Synthesis and Mechanism of a Green Scale and Corrosion Inhibitor
Abstract
:1. Introduction
2. Results and Discussion
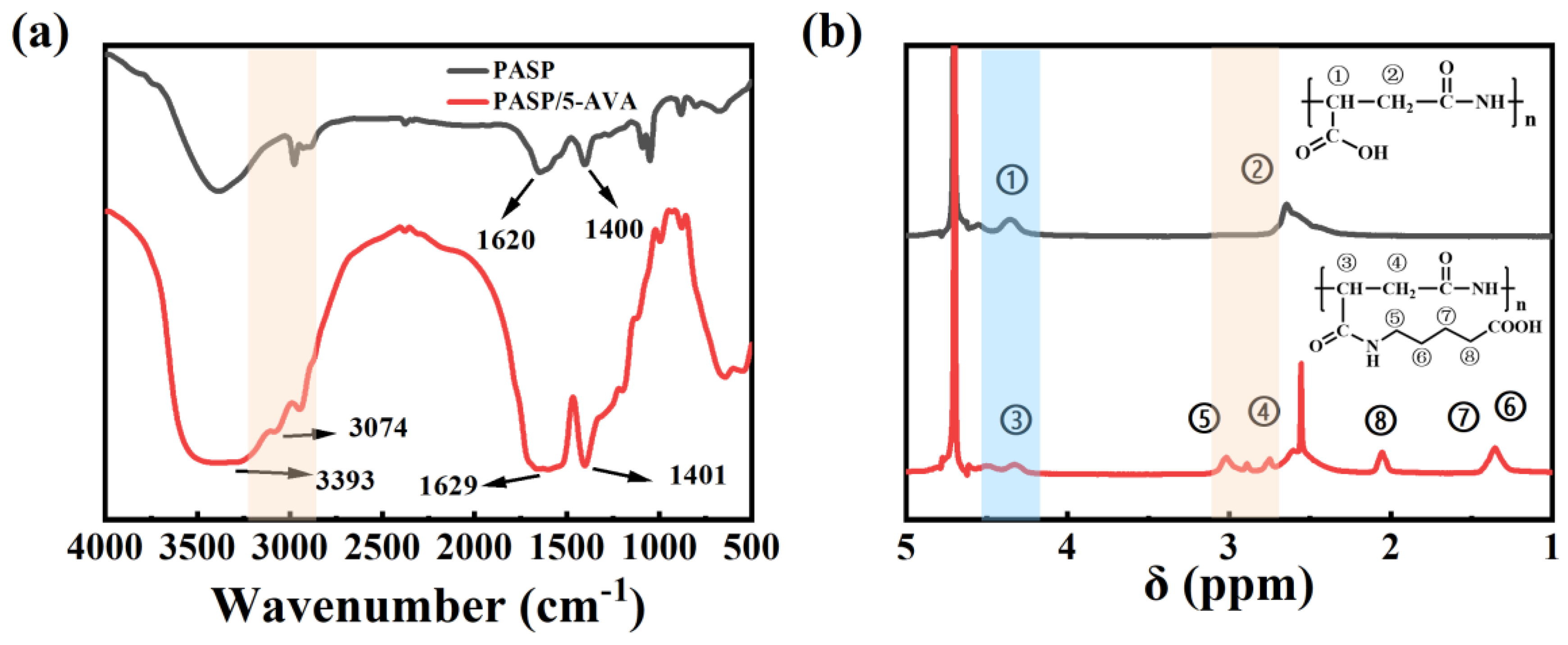
2.1. Structural Characterization of PASP/5–AVA
2.1.1. The Results Analysis of FT-IR and 1H-NMR
2.1.2. Gel Chromatography (GPC) Analysis
2.1.3. Zeta Potential Analysis
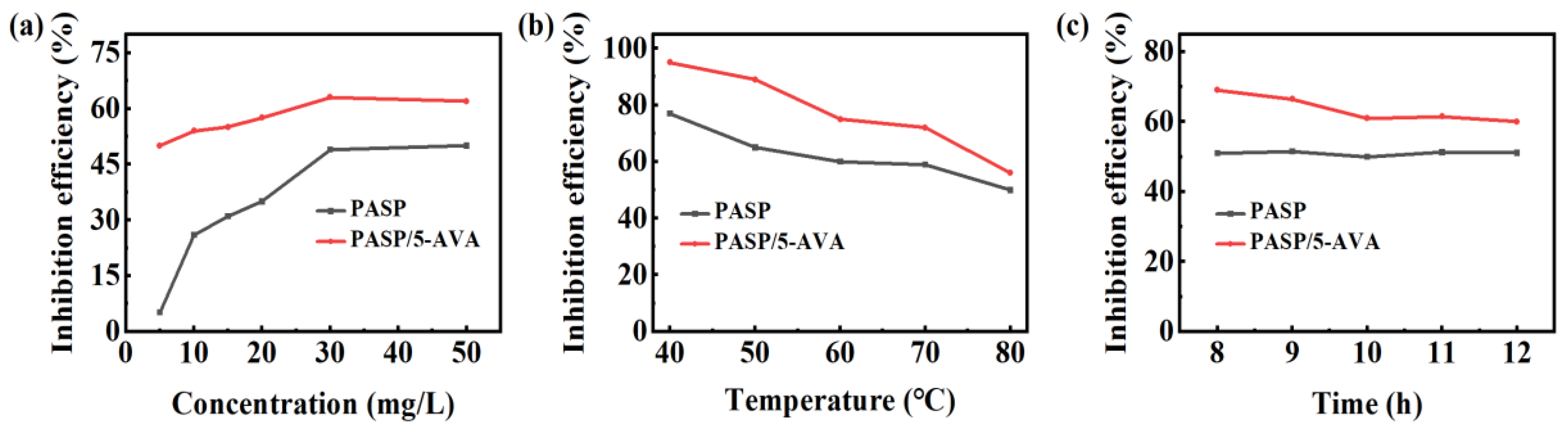
2.2. Scale Inhibition Results Analysis of PASP/5–AVA
2.2.1. Analysis of CaCO3 Scale Results
2.2.2. Analysis of CaSO4 Scale Results
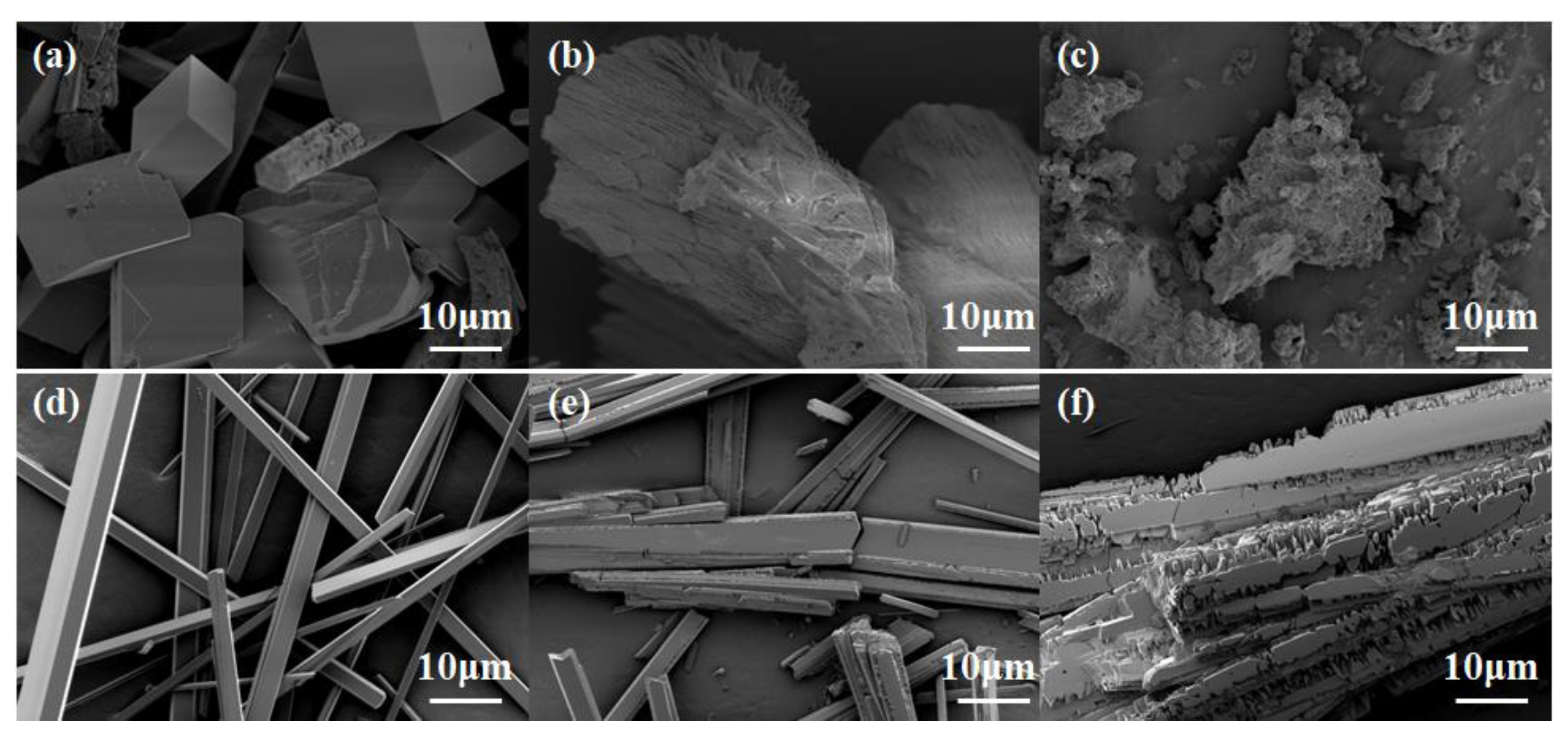
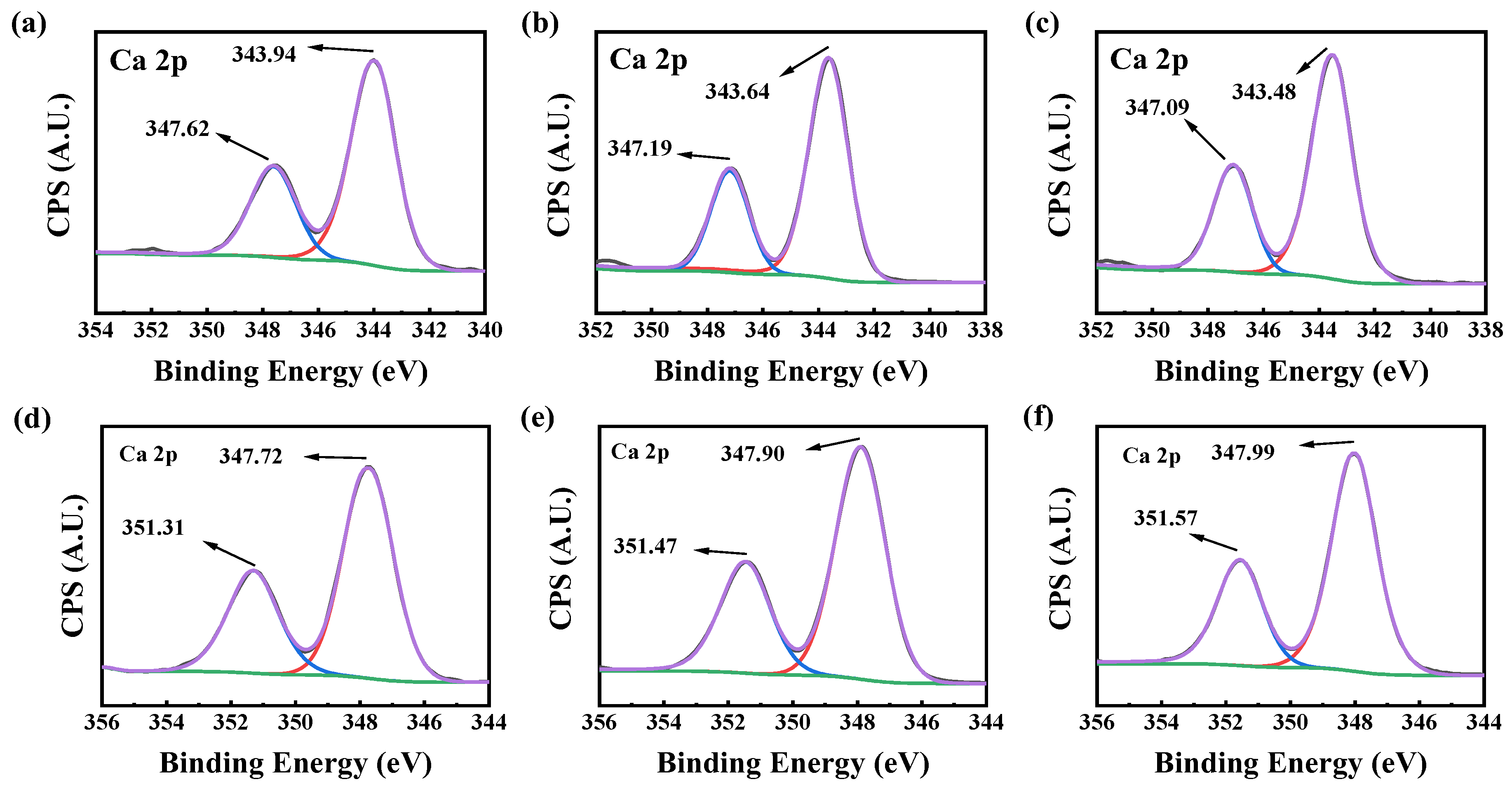
2.3. Calcium Scale Analysis
2.3.1. SEM Analysis of Calcium Scales
2.3.2. XRD Analysis of Calcium Scales
2.3.3. XPS Analysis of Calcium Scales
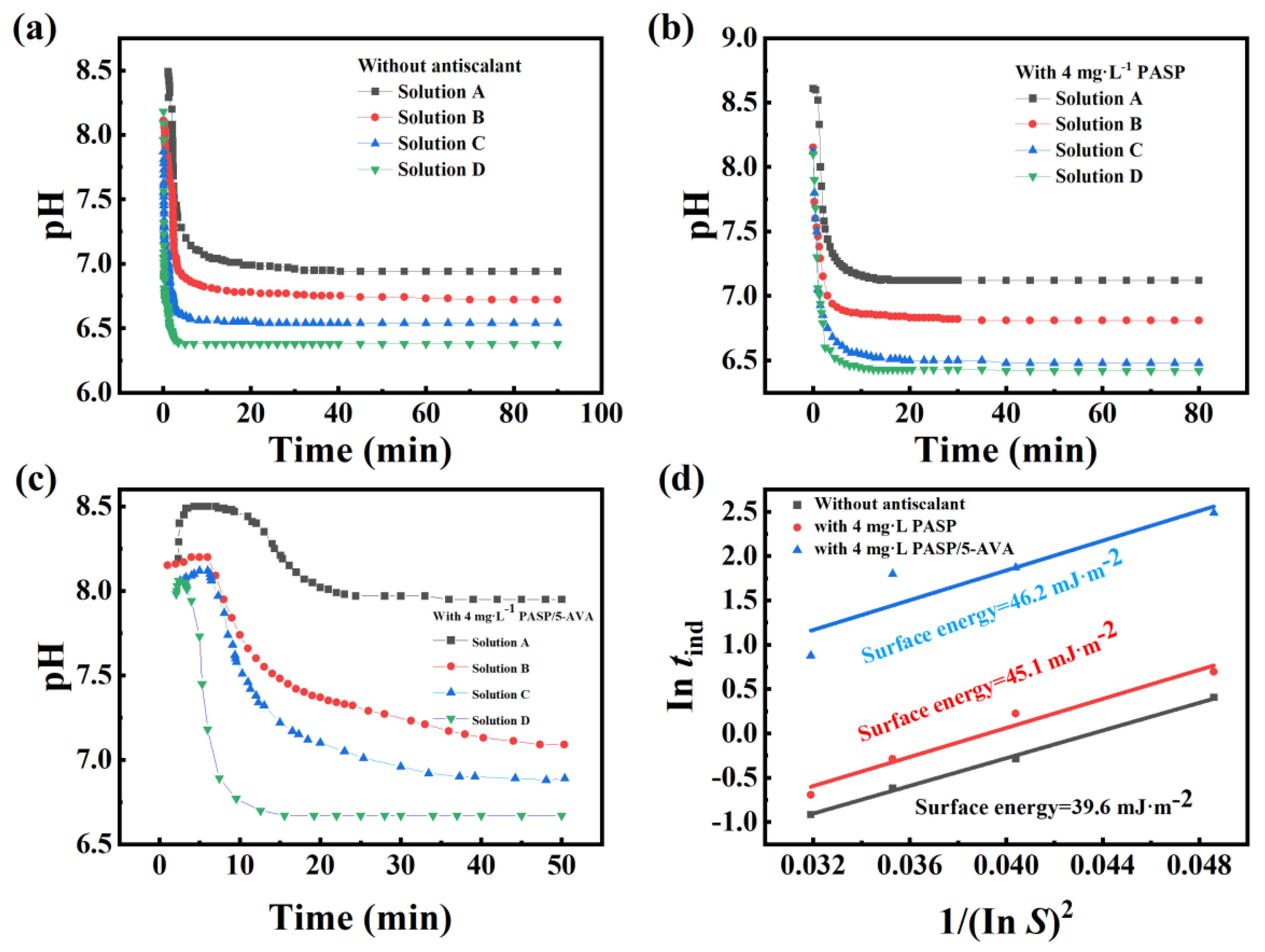
2.3.4. Surface Energy Analysis of CaCO3 Scale
2.4. Corrosion Inhibition Analysis of PASP/5–AVA
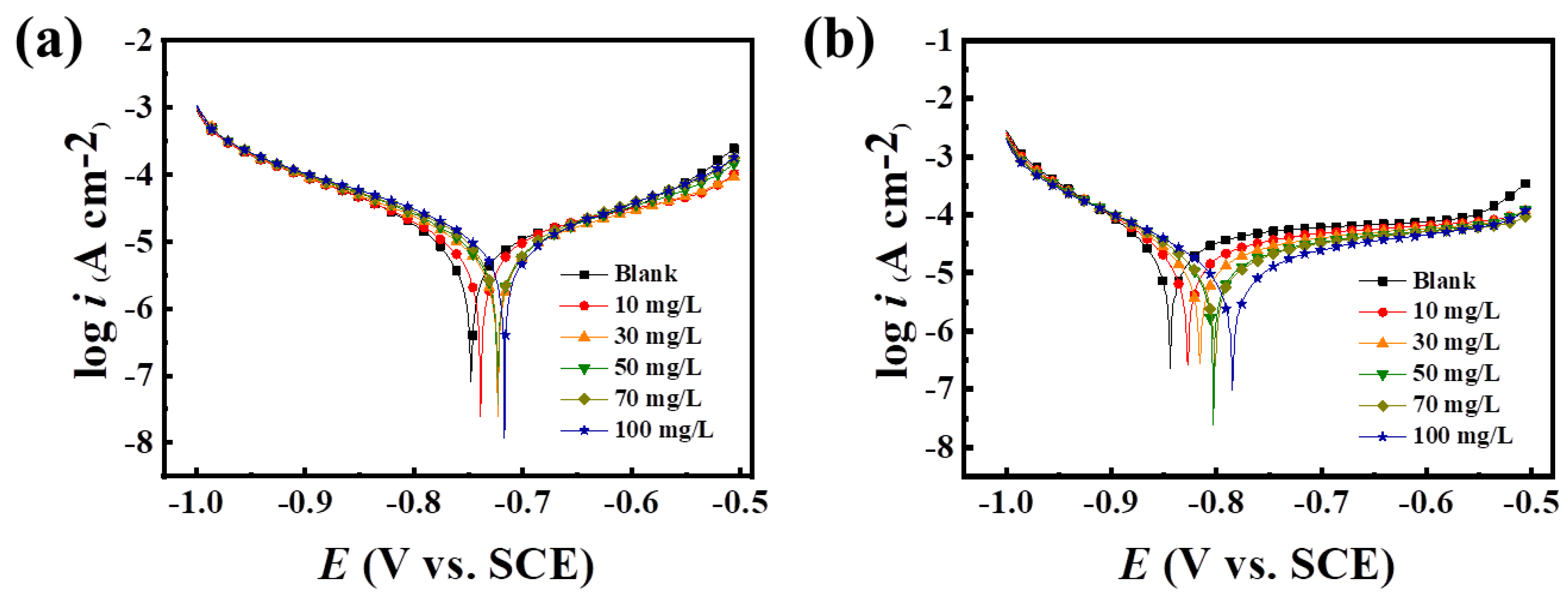
2.4.1. Analysis of Electrochemical Polarization Curves
2.4.2. Analysis of Electrochemical AC Impedance Spectra
2.5. Action Mechanism of PASP/5–AVA
2.5.1. Mechanism Analysis of Scale Inhibition
2.5.2. Analysis of Corrosion Inhibition Mechanism
3. Materials and Methods
3.1. Reagents and Equipment
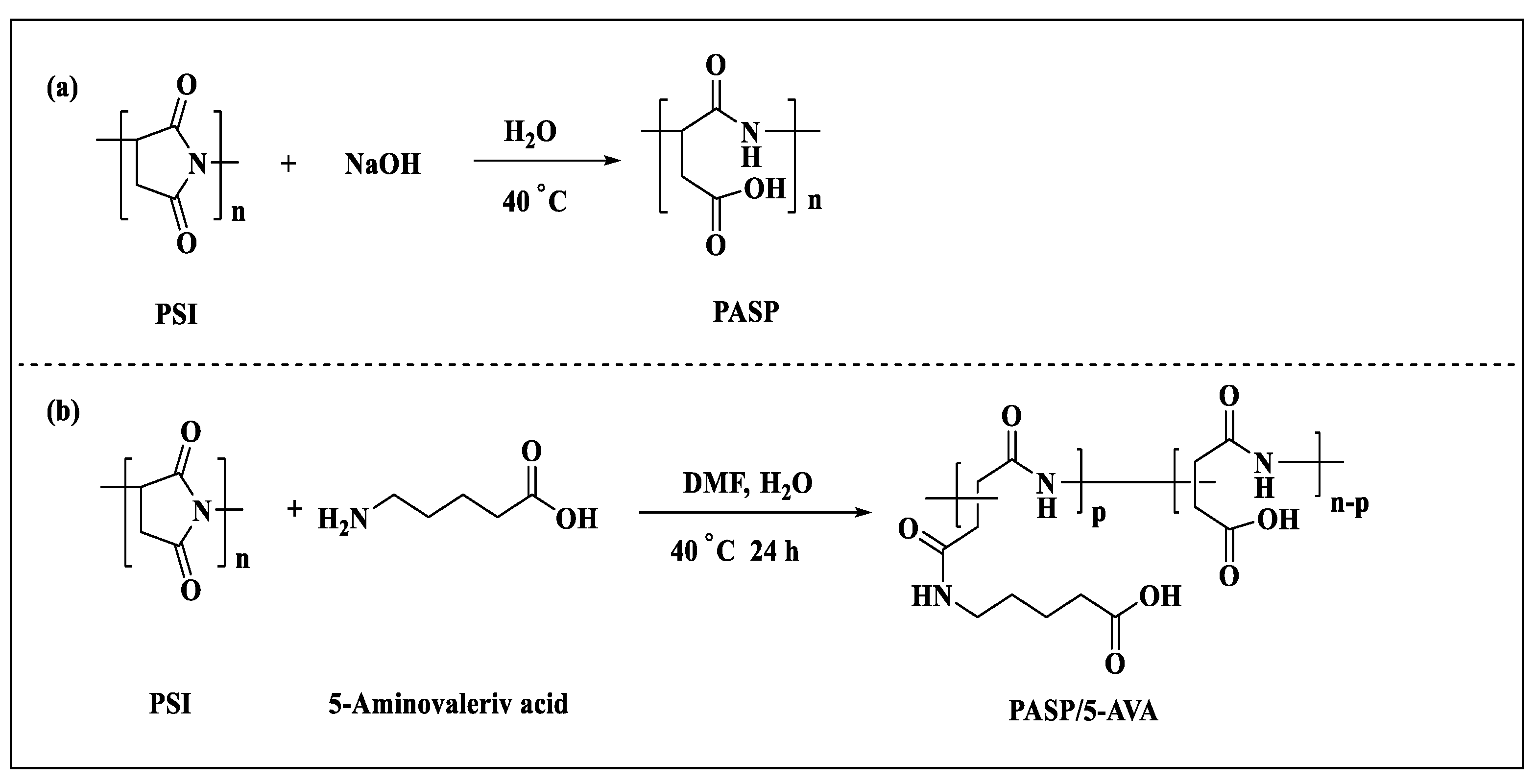
3.2. Synthesis of PASP and PASP/5–AVA
3.3. Characterization of PASP/5–AVA
3.4. Static Scale Inhibition Experiment
3.5. Calcium Scale Characterization
3.6. Determination of Scale Crystal Surface Energy
3.7. Determination of Corrosion Inhibition Efficiency
3.8. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, M. Global water shortage and potable water safety; Today’s concern and tomorrow’s crisis. Environ. Int. 2022, 158, 106936. [Google Scholar] [CrossRef] [PubMed]
- Kaekratoke, N.; Flood, A.; Crespy, D. Homopolymers and copolymers with iminodiacetic acid chelating units for scale inhibition. Desalination 2024, 583, 117643. [Google Scholar] [CrossRef]
- Li, C.G.; Liu, C.; Xu, W.H.; Shan, M.G.; Wu, H.X. Formation mechanisms and supervisory prediction of scaling in water supply pipelines: A review. Water Res. 2022, 222, 118922. [Google Scholar] [CrossRef]
- Dou, W.Y.; Peng, X.J.; Kong, L.H.; Hu, X.Y. A review on the removal of Cl (-I) with high concentration from industrial wastewater: Approaches and mechanisms. Sci. Total Environ. 2022, 824, 153909. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chang, Y.X.; Yang, D.W.; Yang, L.; Jiang, B.; Yan, W.; Xu, H.; Xu, X. A novel membrane-free electrochemical separation-filtering crystallization coupling process for treating circulating cooling water. Water Res. 2024, 256, 121617. [Google Scholar] [CrossRef]
- Taqieddin, A.; Sarrouf, S.; Ehsan, M.F.; Alshawabkeh, A.N. New insights on designing the next-generation materials for electrochemical synthesis of reactive oxidative species towards efffcient and scalable water treatment: A review and perspectives. J. Environ. Chem. Eng. 2023, 11, 111384. [Google Scholar] [CrossRef]
- Zhu, H.F.; Zheng, F.; Lu, S.J.; Hao, L.S.; Li, B.J.; Mao, Z.X.; Long, Y.Y.; Yao, C.T.; Wu, H.; Zheng, X.; et al. Effect of electrochemical pretreatment on the control of scaling and fouling caused by circulating cooling water on heat exchanger and side-stream reverse osmosis membrane. J. Water Process Eng. 2021, 43, 102261. [Google Scholar] [CrossRef]
- Bharti, M.; Das, P.P.; Purkait, M.K. A review on the treatment of water and wastewater by electrocoagulation process: Advances and emerging applications. J. Environ. Chem. Eng. 2023, 11, 111558. [Google Scholar] [CrossRef]
- Yu, W.; Xiong, P.; Teng, J.H.; Chen, C.; Li, B.S.; Zhao, L.H.; Lin, H.J.; Shen, L.G. Advances in synthesis and application of amphoteric polymer-based water treatment agents. Desalination 2024, 574, 117280. [Google Scholar] [CrossRef]
- Hu, Y.L.; Chen, C.M.; Liu, S.T. State of art bio-materials as scale inhibitors in recirculating cooling water system: A review article. Water Sci. Technol. 2022, 85, 1500–1521. [Google Scholar] [CrossRef]
- Yi, X.Y.; Yang, S.M.; He, X.; Wang, Z.W.; Rui, M.; Tang, Y.L. Use of modified poly-epoxysuccinic acid as an efficient scale inhibitor to control CaSO4 scaling in NF processes: Performance and mechanisms. Desalination 2024, 586, 117821. [Google Scholar] [CrossRef]
- Adelnia, H.; Sirous, F.; Blakey, I.; Ta, H.T. Metal ion chelation of poly(aspartic acid): From scale inhibition to therapeutic potentials. Int. J. Biol. Macromol. 2023, 229, 974–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Zhang, D.Q.; Liu, D.D.; Cai, Y.J.; Gao, L.X. Polyaspartic acid modified by fluorescent carbon quantum dots as an environmentally friendly scale inhibitor for calcium sulphate. Desalination 2024, 584, 117740. [Google Scholar] [CrossRef]
- Husna, U.Z.; Elraies, K.A.; Shuhili, J.A.B.M.; Elryes, A.A. A review: The utilization potency of biopolymer as an eco-friendly scale inhibitors. J. Pet. Explor. Prod. Technol. 2021, 12, 1075–1094. [Google Scholar] [CrossRef]
- Chai, C.X.; Xu, Y.H.; Shi, S.C.; Zhao, X.W.; Wu, Y.F.; Xu, Y.; Zhang, L. Functional polyaspartic acid derivatives as eco-friendly corrosion inhibitors for mild steel in 0.5 M H2SO4 solution. RSC Adv. 2018, 8, 24970–24981. [Google Scholar] [CrossRef]
- Zhao, J.L.; Zhang, M.L.; Zhang, Z.K.; Ma, C.; Cao, Z.Y.; Ni, S.F.; Xu, Y. Polyaspartic acid-capped (4-[(2-aminoethyl) amino]-4-oxobutanoic acid as an efficient and green gypsum scale inhibitor and its scale inhibition mechanism. Desalination 2022, 543, 116101. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, Y.J.; Miao, S.F.; Wan, D.Q. Fabrication of a novel chitosan/polyaspartic acid composite: A pH-tunable sorbent for efffcient adsorption of heavy metal ions and dyes from water. J. Water Process Eng. 2023, 56, 104554. [Google Scholar] [CrossRef]
- Yu, X.C.; Kan, J.h.; Han, J.; Liu, K.L.; Li, J.H.; Zhang, H.X.; Chen, J.X. Synthesis, scale and corrosion inhibition evaluation and mechanism of 2-aminobenzimidazole modified polyaspartic acid. J. Environ. Chem. Eng. 2024, 12, 112950. [Google Scholar] [CrossRef]
- Tkachenko, S.; Trukhina, M.; Ryabova, A.; Oshchepkov, M.; Kamagurov, S.; Popov, K. Fluorescent-tagged antiscalants-the new materials for scale inhibition mechanism studies, antiscalant traceability and antiscaling efficacy optimization during CaCO3 and CaSO4·2H2O scale formation. Int. J. Mol. Sci. 2023, 24, 3087. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, H.; Chen, E.; Li, M.; Liu, S. Physicochemical characteristics and the scale inhibition effect of air nanobubbles (A-NBs) in a circulating cooling water system. Langmuir 2023, 39, 1629–1639. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Srinivasan, S.; Samrat, K.; Priyalakshmi, B.; Kumar, R.D.; Bharani, A.; Kumar, R.G.; Kavisri, M.; Moovendhan, M. Sustainable approach to manage the vulnerable rodents using eco-friendly green rodenticides formulation through nanotechnology principles—A review. Process Saf. Environ. 2023, 171, 591–606. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Zhao, P.; Niu, Z.G. Characteristics of molecular weight distribution of dissolved organic matter in bromide-containing water and disinfection by-product formation properties during treatment processes. J. Environ. Sci. 2017, 65, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.F.; Tan, Q.Q.; Liu, Z.; Li, H.H.; Zheng, Y.X.; Zhang, L.H.; Liu, Z.F. Synthesis and application of a phosphorous-free and non-nitrogen polymer as an environmentally friendly scale inhibition and dispersion agent in simulated cooling water systems. ACS Omega 2020, 5, 15487–15494. [Google Scholar] [CrossRef] [PubMed]
- Timothy, U.J.; Umoren, P.S.; Solomon, M.M.; Igwe, I.O.; Umoren, S.A. An appraisal of the utilization of natural gums as corrosion inhibitors: Prospects, challenges, and future perspectives. Int. J. Biol. Macromol. 2023, 253, 126904. [Google Scholar] [CrossRef] [PubMed]
- Nassar, L.; Wadi, V.S.; Hegab, H.M.; Khalil, H.; Banat, F.; Naddeo, V.; Hasan, S.W. Sustainable and green polylactic acid-based membrane embedded with self-assembled positively charged f-MWCNTs/GO nanohybrids for the removal of nutrients from wastewater. npj Clean Water 2022, 5, 57. [Google Scholar] [CrossRef]
- Hou, J.J.; Liu, Z.D.; Ni, X.Y. Unlocking the mysteries: Investigating the kinetic mechanism of calcium carbonate formation from phosphogypsum. Process. Saf. Environ. 2024, 182, 625–637. [Google Scholar] [CrossRef]
- Liu, X.; Sheng, X.; Yao, Q.; Zhao, L.; Xu, Z.; Zhou, Y. Synthesis of a new type of 2-phosphonobutane-1,2,4-tricarboxylic-acid-modified terpolymer scale inhibitor and its application in the oil field. Energy Fuels 2021, 35, 6136–6143. [Google Scholar] [CrossRef]
- Varvara, S.; Berghian-Grosan, C.; Damian, G.; Popa, M.; Popa, F. Combined electrochemical, raman analysis and machine learning assessments of the inhibitive properties of an 1,3,4-oxadiazole-2-thiol derivative against carbon steel corrosion in HCl solution. Materials 2022, 15, 2224. [Google Scholar] [CrossRef]
- Gao, X.; Wu, Y.; Huang, Q.; Jiang, Y.; Ma, D.; Ren, T. The inhibition behavior of novel ionic liquids for magnesium alloy in NaCl solution: Experimental and theoretical investigation. J. Mol. Liq. 2021, 324, 114732. [Google Scholar] [CrossRef]
- Pandiyarajan, S.; Manickaraj, S.S.M.; Liao, A.H.; Ramachandran, A.; Lee, K.Y.; Chuang, H.C. Recovery of Al2O3 from hazardous Al waste as a reinforcement particle for high-performance Ni/Al2O3 corrosion resistance coating via ultrasonic-aided supercritical-CO2 electrodeposition. Chemosphere 2023, 313, 137626. [Google Scholar] [CrossRef]
- GB/T 16632−2019; Determination of Scale Inhibition Performance of Water Treatment Chemicals-Calcium Carbonate Precipitation Method. The Standardization Administration of China: Beijing, China, 2019.
- QSY126−2014; Specifications of Corrosion and Scale Inhibitors for Oil Field Water Treatment. The China National Petroleum Corporation China: Beijing, China, 2014.
- Benslimane, S.; Bouhidel, K.E.; Ferfache, A.; Farhi, S. Mechanistic study of the synergetic inhibiting effects of Zn2+, Cu2+ and Mg2+ ions on calcium carbonate precipitation. Water Res. 2020, 186, 116323. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ding, X.L.; Dai, Y.M.; Wan, L. A study of guar gum derivatives as green kinetic hydrate inhibitors. J. Environ. Chem. Eng. 2024, 12, 112075. [Google Scholar] [CrossRef]
- Raj, R.M.; Ganesan, S.; Suganthi, S.; Vignesh, S.; Hatamleh, A.A.; Alnafisi, B.K.; Venkatesan, R.; Raj, V.; Lo, H.M. Facile construction of cost-effective zinc-aluminium polymeric framework for efficient removal of selective both drug and dye from an aqueous medium. Chemosphere 2023, 311, 137105. [Google Scholar] [CrossRef]
- Zeng, T.T.; Ma, D.L.; Gui, Y.G. Gas-Sensing Performance of Metal Oxide Heterojunction Materials for SF6 Decomposition Gases: A DFT Study. Int. J. Mol. Sci. 2024, 25, 8009. [Google Scholar] [CrossRef] [PubMed]





| Sample | Mn | Mw | Ð |
|---|---|---|---|
| PASP | 1778 | 2039 | 1.147 |
| PASP/5–AVA | 11,328 | 14,109 | 1.245 |
| Different Concentrations of CaCO3 Solution (mol/L) | Antiscalants | |||
|---|---|---|---|---|
| Blank | PASP | PASP/5–AVA | ||
| tind (min) | 0.015 | 1.50 | 2.25 | 53.71 |
| 0.02 | 0.75 | 1.25 | 6.00 | |
| 0.025 | 0.54 | 0.75 | 2.19 | |
| 0.03 | 0.4 | 0.5 | 1.05 | |
| Inhibitors | C (mg/L) | −Ecorr (Vvs.SCE) | βa (mV/dec) | −βc (mV/dec) | Icorr (mA/cm2) | ηp (%) | θ |
|---|---|---|---|---|---|---|---|
| Blank | — | 0.6775 | 202.55 | 102.99 | 3.516 | - | - |
| PASP | 30 | 0.6228 | 267.81 | 109.97 | 3.129 | 10.72 | 0.11 |
| 50 | 0.6296 | 250.06 | 104.34 | 2.955 | 15.96 | 0.16 | |
| 70 | 0.6294 | 278.71 | 100.39 | 2.883 | 18.12 | 0.18 | |
| 100 | 0.6208 | 259.27 | 104.13 | 2.749 | 21.84 | 0.22 | |
| PASP/5–AVA | 30 | 0.6050 | 153.0 | 117.6 | 1.395 | 31.7 | 0.32 |
| 50 | 0.6142 | 141.8 | 113.4 | 1.386 | 32.1 | 0.32 | |
| 70 | 0.6166 | 149.4 | 114.9 | 1.118 | 45.2 | 0.45 | |
| 100 | 0.6047 | 143.9 | 118.0 | 1.001 | 51.0 | 0.51 |
| Inhibitors | C (mg/L) | Rct (Ohm.cm2) | Cdl (F/cm2) | χ2 (×10−3) | ηp (%) |
|---|---|---|---|---|---|
| Blank | — | 144.3 | 0.40937 | 0.3777 | - |
| PASP | 10 | 144.8 | 0.37148 | 0.3126 | 6.73 |
| 30 | 145.6 | 0.35909 | 0.4240 | 12.28 | |
| 50 | 147.8 | 0.32399 | 0.4360 | 20.86 | |
| 70 | 154.5 | 0.31749 | 0.4178 | 22.44 | |
| 100 | 205.4 | 0.31148 | 0.3962 | 23.91 | |
| PASP/5–AVA | 10 | 57.4 | 0.33137 | 0.2571 | 19.96 |
| 30 | 68.52 | 0.29042 | 0.4167 | 29.01 | |
| 50 | 70.46 | 0.27168 | 0.3654 | 33.63 | |
| 70 | 74.02 | 0.21842 | 0.3600 | 46.64 | |
| 100 | 75.27 | 0.20722 | 0.39947 | 49.38 |
| Inhibitor | EHOMO (eV) | ELUMO (eV) | ∆E (eV) | χ (eV) | η (eV) | ∆N |
|---|---|---|---|---|---|---|
| PASP | −7.1724 | −1.2743 | 5.8981 | 4.2233 | 2.9491 | 0.4708 |
| PASP/5–AVA | −6.4474 | −1.4085 | 5.0389 | 4.0379 | 2.6294 | 0.5633 |
| Name | Purchasing Company | |
|---|---|---|
| Reagents | Polysuccinimide (PSI, Mw = 7000, AR) | Wuhan Yuancheng Gongyi Technology Company Limited (Wuhan, China) |
| 5-Aminovaleric acid (AR) | Tianjin De’en Chemical Reagents Company Limited (Tianjin, China) | |
| Anhydrous sodium sulfate (AR, 99%) | Tianjin Komi Chemical Reagent Company Limited (Tianjin, China) | |
| Sodium carbonate (AR, 99.8%) | Tianjin Komi Chemical Reagent Co., Ltd. (Tianjin, China) | |
| Potassium hydroxide (AR, 90%) | Tianjin Komi Chemical Reagent Co., Ltd. (Tianjin, China) | |
| Anhydrous borax (AR, 95%) | Shanghai Shaoyuan Chemical Reagent Co., Ltd. (Shanghai, China) | |
| Calcium chloride (AR, 99.9%) | Shanghai Shaoyuan Chemical Reagent Co., Ltd. (Shanghai, China) | |
| Potassium chloride (AR, 99.999%) | Shanghai Shaoyuan Chemical Reagent Co., Ltd. (Shanghai, China) | |
| Sodium chloride (AR, 99%) | Shanghai Shaoyuan Chemical Reagent Co., Ltd. (Shanghai, China) | |
| Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA) | Shanghai Energy Chemical Reagent Co. Ltd. (Shanghai, China) | |
| Absolute ethanol | Anhui Ante Food Co., Ltd. (Anhui China) | |
| Ethanol absolute (AR, 99.5%) | Anhui Ante Food Co., Ltd. (Anhui, China) | |
| Hydrochloric acid (ω = 36%) | China Pingmei Shenma Group Kaifeng Dongda Chemical Co., Ltd. (Kaifeng, China) | |
| Equipment | An AVANCE 400 nuclear magnetic resonance spectrometer | Bruker Co., Ltd. (Bilerika, Germany) |
| An ESCALAB 250Xi X-ray photoelectron spectrometer | Thermo Fisher Scientific Co., Ltd. (waltham, USA) | |
| A JSM-7610F SEM | Japan Electronics Co., Ltd. (Tokyo Metropolis, Japan) | |
| An HH-601 constant-temperature water tank | Jintan Jingda Instrument Manufacturing Co., Ltd. (Changzhou, China) | |
| A Nano ZS particle size and zeta potential analyzer | Malvery Instruments Ltd. (Marvin, England) | |
| A PL-GPC50 gel permeation chromatograph | Agilent Technology Co., Ltd. (Palo Alto City, USA) | |
| A DDS-11A conductivity meter | INASE Scientific Instrument Co., Ltd. (Shanghai, China) | |
| A D8 Advance X-ray powder diffractometer | Bruker Co., Ltd. (Bilerika, Germany) | |
| A CHI660E electrochemical workstation | Chenhua Instrument Co., Ltd. (Shanghai, China) | |
| A VERTEX 70 Fourier transform infrared spectrometer | Bruker Co., Ltd. (Bilerika, Germany) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Han, Y.; Zhang, X.; Cao, Z.; Zhao, X.; Wang, Y.; Cai, Y.; Wu, Y.; Xu, Y. Synthesis and Mechanism of a Green Scale and Corrosion Inhibitor. Int. J. Mol. Sci. 2024, 25, 10150. https://doi.org/10.3390/ijms251810150
Zhao L, Han Y, Zhang X, Cao Z, Zhao X, Wang Y, Cai Y, Wu Y, Xu Y. Synthesis and Mechanism of a Green Scale and Corrosion Inhibitor. International Journal of Molecular Sciences. 2024; 25(18):10150. https://doi.org/10.3390/ijms251810150
Chicago/Turabian StyleZhao, Linlin, Yu Han, Xiaojuan Zhang, Zhongyan Cao, Xiaowei Zhao, Yuxia Wang, Yonghong Cai, Yufeng Wu, and Ying Xu. 2024. "Synthesis and Mechanism of a Green Scale and Corrosion Inhibitor" International Journal of Molecular Sciences 25, no. 18: 10150. https://doi.org/10.3390/ijms251810150






