu, J.; et al. Reactivation of HIV-1 from Latency by an Ingenol Derivative from Euphorbia Kansui. Sci. Rep. 2017, 7, 9451. [Google Scholar] [CrossRef]
- Oh, S.; Oh, H.W.; Lee, H.R.; Yoon, S.Y.; Oh, S.R.; Ko, Y.E.; Yoo, N.; Jeong, J.; Kim, J.W. Ingenane-type diterpene compounds from Euphorbia kansui modulate IFN-gamma production through NF-kappaB activation. J. Sci. Food Agric. 2016, 96, 2635–2640. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, J.W.; Kang, A.; Zhang, Q.; Zhou, S.K.; Bao, B.H.; Cao, Y.D.; Yao, W.F.; Tang, Y.P.; Zhang, L.; et al. ameliorate malignant ascites by modulating gut microbiota and related metabolic functions. J. Ethnopharmacol. 2020, 249, 112423. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.L.; Zhang, Y.; Wang, K.; Zhang, M.; Chen, P.D.; Yao, W.F.; Tang, Y.P.; Wu, J.H.; Zhang, L. Effect of the vinegar-process on chemical compositions and biological activities of Euphorbia kansui: A review. J. Ethnopharmacol. 2020, 252, 112557. [Google Scholar] [CrossRef]
- Hampson, P.; Chahal, H.; Khanim, F.; Hayden, R.; Mulder, A.; Assi, L.K.; Bunce, C.M.; Lord, J.M. PEP005, a selective small-molecule activator of protein kinase C, has potent antileukemic activity mediated via the delta isoform of PKC. Blood 2005, 106, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Ersvaer, E.; Kittang, A.O.; Hampson, P.; Sand, K.; Gjertsen, B.T.; Lord, J.M.; Bruserud, O. The protein kinase C agonist PEP005 (ingenol 3-angelate) in the treatment of human cancer: A balance between efficacy and toxicity. Toxins 2010, 2, 174–194. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Inokuchi, J.; Eto, M.; Murata, M.; Kang, J.H. Protein Kinase C (PKC) Isozymes as Diagnostic and Prognostic Biomarkers and Therapeutic Targets for Cancer. Cancers 2022, 14, 5425. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, F.; Wilkinson, M.; Baxter, E.; Brennan, D.J. Mitogen-Activated Protein Kinase (MAPK) and Obesity-Related Cancer. Int. J. Mol. Sci. 2020, 21, 1241. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, Y.; Xu, J.; Yang, M.; Chen, P.; Xu, W.; Zhao, J.; Geng, L.; Gong, S. PKN2 in colon cancer cells inhibits M2 phenotype polarization of tumor-associated macrophages via regulating DUSP6-Erk1/2 pathway. Mol. Cancer 2018, 17, 13. [Google Scholar] [CrossRef]
- Wang, W.; Qian, B.; Zhao, C.; Peng, M.; Liu, L.; Xie, M.; Peng, N.; He, Q.; Ying, S.; Zhu, Y.; et al. Sublytic C5b-9 Induces CCL3/4 Production and Macrophage Accumulation in Thy-1N Rats via PKC-alpha/p65/IRF-8 Axis. Int. J. Biol. Sci. 2022, 18, 3178–3193. [Google Scholar] [CrossRef]
- Simpson, R.U.; O’Connell, T.D.; Pan, Q.; Newhouse, J.; Somerman, M.J. Antisense oligonucleotides targeted against protein kinase Cbeta and CbetaII block 1,25-(OH)2D3-induced differentiation. J. Biol. Chem. 1998, 273, 19587–19591. [Google Scholar] [CrossRef]
- Li, J.-C.; Li, S.-Y.; Tang, J.-X.; Liu, D.; Feng, X.-Y.; Rao, K.-R.; Zhao, X.-D.; Li, H.-M.; Li, R.-T. Triterpenoids, steroids and other constituents from Euphorbia kansui and their anti-inflammatory and anti-tumor properties. Phytochemistry 2022, 204, 113449. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Huang, L.; Asada, Y.; Morris-Natschke, S.L.; Chen, C.H.; Lee, K.H.; Koike, K. Identification, structural modification, and dichotomous effects on human immunodeficiency virus type 1 (HIV-1) replication of ingenane esters from Euphorbia kansui. Eur. J. Med. Chem. 2018, 156, 618–627. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef]
- Wang, H.; Yung, M.M.H.; Ngan, H.Y.S.; Chan, K.K.L.; Chan, D.W. The Impact of the Tumor Microenvironment on Macrophage Polarization in Cancer Metastatic Progression. Int. J. Mol. Sci. 2021, 22, 6560. [Google Scholar] [CrossRef] [PubMed]
- Saeedifar, A.M.; Mosayebi, G.; Ghazavi, A.; Bushehri, R.H.; Ganji, A. Macrophage polarization by phytotherapy in the tumor microenvironment. Phytother. Res. 2021, 35, 3632–3648. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, D.; Hornia, A.; Devonish, W.; Pagano, M.; Foster, D.A. Activation of protein kinase C triggers its ubiquitination and degradation. Mol. Cell Biol. 1998, 18, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, S.; Wang, Y. Diannexin alleviates myocardial ischemia-reperfusion injury by orchestrating cardiomyocyte oxidative damage, macrophage polarization and fibrotic process by TLR4-NF-kB-mediated inactivation of NLRP3 inflammasome. Int. Immunopharmacol. 2024, 130, 111668. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.N.; Lee, J.W.; Ryu, H.W.; Lee, J.K.; Oh, E.S.; Kim, D.Y.; Ro, H.; Yoon, D.; Park, J.Y.; Hong, S.T.; et al. Black Ginseng Extract Exerts Potentially Anti-Asthmatic Activity by Inhibiting the Protein Kinase Ctheta-Mediated IL-4/STAT6 Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 11970. [Google Scholar] [CrossRef]
- Kloosterman, D.J.; Akkari, L. Macrophages at the interface of the co-evolving cancer ecosystem. Cell 2023, 186, 1627–1651. [Google Scholar] [CrossRef]
- Li, Z.; Li, D.; Chen, R.; Gao, S.; Xu, Z.; Li, N. Cell death regulation: A new way for natural products to treat osteoporosis. Pharmacol. Res. 2023, 187, 106635. [Google Scholar] [CrossRef]


| Drug | A549 IC50 (μM) | MCF-7 IC50 (μM) | HepG2 IC50 (μM) |
|---|---|---|---|
| Control | - | - | - |
| Adr 1 | 5.98 ± 2.06 | 3.31 ± 1.01 | 4.37 ± 1.21 |
| Compound A | 21.97 ± 5.01 | 27.12 ± 3.34 | 20.97 ± 4.53 |
| Compounds | RAW264.7 | |
|---|---|---|
| CC50 1 (μM) | IC50 2 (μM) | |
| Control | - | - |
| LPS 3 | - | - |
| Compound A | 20.34 ± 3.62 | 0.57 ± 0.07 |
| l-NMMA4 | >50 | 21.80 ± 3.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Wang, L.; Pu, L.; Li, J.; Li, H.; Liu, D.; Li, R. An Ingenane-Type Diterpene from Euphorbia kansui Promoted Cell Apoptosis and Macrophage Polarization via the Regulation of PKC Signaling Pathways. Int. J. Mol. Sci. 2024, 25, 10123. https://doi.org/10.3390/ijms251810123
Feng X, Wang L, Pu L, Li J, Li H, Liu D, Li R. An Ingenane-Type Diterpene from Euphorbia kansui Promoted Cell Apoptosis and Macrophage Polarization via the Regulation of PKC Signaling Pathways. International Journal of Molecular Sciences. 2024; 25(18):10123. https://doi.org/10.3390/ijms251810123
Chicago/Turabian StyleFeng, Xiaoyi, Lizhong Wang, Li Pu, Jianchun Li, Hongmei Li, Dan Liu, and Rongtao Li. 2024. "An Ingenane-Type Diterpene from Euphorbia kansui Promoted Cell Apoptosis and Macrophage Polarization via the Regulation of PKC Signaling Pathways" International Journal of Molecular Sciences 25, no. 18: 10123. https://doi.org/10.3390/ijms251810123








