Graphene-Oxide Peptide-Containing Materials for Biomedical Applications
Abstract
:1. Introduction
2. Understanding GO and Its Derivatives
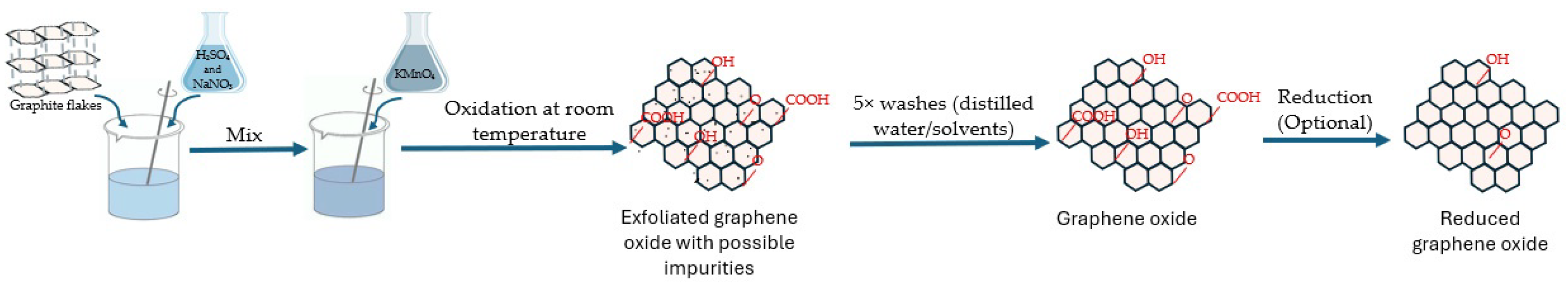
3. Methods for Synthesizing GO
- Oxidation of graphite:
- -
- Graphite is mixed with concentrated H2SO4 and NaNO3.
- -
- KMnO4 is added slowly to the mixture while stirring.
- Exfoliation and expansion:
- -
- The resulting mixture is stirred and maintained at a specific temperature to initiate the oxidation of graphite.
- Purification:
- Optional reduction:
- -
- GO obtained from the previous steps may undergo reduction to remove some oxygen-containing functional groups and restore sp2 carbon–carbon bonds, resulting in rGO.
- -
- This reduction can be achieved through various methods, such as chemical reduction using hydrazine or thermal treatment.
4. Structural and Property Analyses of GO Derivatives
5. Functionalization of GO Derivatives with Peptides and Proteins
5.1. Peptides for Functionalizing GO
| Peptide/ Protein | Sequence | Peptide/Protein Binding Target: | Functional Behavior | References |
|---|---|---|---|---|
| HHC-36 | KRWWKWWRR | G/GO | Demonstrates antimicrobial activity when functionalized with graphene. | [53] |
| CATH-2 | RFGRFLRKIRRFRPKVTITIQGSARF | G/GO | Enhances antimicrobial properties when functionalized with G/GO. | [52] |
| Octaarginine (R8) | RRRRRRRR | G/GO | Evaluated for efficient gene delivery with GO. | [54] |
| Chlorotoxin | MCMPCFTTDHQMARKCDDCCGGKGRGKCYGPQCLCR | G/GO | Used for targeted glioma therapy with GO. | [55] |
| RGD2 | RGD | G/GO | Improves efficiency of chemotherapeutic agents with GO. | [56] |
| GAMHLP-WHMGTL | GAMHLPWHMGTL | G/GO | Facilitates the separation and stabilization of graphene sheets. | [51] |
| P1 peptide | HSSYWYAFNNKT | G/GO | Used for exfoliating graphene and functionalizing it for biosensing applications. | [4] |
| GR3R | GGGGAAGGGGRRR | G/GO | Forms stable layers on graphene for molecular film creation and surface interaction studies. | [57] |
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ | G/GO | Enhances antimicrobial efficacy of graphene-based composites. | [58] |
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | G/GO | Exhibits antimicrobial properties for wound healing with G/GO. | [59] |
| Magainin | GIGKFLHSAKKFGKAFVGEIMKS | G/GO | Shows antimicrobial activity in GO-functionalized materials. | [60] |
| β-amyloid peptide | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA | G/GO | Studied for amyloid aggregation and Alzheimer’s intervention with GO. | [61] |
| α-synuclein | MDVFMKGLSKAKEGVVAAEKTKQGVAEAAGKTKEGVLYVGSKTREGVVQGVVTGVTAVA | G/GO | Studied for neurotoxic effects and therapeutic interventions with graphene. | [62] |
| β-defensin 2 | GIGDPVTCLKSGAICHPVFCPRRYKQIGTCGLPGTKCCKKP | G/GO | Enhances antimicrobial properties in G/GO-based surfaces and is effective in bone reconstruction. | [63] |
| Bovine serum albumin (BSA) | 583 amino acids | G/GO | Enhanced stability and functionality are potentially useful for biomedical applications such as biosensing and drug delivery. | [64] |
| Lysozyme | 129 amino acids | G/GO | Preservation of enzymatic activity, facilitating its use in various industrial and biomedical applications, including antimicrobial coatings and biosensors. | [65] |
| Hemoglobin | Α-chain: 141 amino acids B-chain: 146 amino acids | G/GO | Oxygen-carrying capabilities, making it suitable for applications such as artificial blood substitutes and biosensors. | [66] |
| Streptavidin | 159 amino acids | G/GO | Enhanced sensitivity and specificity in biosensing applications, facilitating the detection of various biomolecules. | [67] |
| Fibronectin | Several hundred to over a thousand amino acids | G/GO | Promotion of cell adhesion and growth, enhancing tissue regeneration for wound healing and regenerative medicine applications. | [68] |
| Pepsin A | 326 amino acids | G/GO | Preservation of enzymatic activity and stability, enabling its use in biocatalysis and enzymatic systems for protein digestion and pharmaceutical manufacturing. | [69] |
| Myoglobin | 153 amino acids | G/GO | Enhanced electrochemical performance in biosensing and bioelectronic devices, particularly for detecting oxygen levels and monitoring physiological conditions. | [70] |
5.2. Proteins for Functionalizing GO
5.3. Interaction Mechanisms
5.3.1. Non-Covalent Interactions
- π–π stacking: Aromatic residues in peptides/proteins can interact with the aromatic rings of GO via π–π stacking interactions. For example, melittin, which contains aromatic residues such as phenylalanine, tyrosine, and tryptophan, can engage in π–π stacking with GO, enhancing binding affinity and stability [89].
- Electrostatic interactions: Positively charged amino-acid residues in peptides/proteins can interact with the negatively charged functional groups on the surface of GO. For instance, histone proteins rich in lysine and arginine residues can form electrostatic interactions with the carboxyl and hydroxyl groups on GO [90].
- Hydrophobic interactions: Hydrophobic residues in peptides/proteins can interact with the hydrophobic regions of GO, promoting strong binding. An example is the β-amyloid peptide, which contains hydrophobic residues such as valine, isoleucine, and leucine, facilitating hydrophobic interactions with GO [91].
- Hydrogen bonding: Functional groups capable of hydrogen bonding in peptides/proteins can interact with similar groups on GO. Silk fibroin, with its hydroxyl, carboxyl, and amino groups, can form hydrogen bonds with GO, enhancing the stability of the conjugate [92].
- Van der Waals forces: Non-specific van der Waals forces between peptides/proteins and the surface of GO contribute to binding stability. BSA, for instance, can engage in van der Waals interactions with GO, aiding in forming a stable conjugate [64].
5.3.2. Covalent Interactions
- Amide bond formation is the primary interaction in peptide/protein conjugation with GO. It occurs between the carboxyl groups of the peptides/proteins and the amine or hydroxyl groups on the surface of GO. The reaction typically proceeds via condensation reactions, resulting in the formation of stable amide bonds. For example, LL-37 can form amide bonds through a condensation reaction between its carboxyl group and the amine or hydroxyl groups on GO [96].
- Esterification involves the reaction between carboxyl groups of peptides/proteins and hydroxyl groups on the surface of GO. This reaction leads to the forming of ester bonds, contributing to the covalent attachment of peptides/proteins to GO. For instance, lysozyme can react with the hydroxyl groups on GO to form ester bonds, contributing to the covalent attachment [97].
- Thiol chemistry: thiol groups (-SH) present in peptides/proteins can react with various functional groups on the surface of GO, such as epoxides or carboxyl groups, through thiol-ene or thiol-epoxy reactions. This results in the formation of thioether bonds, which contribute to covalent binding. For example, insulin, known for its structural flexibility and containing cysteine residues with thiol groups, can form thioether bonds with GO [98].
- Click chemistry: click chemistry reactions, such as azide–alkyne cycloaddition (CuAAC) or strain-promoted alkyne–azide cycloaddition (SPAAC), can be utilized for covalent conjugation of peptides/proteins to GO. Functional groups, such as azide or alkyne, are introduced onto peptides/proteins and GO surfaces, enabling selective and efficient covalent binding via click chemistry reactions [99]. CPPs (such as transportan, TAT, penentratin, R8, and MAP) modified with azide or alkyne tags can undergo click chemistry reactions with GO surfaces functionalized with complementary groups [10].
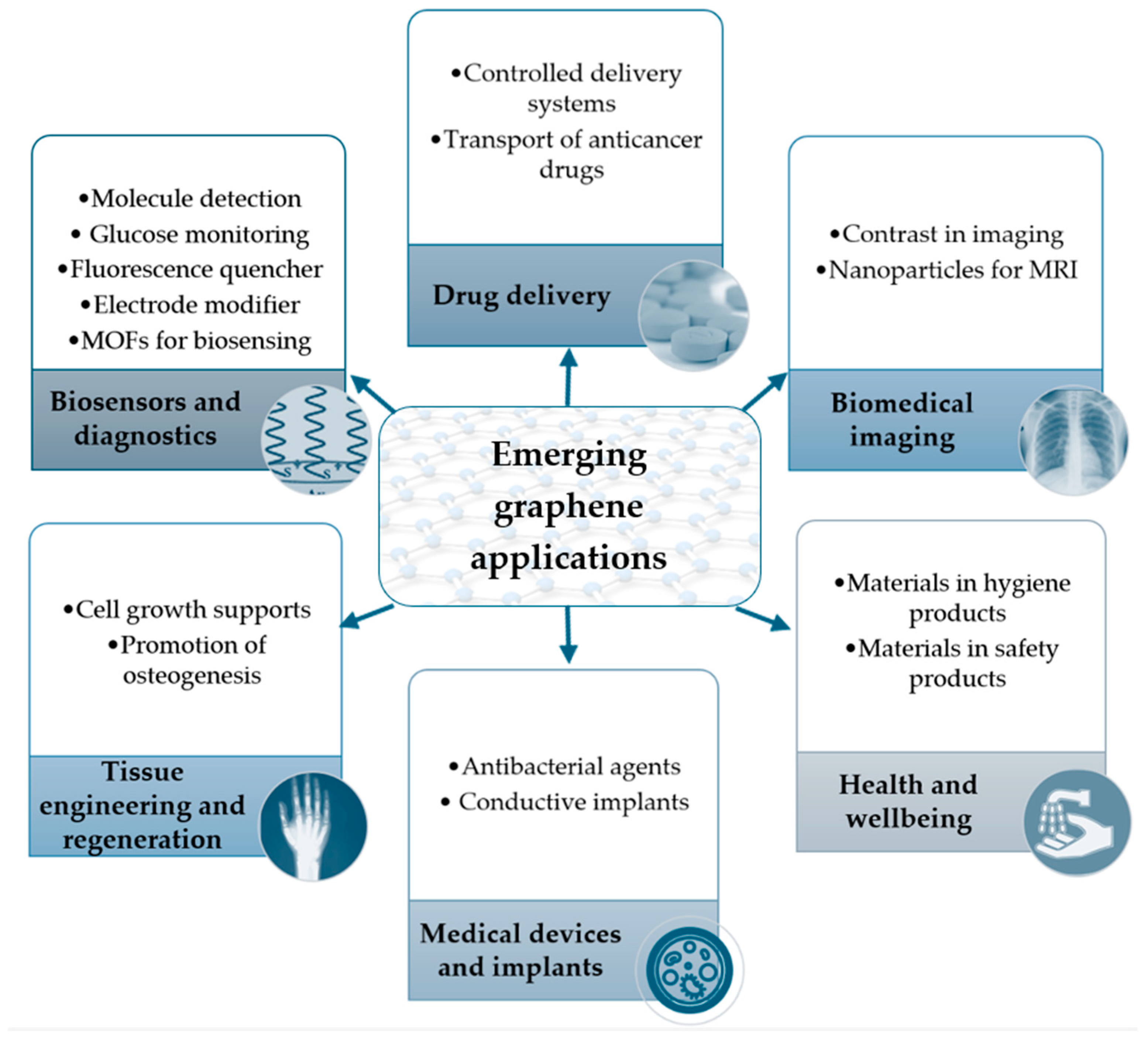
6. Utilization of Graphene-Based Materials in the Biomedical Sector
6.1. Graphene Used for Biosensors and Diagnoses
6.1.1. Graphene Oxide as a Fluorescence Quencher in Biosensors
6.1.2. Graphene/Graphene Oxide as an Electrode Modifier for Peptide Functionalization
6.1.3. Enhancing Metal–Organic Frameworks with Graphene Materials for Advanced Biosensing Applications
6.2. Graphene’s Utility in Drug Delivery
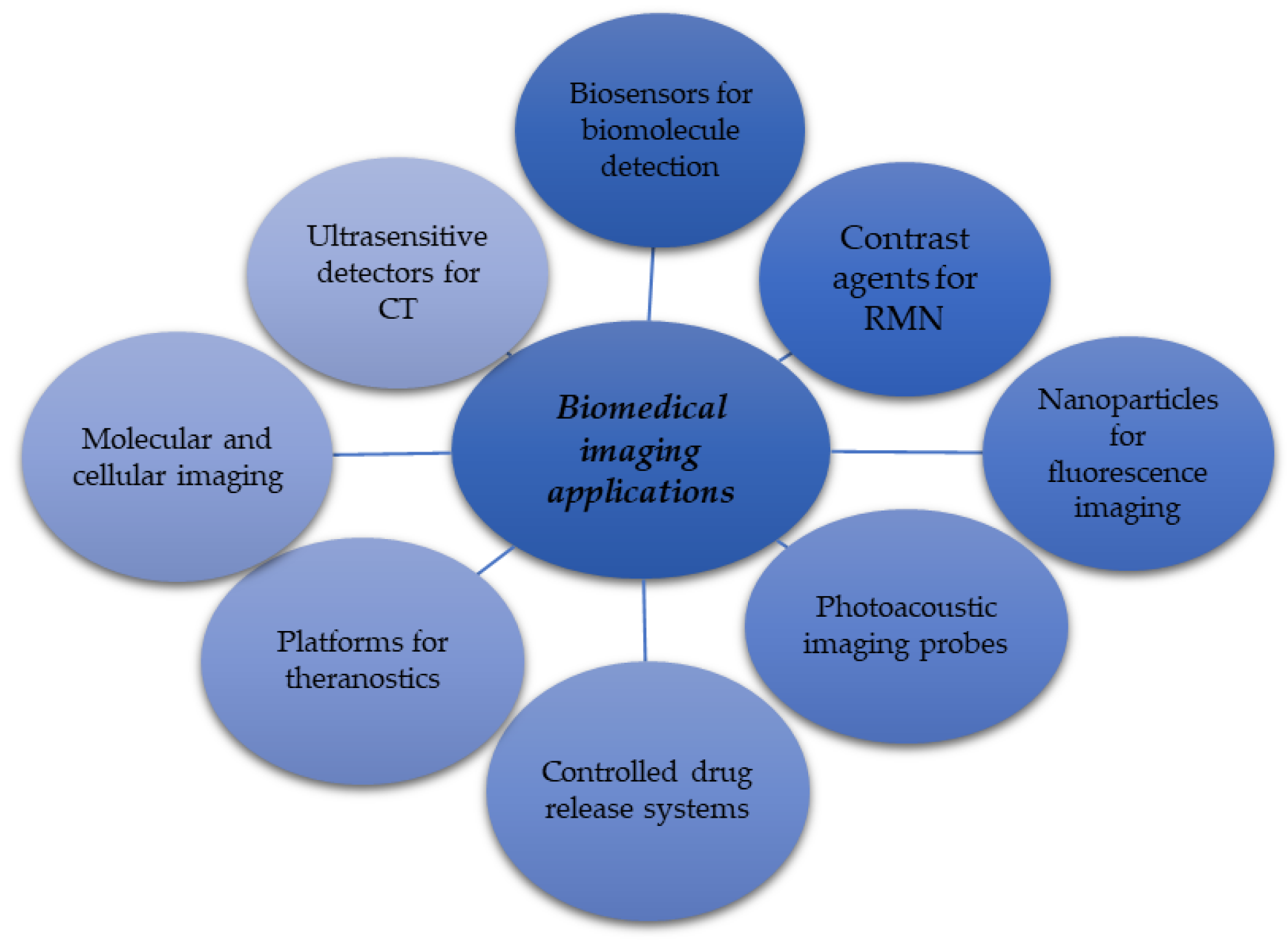
6.3. Graphene Application for Biomedical Imaging Purposes
6.4. Graphene Use in Tissue Engineering and Regeneration
6.5. Graphene Applications as Medical Devices and Implants Based on Its Antimicrobial Properties
7. Limitations
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Li, S.S.; Yang, H.Y.; Luo, J. Progress in the functional modification of graphene/graphene oxide: A review. Rsc Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, D.P.; Muñoz, R.; Amami, M.; Singh, R.K.; Singh, S.; Kumar, V. Graphene-based materials for biotechnological and biomedical applications: Drug delivery, bioimaging and biosensing. Mater. Today Chem. 2023, 33, 101750. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, G.; Wang, Z.; Wu, K.; Deng, A.; Li, J. Electrochemiluminescence immunosensor based on tin dioxide quantum dots and palladium-modified graphene oxide for the detection of zearalenone. Talanta 2024, 271, 125740. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Brljak, N.; Slocik, J.M.; Rao, R.; Knecht, M.R.; Walsh, T.R. Graphene exfoliation using multidomain peptides. J. Mater. Chem. B 2024, 12, 4824–4832. [Google Scholar] [CrossRef]
- Singh, D. Graphene-tethered peptide nanosheets—A facile approach for cargo molecules in cancer. Nano-Struct. Nano-Objects 2024, 37, 101115. [Google Scholar] [CrossRef]
- Gupta, S.; Azadvari, N.; Hosseinzadeh, P. Design of Protein Segments and Peptides for Binding to Protein Targets. Biodes Res. 2022, 2022, 9783197. [Google Scholar] [CrossRef]
- Molhoek, E.M.; van Dijk, A.; Veldhuizen, E.J.; Dijk-Knijnenburg, H.; Mars-Groenendijk, R.H.; Boele, L.C.; Kaman-van Zanten, W.E.; Haagsman, H.P.; Bikker, F.J. Chicken cathelicidin-2-derived peptides with enhanced immunomodulatory and antibacterial activities against biological warfare agents. Int. J. Antimicrob. Agents 2010, 36, 271–274. [Google Scholar] [CrossRef]
- Imani, R.; Emami, S.H.; Faghihi, S. Synthesis and characterization of an octaarginine functionalized graphene oxide nano-carrier for gene delivery applications. Phys. Chem. Chem. Phys. 2015, 17, 6328–6339. [Google Scholar] [CrossRef]
- Sarkar, S.; Ahuja, S. Chapter 11—Applications and limitations of graphene oxide for remediating contaminants of emerging concern in wastewater. In Separation Science and Technology; Ahuja, S., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 15, pp. 209–222. [Google Scholar]
- Gessner, I.; Neundorf, I. Nanoparticles Modified with Cell-Penetrating Peptides: Conjugation Mechanisms, Physicochemical Properties, and Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2020, 21, 2536. [Google Scholar] [CrossRef]
- Biru, E.I.; Necolau, M.I.; Zainea, A.; Iovu, H. Graphene Oxide-Protein-Based Scaffolds for Tissue Engineering: Recent Advances and Applications. Polymers 2022, 14, 1032. [Google Scholar] [CrossRef]
- Kumar, S.; Balayaa, R.D.A.; Kanekar, S.; Raju, R.; Prasad, T.S.K.; Kandasamy, R.K. Computational Tools for Exploring Peptide-Membrane Interactions in Gram-Positive Bacteria. Comput. Struct. Biotechnol. J. 2023, 21, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Cheng, H.M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Perala, R.S.; Chandrasekar, N.; Balaji, R.; Alexander, P.S.; Humaidi, N.Z.N.; Hwang, M.T. A comprehensive review on graphene-based materials: From synthesis to contemporary sensor applications. Mater. Sci. Eng. R-Rep. 2024, 159, 100805. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Zhai, G. Biomedical applications of the graphene-based materials. Mater. Sci. Eng. C 2016, 61, 953–964. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Fallahazad, P. Rational and key strategies toward enhancing the performance of graphene/silicon solar cells. Mater. Adv. 2023, 4, 1876–1899. [Google Scholar] [CrossRef]
- Machado, B.F.; Serp, P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Al-Maliki, R.M.; Alsalhy, Q.F.; Al-Jubouri, S.; Salih, I.K.; AbdulRazak, A.A.; Shehab, M.A.; Németh, Z.; Hernadi, K. Classification of Nanomaterials and the Effect of Graphene Oxide (GO) and Recently Developed Nanoparticles on the Ultrafiltration Membrane and Their Applications: A Review. Membranes 2022, 12, 1043. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification Framework for Graphene-Based Materials. Angew. Chem. Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef]
- Qi, K.; Sun, B.; Liu, S.-y.; Zhang, M. Research progress on carbon materials in tumor photothermal therapy. Biomed. Pharmacother. 2023, 165, 115070. [Google Scholar] [CrossRef]
- Du Preez, H.; Halma, M. Graphene-Based Nanomaterials: Uses, Environmental Fate and Human Health Hazards. Nano Biomed. Eng. 2023, 16, 219–231. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Liu, B.; Xie, J.; Ma, H.; Zhang, X.; Pan, Y.; Lv, J.; Ge, H.; Ren, N.; Su, H.; Xie, X.; et al. Graphene: From Graphite to Graphene Oxide and Graphene Oxide Quantum Dots. Small 2017, 13, 1601001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. Engl. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J.; et al. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef]
- Kumar, N.; Salehiyan, R.; Chauke, V.; Joseph Botlhoko, O.; Setshedi, K.; Scriba, M.; Masukume, M.; Sinha Ray, S. Top-down synthesis of graphene: A comprehensive review. FlatChem 2021, 27, 100224. [Google Scholar] [CrossRef]
- Brodie, B.C. XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Berichte Der Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Anegbe, B.; Ifijen, I.H.; Maliki, M.; Uwidia, I.E.; Aigbodion, A.I. Graphene oxide synthesis and applications in emerging contaminant removal: A comprehensive review. Environ. Sci. Eur. 2024, 36, 15. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Zheng-Guang, Z.; Hui-Jiang, Y.; Fei, L.; Yan-Huang, F. Preparation of Graphene Oxide by Ultrasound-Assisted Hummers Method. Chin. J. Inorg. Chem. 2011, 27, 1753–1757. [Google Scholar]
- Méndez-Lozano, N.; Pérez-Reynoso, F.; González-Gutiérrez, C. Eco-Friendly Approach for Graphene Oxide Synthesis by Modified Hummers Method. Materials 2022, 15, 7228. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.J.; Pomberger, A.; Felton, K.C.; Grainger, R.; Barecka, M.; Chamberlain, T.W.; Bourne, R.A.; Johnson, C.N.; Lapkin, A.A. A Brief Introduction to Chemical Reaction Optimization. Chem. Rev. 2023, 123, 3089–3126. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H.; Khraisheh, M.; Atieh, M.; Al-Ghouti, M. Synthesis of graphene oxides particle of high oxidation degree using a modified Hummers method. Ceram. Int. 2020, 46, 23997–24007. [Google Scholar] [CrossRef]
- Pinto, A.M.; Gonçalves, I.C.; Magalhães, F.D. Graphene-based materials biocompatibility: A review. Colloids Surf. B Biointerfaces 2013, 111, 188–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of chemical vapor deposition of graphene and related applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef]
- Drewniak, S.; Muzyka, R.; Stolarczyk, A.; Pustelny, T.; Kotyczka-Morańska, M.; Setkiewicz, M. Studies of Reduced Graphene Oxide and Graphite Oxide in the Aspect of Their Possible Application in Gas Sensors. Sensors 2016, 16, 103. [Google Scholar] [CrossRef]
- Dave, S.H.; Gong, C.; Robertson, A.W.; Warner, J.H.; Grossman, J.C. Chemistry and Structure of Graphene Oxide via Direct Imaging. ACS Nano 2016, 10, 7515–7522. [Google Scholar] [CrossRef]
- Nanda, S.S.; Yi, D.K.; Kim, K. Study of antibacterial mechanism of graphene oxide using Raman spectroscopy. Sci. Rep. 2016, 6, 28443. [Google Scholar] [CrossRef]
- Blanton, T.N.; Majumdar, D. X-ray diffraction characterization of polymer intercalated graphite oxide. Powder Diffr. 2012, 27, 104–107. [Google Scholar] [CrossRef]
- Teklu, A.; Barry, C.; Palumbo, M.; Weiwadel, C.; Kuthirummal, N.; Flagg, J. Mechanical Characterization of Reduced Graphene Oxide Using AFM. Adv. Condens. Matter Phys. 2019, 2019, 8713965. [Google Scholar] [CrossRef]
- Guo, S.; Garaj, S.; Bianco, A.; Ménard-Moyon, C. Controlling covalent chemistry on graphene oxide. Nat. Rev. Phys. 2022, 4, 247–262. [Google Scholar] [CrossRef]
- Rudenko, R.M.; Voitsihovska, O.O.; Poroshin, V.N. Enhancement of electrical conductivity of hydrazine-reduced graphene oxide under thermal annealing in hydrogen atmosphere. Mater. Lett. 2023, 331, 133476. [Google Scholar] [CrossRef]
- Joshi, A.S.; Elamurugu, E.; Leela.S. Impact of Graphene oxide (GO) and reduced Graphene Oxide (rGO) on the TiO2 thin film composite (TiO2: GO/ rGO) photoanodes. Chem. Phys. Impact 2024, 9, 100667. [Google Scholar] [CrossRef]
- Mohammed, S. Graphene oxide: A mini-review on the versatility and challenges as a membrane material for solvent-based separation. Chem. Eng. J. Adv. 2022, 12, 100392. [Google Scholar] [CrossRef]
- Ou, L.; Luo, Y.; Wei, G. Atomic-Level Study of Adsorption, Conformational Change, and Dimerization of an α-Helical Peptide at Graphene Surface. J. Phys. Chem. B 2011, 115, 9813–9822. [Google Scholar] [CrossRef]
- Rajesh, C.; Majumder, C.; Mizuseki, H.; Kawazoe, Y. A theoretical study on the interaction of aromatic amino acids with graphene and single walled carbon nanotube. J. Chem. Phys. 2009, 130, 124911. [Google Scholar] [CrossRef]
- Shen, J.; Shi, M.; Yan, B.; Ma, H.; Li, N.; Hu, Y.; Ye, M. Covalent attaching protein to graphene oxide via diimide-activated amidation. Colloids Surf. B Biointerfaces 2010, 81, 434–438. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Guo, S.; Zhang, J. Interactions of graphene and graphene oxide with proteins and peptides. Nanotechnol. Rev. 2013, 2, 27–45. [Google Scholar] [CrossRef]
- Joshi, S.; Siddiqui, R.; Sharma, P.; Kumar, R.; Verma, G.; Saini, A. Green synthesis of peptide functionalized reduced graphene oxide (rGO) nano bioconjugate with enhanced antibacterial activity. Sci. Rep. 2020, 10, 9441. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mei, L.; Jin, M.; Jiang, X.; Li, X.; Li, J.; Xu, Y.; Meng, Z.; Zhu, J.; Wu, F. Composite Coating of Graphene Oxide/TiO2 Nanotubes/HHC-36 Antibacterial Peptide Construction and an Exploration of Its Bacteriostat and Osteogenesis Effects. J. Biomed. Nanotechnol. 2021, 17, 662–676. [Google Scholar] [CrossRef]
- Imani, R.; Prakash, S.; Vali, H.; Faghihi, S. Polyethylene glycol and octa-arginine dual-functionalized nanographene oxide: An optimization for efficient nucleic acid delivery. Biomater. Sci. 2018, 6, 1636–1650. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gu, W.; Xiao, N.; Ye, L.; Xu, Q. Chlorotoxin-conjugated graphene oxide for targeted delivery of an anticancer drug. Int. J. Nanomed. 2014, 9, 1433–1442. [Google Scholar] [CrossRef]
- Wang, C.; Chen, B.; Zou, M.; Cheng, G. Cyclic RGD-modified chitosan/graphene oxide polymers for drug delivery and cellular imaging. Colloids Surf. B Biointerfaces 2014, 122, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Homma, C.; Tsukiiwa, M.; Noguchi, H.; Tanaka, M.; Okochi, M.; Tomizawa, H.; Sugizaki, Y.; Isobayashi, A.; Hayamizu, Y. Designable peptides on graphene field-effect transistors for selective detection of odor molecules. Biosens. Bioelectron. 2023, 224, 115047. [Google Scholar] [CrossRef]
- Zhang, C.; Ge, Y.; Lu, X.; Chen, Z.; Liu, J.; Zhang, M.; Yang, K.; Yuan, B. Membrane perturbation of fullerene and graphene oxide distinguished by pore-forming peptide melittin. Carbon 2021, 180, 67–76. [Google Scholar] [CrossRef]
- Häffner, S.M.; Malmsten, M. Interplay between amphiphilic peptides and nanoparticles for selective membrane destabilization and antimicrobial effects. Curr. Opin. Colloid Interface Sci. 2019, 44, 59–71. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Gou, L.; Li, J.; Yuan, B.; Yang, K.; Ma, Y. Designing melittin-graphene hybrid complexes for enhanced antibacterial activity. Adv. Healthc. Mater. 2019, 8, 1801521. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.-P.; Dai, W.; Dong, H.; Wen, Y.; Zhang, X. Graphene quantum dots for the inhibition of β amyloid aggregation. Nanoscale 2015, 7, 19060–19065. [Google Scholar] [CrossRef]
- Aminabad, E.D.; Mobed, A.; Hasanzadeh, M.; Feizi, M.A.H.; Safaralizadeh, R.; Seidi, F. Sensitive immunosensing of α-synuclein protein in human plasma samples using gold nanoparticles conjugated with graphene: An innovative immuno-platform towards early stage identification of Parkinson’s disease using point of care (POC) analysis. RSC Adv. 2022, 12, 4346–4357. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, L.; Yuan, Q.; Gu, P.; You, Z.; Zhuang, A.; Bi, X. Effect of bifunctional β defensin 2-modified scaffold on bone defect reconstruction. ACS Omega 2020, 5, 4302–4312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, Z.; Wang, Y.; Fei, Z.; Cao, J. Changing the activities and structures of bovine serum albumin bound to graphene oxide. Appl. Surf. Sci. 2018, 427, 1019–1029. [Google Scholar] [CrossRef]
- Zuñiga, J.; Akashi, L.; Pinheiro, T.; Rivera, M.; Barreto, L.; Albertin, K.; Champi, A. Synthesis of lysozyme-reduced graphene oxide films for biosensor applications. Diam. Relat. Mater. 2022, 126, 109093. [Google Scholar] [CrossRef]
- Liu, C.; Xu, B.J.; Zhou, L.; Sun, Z.; Mao, H.J.; Zhao, J.L.; Zhang, L.; Chen, X. Graphene oxide functionalized long period fiber grating for highly sensitive hemoglobin detection. Sens. Actuators B Chem. 2018, 261, 91–96. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, L.; Galli, F.; Nederlof, I.; Olsthoorn, R.C.L.; Lamers, G.E.M.; Oosterkamp, T.H.; Abrahams, J.P. A Graphene Oxide Streptavidin Complex for Biorecognition—Towards Affinity Purification. Adv. Funct. Mater. 2010, 20, 2857–2865. [Google Scholar] [CrossRef]
- Subbiah, R.; Du, P.; Van, S.Y.; Suhaeri, M.; Hwang, M.P.; Lee, K.; Park, K. Fibronectin-tethered graphene oxide as an artificial matrix for osteogenesis. Biomed. Mater. 2014, 9, 065003. [Google Scholar] [CrossRef]
- Kang, X.; Wang, R.; Jiang, M.; Li, E.; Li, Y.; Wang, T.; Ren, Z. A label-free biosensor for pepsin detection based on graphene oxide functionalized micro-tapered long period fiber grating. Sens. Actuators Rep. 2023, 5, 100139. [Google Scholar] [CrossRef]
- Guo, C.; Sun, H.; Zhao, X. Myoglobin within graphene oxide sheets and Nafion composite films as highly sensitive biosensor. Sens. Actuators B Chem. 2012, 164, 82–89. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Alayande, A.B.; Yang, E.; Aung, M.; Kim, I.S. Bacterial adhesion inhibition on water treatment membrane by a modified HHC-36 antimicrobial peptide. Environ. Eng. Res. 2022, 28, 220155. [Google Scholar] [CrossRef]
- Schneider, V.A.F.; Coorens, M.; Bokhoven, J.L.M.T.-v.; Posthuma, G.; Dijk, A.v.; Veldhuizen, E.J.A.; Haagsman, H.P. Imaging the Antistaphylococcal Activity of CATH-2: Mechanism of Attack and Regulation of Inflammatory Response. mSphere 2017, 2, e00370-17. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Q.; Cui, C.; Li, J.; Wang, Y. Anti-HER2 functionalized graphene oxide as survivin-siRNA delivery carrier inhibits breast carcinoma growth in vitro and in vivo. Drug Des. Dev. Ther. 2018, 12, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Huang, X.; Ma, Y.; Huang, Y.; Yang, R.; Duan, H.; Chen, Y. Multi-functionalized graphene oxide based anticancer drug-carrier with dual-targeting function and pH-sensitivity. J. Mater. Chem. 2011, 21, 3448–3454. [Google Scholar] [CrossRef]
- Gostaviceanu, A.; Gavrilaş, S.; Copolovici, L.; Copolovici, D.M. Membrane-Active Peptides and Their Potential Biomedical Application. Pharmaceutics 2023, 15, 2091. [Google Scholar] [CrossRef]
- Zitzmann, S.; Ehemann, V.; Schwab, M. Arginine-Glycine-Aspartic Acid (RGD)-Peptide Binds to Both Tumor and Tumor-Endothelial Cells in Vivo. Cancer Res. 2002, 62, 5139–5143. [Google Scholar]
- Katoch, J.; Kim, S.N.; Kuang, Z.; Farmer, B.L.; Naik, R.R.; Tatulian, S.A.; Ishigami, M. Structure of a peptide adsorbed on graphene and graphite. Nano Lett. 2012, 12, 2342–2346. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S. Biomolecule-assisted exfoliation and dispersion of graphene and other two-dimensional materials: A review of recent progress and applications. Nanoscale 2016, 8, 15389–15413. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hitomi, T.; Homma, C.; Rungreungthanapol, T.; Tanaka, M.; Yamada, K.; Hamasaki, H.; Sugizaki, Y.; Isobayashi, A.; Tomizawa, H.; et al. Enantioselective Detection of Gaseous Odorants with Peptide–Graphene Sensors Operating in Humid Environments. ACS Appl. Mater. Interfaces 2024, 16, 18564–18573. [Google Scholar] [CrossRef]
- Yang, Y.; Han, P.; Xie, X.; Yin, X.; Duan, G.; Wen, L. Protein corona reduced graphene oxide cytotoxicity by inhibiting endocytosis. Colloid Interface Sci. Commun. 2021, 45, 100514. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein Corona-Mediated Mitigation of Cytotoxicity of Graphene Oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.R.; Yu, H.L.; Wu, H.; Shen, X.Z.; Deng, C.H. Advanced nanomaterials as sample technique for bio-analysis. Trac-Trends Anal. Chem. 2021, 135, 116168. [Google Scholar] [CrossRef]
- Kumar, S.; Parekh, S.H. Molecular Control of Interfacial Fibronectin Structure on Graphene Oxide Steers Cell Fate. ACS Appl. Mater. Interfaces 2021, 13, 2346–2359. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Y.; Kang, Y.; Zhang, H.; Zhang, Z.; Fei, Z.; Cao, J. Graphene oxide destabilizes myoglobin and alters its conformation. Carbon 2017, 114, 449–456. [Google Scholar] [CrossRef]
- Bai, Y.; Ming, Z.; Cao, Y.; Feng, S.; Yang, H.; Chen, L.; Yang, S.-T. Influence of graphene oxide and reduced graphene oxide on the activity and conformation of lysozyme. Colloids Surf. B Biointerfaces 2017, 154, 96–103. [Google Scholar] [CrossRef]
- Bera, S.; Dhar, J.; Dasgupta, R.; Basu, G.; Chakraborti, S.; Chakrabarti, P. Molecular features of interaction involving hen egg white lysozyme immobilized on graphene oxide and the effect on activity. Int. J. Biol. Macromol. 2018, 120, 2390–2398. [Google Scholar] [CrossRef]
- Sainz-Urruela, C.; Vera-López, S.; Paz San Andrés, M.; Díez-Pascual, A.M. Surface functionalization of graphene oxide with tannic acid: Covalent vs non-covalent approaches. J. Mol. Liq. 2022, 357, 119104. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chatterjee, R.; Chakravortty, D. Evolving and assembling to pierce through: Evolutionary and structural aspects of antimicrobial peptides. Comput. Struct. Biotechnol. J. 2022, 20, 2247–2258. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, B.; Hu, Y.; Deng, N.; Zhao, B.; Li, X.; Liang, Z.; Zhang, L.; Zhang, Y. Aptamer functionalized magnetic graphene oxide nanocomposites for highly selective capture of histones. Electrophoresis 2019, 40, 2135–2141. [Google Scholar] [CrossRef]
- Huang, Y.; Chang, Y.; Liu, L.; Wang, J. Nanomaterials for Modulating the Aggregation of β-Amyloid Peptides. Molecules 2021, 26, 4301. [Google Scholar] [CrossRef]
- Tadepalli, S.; Hamper, H.; Park, S.H.; Cao, S.; Naik, R.R.; Singamaneni, S. Adsorption Behavior of Silk Fibroin on Amphiphilic Graphene Oxide. ACS Biomater. Sci. Eng. 2016, 2, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kyratzis, I. Covalent Immobilization of Proteins on Carbon Nanotubes Using the Cross-Linker 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide—A Critical Assessment. Bioconjugate Chem. 2008, 19, 1945–1950. [Google Scholar] [CrossRef]
- Su, R.; Shi, P.; Zhu, M.; Hong, F.; Li, D. Studies on the properties of graphene oxide–alkaline protease bio-composites. Bioresour. Technol. 2012, 115, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Hermanová, S.; Zarevúcká, M.; Bouša, D.; Pumera, M.; Sofer, Z. Graphene oxide immobilized enzymes show high thermal and solvent stability. Nanoscale 2015, 7, 5852–5858. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Sharma, P.C.; Patibandla, S.; Gao, Y.; Ruppa-Kasani, V.; Goli, J.; Kumar, A.; Chatterjee, A.; Sinha, S.S.; Bates, J.T.; et al. Blocking SARS-CoV-2 Delta Variant (B.1.617.2) Spike Protein Receptor-Binding Domain Binding with the ACE2 Receptor of the Host Cell and Inhibiting Virus Infections Using Human Host Defense Peptide-Conjugated Graphene Quantum Dots. ACS Omega 2022, 7, 8150–8157. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, T.R.B.; Nalder, T.D.; Yang, W.; Marshall, S.N.; Barrow, C.J. Controlling enzyme function through immobilisation on graphene, graphene derivatives and other two dimensional nanomaterials. J. Mater. Chem. B 2018, 6, 3200–3218. [Google Scholar] [CrossRef]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef]
- Cernat, A.; Györfi, S.J.; Irimes, M.-B.; Tertiș, M.; Bodoki, A.; Pralea, I.-E.; Suciu, M.; Cristea, C. Click chemistry on azide-functionalized graphene oxide. Electrochem. Commun. 2019, 98, 23–27. [Google Scholar] [CrossRef]
- Shirvalilou, S.; Khoei, S.; Khoee, S.; Raoufi, N.J.; Karimi, M.R.; Shakeri-Zadeh, A. Development of a magnetic nano-graphene oxide carrier for improved glioma-targeted drug delivery and imaging: In vitro and in vivo evaluations. Chem. Biol. Interact. 2018, 295, 97–108. [Google Scholar] [CrossRef]
- Song, M.-M.; Xu, H.-L.; Liang, J.-X.; Xiang, H.-H.; Liu, R.; Shen, Y.-X. Lactoferrin modified graphene oxide iron oxide nanocomposite for glioma-targeted drug delivery. Mater. Sci. Eng. C 2017, 77, 904–911. [Google Scholar] [CrossRef]
- Torkashvand, H.; Dehdast, S.A.; Nateghpour, M.; Haghi, A.M.; Fard, G.C.; Elmi, T.; Shabani, M.; Tabatabaie, F. Antimalarial nano-drug delivery system based on graphene quantum dot on Plasmodium falciparum: Preparation, characterization, toxicological evaluation. Diam. Relat. Mater. 2023, 132, 109670. [Google Scholar] [CrossRef]
- Yektaniroumand Digehsaraei, S.; Salouti, M.; Amini, B.; Mahmazi, S.; Kalantari, M.; Kazemizadeh, A.; Mehrvand, J. Developing a fluorescence immunosensor for detection of HER2-positive breast cancer based on graphene and magnetic nanoparticles. Microchem. J. 2021, 167, 106300. [Google Scholar] [CrossRef]
- Kenry; Lee, W.C.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Raslan, A.; del Burgo, L.S.; Ciriza, J.; Pedraz, J.L. Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine. Int. J. Pharm. 2020, 580, 119226. [Google Scholar] [CrossRef]
- Shin, S.R.; Li, Y.C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef]
- Goodrum, R.; Weldekidan, H.; Li, H.; Mohanty, A.K.; Misra, M. Graphene-based nanostructures from green processes and their applications in biomedical sensors. Adv. Ind. Eng. Polym. Res. 2024, 7, 37–53. [Google Scholar] [CrossRef]
- Carrasco, S. Metal-Organic Frameworks for the Development of Biosensors: A Current Overview. Biosensors 2018, 8, 92. [Google Scholar] [CrossRef]
- Arshad, F.; Nabi, F.; Iqbal, S.; Khan, R.H. Applications of graphene-based electrochemical and optical biosensors in early detection of cancer biomarkers. Colloids Surf. B Biointerfaces 2022, 212, 112356. [Google Scholar] [CrossRef]
- Sadrabadi, E.A.; Benvidi, A.; Azimzadeh, M.; Asgharnejad, L.; Dezfuli, A.S.; Khashayar, P. Novel electrochemical biosensor for breast cancer detection, based on a nanocomposite of carbon nanofiber, metal–organic framework, and magnetic graphene oxide. Bioelectrochemistry 2024, 155, 108558. [Google Scholar] [CrossRef]
- Jiang, Z.; Feng, B.; Xu, J.; Qing, T.; Zhang, P.; Qing, Z. Graphene biosensors for bacterial and viral pathogens. Biosens. Bioelectron. 2020, 166, 112471. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-Z.; Jian, C.R.; Govindasamy, M.; Li, Y.-C.; Lin, Y.-T.; Su, C.-Y.; Samukawa, S.; Huang, C.-H. Crumpled graphene induced by commercial Heat-Shrinkable material for chemiresistive biosensors toward cancer biomarker detection. Microchem. J. 2023, 195, 109469. [Google Scholar] [CrossRef]
- Saber Mirzaei, S.; Mehrdadi, N.; Nabi bidhendi, G.; Pourmadadi, M.; Ahmadi, M.; Meknatkhah, S. Novel detection of H.pylori using ultrasensitive electrochemical aptasensor based on surface modified graphene oxide doped gold nanoparticles conjugated polythiophene. Microchem. J. 2024, 200, 110279. [Google Scholar] [CrossRef]
- Li, W.; Jiang, T.; Pu, Y.; Jiao, X.; Tan, W.; Qin, S. Glucose biosensor using fluorescence quenching with chitosan-modified graphene oxide. Micro Nano Lett. 2019, 14, 344–348. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, W.; Zhang, J.; Hu, Z.; Wu, J.; Ye, G.; Luo, Y. Highly sensitive and specific graphene oxide-based FRET aptasensor for quantitative detection of human soluble growth stimulating gene protein 2. Talanta 2024, 271, 125629. [Google Scholar] [CrossRef]
- Huang, X.; He, Z.; Guo, D.; Liu, Y.; Song, J.; Yung, B.C.; Lin, L.; Yu, G.; Zhu, J.J.; Xiong, Y.; et al. “Three-in-one” Nanohybrids as Synergistic Nanoquenchers to Enhance No-Wash Fluorescence Biosensors for Ratiometric Detection of Cancer Biomarkers. Theranostics 2018, 8, 3461–3473. [Google Scholar] [CrossRef]
- Beitollai, H.; Safaei, M.; Tajik, S. Application of Graphene and Graphene Oxide for Modification of Electrochemical Sensors and Biosensors: A Review. Int. J. Nano Dimens. 2019, 10, 125–140. [Google Scholar]
- Bagheri, H.; Afkhami, A.; Khoshsafar, H.; Rezaei, M.; Sabounchei, S.J.; Sarlakifar, M. Simultaneous electrochemical sensing of thallium, lead and mercury using a novel ionic liquid/graphene modified electrode. Anal. Chim. Acta 2015, 870, 56–66. [Google Scholar] [CrossRef]
- Yiwei, X.; Wen, Z.; Xiaowei, H.; Jiyong, S.; Xiaobo, Z.; Yanxiao, L.; Xueping, C.; Tahir, H.E.; Zhihua, L. A Self-assembled L-Cysteine and Electrodeposited Gold Nanoparticles-reduced Graphene Oxide Modified Electrode for Adsorptive Stripping Determination of Copper. Electroanalysis 2018, 30, 194–203. [Google Scholar] [CrossRef]
- Karbowska, B.; Rębiś, T.; Milczarek, G. Electrode Modified by Reduced Graphene Oxide for Monitoring of Total Thallium in Grain Products. Int. J. Environ. Res. Public Health 2018, 15, 653. [Google Scholar] [CrossRef]
- Promphet, N.; Rattanarat, P.; Rangkupan, R.; Chailapakul, O.; Rodthongkum, N. An electrochemical sensor based on graphene/polyaniline/polystyrene nanoporous fibers modified electrode for simultaneous determination of lead and cadmium. Sens. Actuators B Chem. 2015, 207, 526–534. [Google Scholar] [CrossRef]
- Li, J.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. An electrochemical sensor based on copper-based metal-organic frameworks-graphene composites for determination of dihydroxybenzene isomers in water. Talanta 2018, 181, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Wang, W.; Zhang, Y.; Yang, T. Highly sensitive simultaneous determination of cadmium (II), lead (II), copper (II), and mercury (II) ions on N-doped graphene modified electrode. J. Electroanal. Chem. 2016, 760, 52–58. [Google Scholar] [CrossRef]
- Guo, J.; Duan, Y.; Jia, Y.; Zhao, Z.; Gao, X.; Liu, P.; Li, F.; Chen, H.; Ye, Y.; Liu, Y.; et al. Biomimetic chiral hydrogen-bonded organic-inorganic frameworks. Nat. Commun. 2024, 15, 139. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, C.; Niu, Y.; Chen, J.; Zhao, Y.; Zhang, Y.; Gao, R.; He, J. Target triggered cleavage effect of DNAzyme: Relying on Pd-Pt alloys functionalized Fe-MOFs for amplified detection of Pb2+. Biosens. Bioelectron. 2018, 101, 297–303. [Google Scholar] [CrossRef]
- Tang, Z.; He, J.; Chen, J.; Niu, Y.; Zhao, Y.; Zhang, Y.; Yu, C. A sensitive sandwich-type immunosensor for the detection of galectin-3 based on N-GNRs-Fe-MOFs@ AuNPs nanocomposites and a novel AuPt-methylene blue nanorod. Biosens. Bioelectron. 2018, 101, 253–259. [Google Scholar] [CrossRef]
- Gholamalizadeh, N.; Mazinani, S.; Abdouss, M.; Bazargan, A.M.; Fatemi, F. Stencil printing of a highly conductive graphene ink toward flexible electrochemical biosensors for glucose monitoring. Prog. Org. Coat. 2022, 172, 107083. [Google Scholar] [CrossRef]
- Wang, S.; Hossain, M.Z.; Shinozuka, K.; Shimizu, N.; Kitada, S.; Suzuki, T.; Ichige, R.; Kuwana, A.; Kobayashi, H. Graphene field-effect transistor biosensor for detection of biotin with ultrahigh sensitivity and specificity. Biosens. Bioelectron. 2020, 165, 112363. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, Y.; Sun, W.; Han, R.; Luo, C.; Wang, X.; Wei, Q. A highly selective and sensitive detection of insulin with chemiluminescence biosensor based on aptamer and oligonucleotide-AuNPs functionalized nanosilica @ graphene oxide aerogel. Anal. Chim. Acta 2019, 1089, 152–164. [Google Scholar] [CrossRef]
- Palacio, I.; Moreno, M.; Náñez, A.; Purwidyantri, A.; Domingues, T.; Cabral, P.D.; Borme, J.; Marciello, M.; Mendieta-Moreno, J.I.; Torres-Vázquez, B.; et al. Attomolar detection of hepatitis C virus core protein powered by molecular antenna-like effect in a graphene field-effect aptasensor. Biosens. Bioelectron. 2023, 222, 115006. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, C.; Lin, Y.; Sun, W.; Liu, H.; Zhu, X.; Dai, Y.; Luo, C. Highly selective and sensitive chemiluminescence biosensor for adenosine detection based on carbon quantum dots catalyzing luminescence released from aptamers functionalized graphene@magnetic β-cyclodextrin polymers. Talanta 2018, 186, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.-f.; Snyder, A.; Xie, J.; Stanciu, L.A. Functionalized graphene oxide for the fabrication of paraoxon biosensors. Anal. Chim. Acta 2014, 827, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, Q.H.; Yu, H.L. Graphene-based synthetic peptide electrochemical sensor for colorectal cancer diagnosis. Alex. Eng. J. 2024, 101, 90–97. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Early detection of cancer utilizing biosensors based on “Green Graphene”: An innovative and sustainable methodology for advancing cancer diagnosis. TrAC Trends Anal. Chem. 2023, 167, 117254. [Google Scholar] [CrossRef]
- Zhang, Z.; Xia, X.Y.; Xiang, X.; Huang, F.H.; Han, L. Conjugated cationic polymer-assisted amplified fluorescent biosensor for protein detection via terminal protection of small molecule-linked DNA and graphene oxide. Sens. Actuators B-Chem. 2017, 249, 8–13. [Google Scholar] [CrossRef]
- Sun, X.J.; Fang, F.Y.; Na, J.T.; Yan, R.J.; Huang, Y.; Zhou, Z.D.; Zhao, Y.X.; Li, G.Y. Fluorescent “turn-on” aptamer sensor for sensitive and reliable detection of Golgi glycoprotein 73 based on nitrogen-doped graphene quantum dots and molybdenum disulfide nanosheets. J. Pharm. Biomed. Anal. 2023, 225, 115215. [Google Scholar] [CrossRef]
- Wang, M.K.; Lin, Z.H.; Liu, Q.; Jiang, S.; Liu, H.; Su, X.G. DNA-hosted copper nanoclusters/graphene oxide based fluorescent biosensor for protein kinase activity detection. Anal. Chim. Acta 2018, 1012, 66–73. [Google Scholar] [CrossRef]
- Sancar, T.; Altinbasak, I.; Sanyal, R.; Sanyal, A. Electrospun photothermally active graphene-based nanofibers with a Retro-Diels-Alder reaction to initiate drug release. Eur. Polym. J. 2024, 210, 112946. [Google Scholar] [CrossRef]
- Khudhair, A.M.; Ben Ahmed, A.; Ajeel, F.N.; Mohammed, M.H. Theoretical investigation on the therapeutic applications of C2B and C2O as targeted drug delivery systems for hydroxyurea and 6-thioguanine in cancer treatment. Nano-Struct. Nano-Objects 2024, 38, 101135. [Google Scholar] [CrossRef]
- Mianehrow, H.; Afshari, R.; Mazinani, S.; Sharif, F.; Abdouss, M. Introducing a highly dispersed reduced graphene oxide nano-biohybrid employing chitosan/hydroxyethyl cellulose for controlled drug delivery. Int. J. Pharm. 2016, 509, 400–407. [Google Scholar] [CrossRef]
- Karimi, S.; Namazi, H. Fe3O4@PEG-coated dendrimer modified graphene oxide nanocomposite as a pH-sensitive drug carrier for targeted delivery of doxorubicin. J. Alloys Compd. 2021, 879, 160426. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Baldi, G.; Doumett, S.; Garcia-Hevia, L.; Gallo, J.; Bañobre-López, M.; Dražić, G.; Calhelha, R.C.; Ferreira, I.C.F.R.; Lima, R.; et al. Multifunctional graphene-based magnetic nanocarriers for combined hyperthermia and dual stimuli-responsive drug delivery. Mater. Sci. Eng. C 2018, 93, 206–217. [Google Scholar] [CrossRef]
- Gupta, J.; Prakash, A.; Jaiswal, M.K.; Agarrwal, A.; Bahadur, D. Superparamagnetic iron oxide-reduced graphene oxide nanohybrid-a vehicle for targeted drug delivery and hyperthermia treatment of cancer. J. Magn. Magn. Mater. 2018, 448, 332–338. [Google Scholar] [CrossRef]
- Kumara, B.N.; Shambhu, R.; Prabhu, A.; Prasad, K.S. Novel chitosan—Graphene quantum dots composite for therapeutic delivery and tracking through enzymatic stimuli response. Carbohydr. Polym. 2022, 289, 119426. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, P.; Rao, G.; Kumar, A.S.; Prakash, J.; Rathinasabapathi, P.; Venkatasubbu, G.D. Interaction of BSA with graphene oxide: Influence on the bioactivity of graphene oxide. Diam. Relat. Mater. 2023, 132, 109629. [Google Scholar] [CrossRef]
- Iynoon Jariya, S.A.; Babu, A.A.; Sankara Narayanan, T.S.N.; Vellaichamy, E.; Ravichandran, K. Development of a novel smart carrier for drug delivery: Ciprofloxacin loaded vaterite/reduced graphene oxide/PCL composite coating on TiO2 nanotube coated titanium. Ceram. Int. 2022, 48, 9579–9594. [Google Scholar] [CrossRef]
- Tabish, T.A.; Hussain, M.Z.; Zervou, S.; Myers, W.K.; Tu, W.; Xu, J.; Beer, I.; Huang, W.E.; Chandrawati, R.; Crabtree, M.J.; et al. S-nitrosocysteamine-functionalised porous graphene oxide nanosheets as nitric oxide delivery vehicles for cardiovascular applications. Redox Biol. 2024, 72, 103144. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Surhland, C.; Sanchez, Z.; Chaudhary, P.; Suresh Kumar, M.A.; Lee, S.; Peña, L.A.; Waring, M.; Sitharaman, B.; Naidu, M. Graphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiforme. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 109–118. [Google Scholar] [CrossRef]
- Li, L.; Xiang, F.; Wang, F.; Liu, Y. Preparation and antitumor study of intelligent injectable hydrogel: Carboxymethyl chitosan–aldehyde gum Arabic composite graphene oxide hydrogel. Int. J. Biol. Macromol. 2024, 259, 129429. [Google Scholar] [CrossRef]
- Shim, G.; Kim, J.-Y.; Han, J.; Chung, S.W.; Lee, S.; Byun, Y.; Oh, Y.-K. Reduced graphene oxide nanosheets coated with an anti-angiogenic anticancer low-molecular-weight heparin derivative for delivery of anticancer drugs. J. Control. Release 2014, 189, 80–89. [Google Scholar] [CrossRef]
- Yurdabak Karaca, G.; Bulbul, Y.E.; Oksuz, A.U. Gold-hyaluranic acid micromotors and cold atmospheric plasma for enhanced drug delivery and therapeutic applications. Int. J. Biol. Macromol. 2023, 253, 127075. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, H.; Domb, A.J.; Sharifzadeh, G.; Nahum, V. Gene Therapy for Regenerative Medicine. Pharmaceutics 2023, 15, 856. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.; Campbell, E.; Naumov, A. Multifunctional graphene oxide/iron oxide nanoparticles for magnetic targeted drug delivery dual magnetic resonance/fluorescence imaging and cancer sensing. PLoS ONE 2019, 14, e0217072. [Google Scholar] [CrossRef] [PubMed]
- Mohanaraman, S.P.; Chidambaram, R. A holistic review on red fluorescent graphene quantum dots, its synthesis, unique properties with emphasis on biomedical applications. Heliyon 2024, 10, e35760. [Google Scholar] [CrossRef]
- Othman, F.E.C.; Nordin, N.A.H.M.; Ismail, N.; Zakria, H.S.; Junoh, H.; Aziz, M.H.A. A review on sustainable graphene production from rice husks: Strategies and key considerations. Chem. Eng. J. 2024, 497, 154408. [Google Scholar] [CrossRef]
- Ahmad, F.; Ghazal, H.; Rasheed, F.; Shahid, M.; Vasantham, S.K.; Rafiq, W.; Abbas, Z.; Sarwar, S.; Ain, Q.U.; Waqar, A.; et al. Graphene and its derivatives in medical applications: A comprehensive review. Synth. Met. 2024, 304, 117594. [Google Scholar] [CrossRef]
- Ando, H.; Suzuki, K.; Kaji, H.; Kambe, T.; Nishina, Y.; Nakano, C.; Gotoh, K. Dynamic nuclear polarization—Nuclear magnetic resonance for analyzing surface functional groups on carbonaceous materials. Carbon 2023, 206, 84–93. [Google Scholar] [CrossRef]
- Torkashvand, N.; Sarlak, N. Polymerized graphene oxide/MnCe0.5Fe1.5O4 nanoferrofluid as a T2- and T2*-weighted contrast agent for magnetic resonance imaging. Colloids Surf. B Biointerfaces 2020, 185, 110555. [Google Scholar] [CrossRef]
- Li, Y.; Ma, P.; Tao, Q.; Krause, H.-J.; Yang, S.; Ding, G.; Dong, H.; Xie, X. Magnetic graphene quantum dots facilitate closed-tube one-step detection of SARS-CoV-2 with ultra-low field NMR relaxometry. Sens. Actuators B Chem. 2021, 337, 129786. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Tao, Q.; Ye, C.; Yu, M.; Li, J.; Zhou, H.; Yang, S.; Ding, G.; Xie, X. Enhancing the magnetic relaxivity of MRI contrast agents via the localized superacid microenvironment of graphene quantum dots. Biomaterials 2020, 250, 120056. [Google Scholar] [CrossRef]
- Su, Y.; Wang, N.; Liu, B.; Du, Y.; Li, R.; Meng, Y.; Feng, Y.; Shan, Z.; Meng, S. A phototheranostic nanoparticle for cancer therapy fabricated by BODIPY and graphene to realize photo-chemo synergistic therapy and fluorescence/photothermal imaging. Dye. Pigment. 2020, 177, 108262. [Google Scholar] [CrossRef]
- Liu, J.; Qin, L.; Kang, S.-Z.; Li, G.; Li, X. Gold nanoparticles/glycine derivatives/graphene quantum dots composite with tunable fluorescence and surface enhanced Raman scattering signals for cellular imaging. Mater. Des. 2017, 123, 32–38. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, H.; Ou, M.; Sun, D.; Yang, C. Luminescence and magnetic properties of bifunctional nanoparticles composited by nitrogen-doped graphene quantum dots and gadolinium. J. Rare Earths 2024, 42, 716–723. [Google Scholar] [CrossRef]
- Li, N.; Shi, L.; Zou, X.; Wang, T.; Wang, D.; Gong, Z.; Fan, M. Fluorescence immunoassay rapid detection of 2019-nCoV antibody based on the fluorescence resonance energy transfer between graphene quantum dots and Ag@Au nanoparticle. Microchem. J. 2022, 173, 107046. [Google Scholar] [CrossRef]
- Liu, H.; Hao, C.; Nan, Z.; Qu, H.; Zhang, X.; Zhang, Z.; Sun, R. Fabrication of graphene oxide and sliver nanoparticle hybrids for fluorescence quenching of DNA labeled by methylene blue. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 243, 118802. [Google Scholar] [CrossRef]
- Chu, B.; Chen, Z.; Shi, H.; Wu, X.; Wang, H.; Dong, F.; He, Y. Fluorescence, ultrasonic and photoacoustic imaging for analysis and diagnosis of diseases. Chem. Commun. 2023, 59, 2399–2412. [Google Scholar] [CrossRef]
- Nam, K.-H.; Im, Y.-O.; Park, H.J.; Lee, H.; Park, J.; Jeong, S.; Kim, S.M.; You, N.-H.; Choi, J.-H.; Han, H.; et al. Photoacoustic effect on the electrical and mechanical properties of polymer-infiltrated carbon nanotube fiber/graphene oxide composites. Compos. Sci. Technol. 2017, 153, 136–144. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, Y.; Xu, P.; Zhang, M.; Wu, H.; Yang, S. Graphene oxide / MnWO4 nanocomposite for magnetic resonance / photoacoustic dual-model imaging and tumor photothermo-chemotherapy. Carbon 2018, 138, 397–409. [Google Scholar] [CrossRef]
- Xuan, Y.; Zhang, R.-Y.; Zhao, D.-H.; Zhang, X.-S.; An, J.; Cheng, K.; Hou, X.-L.; Song, X.-L.; Zhao, Y.-D.; Yang, X.-Q. Ultrafast synthesis of gold nanosphere cluster coated by graphene quantum dot for active targeting PA/CT imaging and near-infrared laser/pH-triggered chemo-photothermal synergistic tumor therapy. Chem. Eng. J. 2019, 369, 87–99. [Google Scholar] [CrossRef]
- Sheng, Z.; Song, L.; Zheng, J.; Hu, D.; He, M.; Zheng, M.; Gao, G.; Gong, P.; Zhang, P.; Ma, Y.; et al. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials 2013, 34, 5236–5243. [Google Scholar] [CrossRef]
- Depan, D.; Shah, J.; Misra, R.D.K. Controlled release of drug from folate-decorated and graphene mediated drug delivery system: Synthesis, loading efficiency, and drug release response. Mater. Sci. Eng. C 2011, 31, 1305–1312. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, M.; Fan, Y.; Zhang, Q.; Wang, Y.; Yuan, W.; Zhou, N.; Che, J. Novel controlled drug release system engineered with inclusion complexes based on carboxylic graphene. Colloids Surf. B Biointerfaces 2019, 175, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.H.; Neila, F.; Smida, K.; Li, Z.; Abdelmalek, Z.; Tlili, I. pH-responsive anticancer drug delivery systems: Insights into the enhanced adsorption and release of DOX drugs using graphene oxide as a nanocarrier. Eng. Anal. Bound. Elem. 2023, 157, 157–165. [Google Scholar] [CrossRef]
- Güncüm, E.; Geyik, G.; Işıklan, N. Magnetic graphene oxide functionalized alginate-g-poly(2-hydroxypropylmethacrylamide) nanoplatform for near-infrared light/pH/magnetic field-sensitive drug release and chemo/phototherapy. Int. J. Pharm. 2024, 659, 124287. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.M.; Alqarni, Z.; Hamed, Y.S.; Yang, K.; Nour, H.F.; Lu, J. Biocompatible graphene oxide/carboxymethylated chitosan nanocomposite hydrogel for controlled release of polyphenolic antioxidants. J. Drug Deliv. Sci. Technol. 2024, 95, 105630. [Google Scholar] [CrossRef]
- Liao, R.; Zhang, Y.; Mao, W. Functionalized graphene oxide NPs as a nanocarrier for drug delivery system in quercetin/ lurbinectedin as dual sensitive therapeutics for A549 lung cancer treatment. Heliyon 2024, 10, e31212. [Google Scholar] [CrossRef]
- Lin, J.; Lin, J.-H.; Yeh, T.-Y.; Zheng, J.-H.; Cho, E.-C.; Lee, K.-C. Fabrication of hyaluronic acid with graphene quantum dot as a dual drug delivery system for cancer therapy. FlatChem 2024, 44, 100607. [Google Scholar] [CrossRef]
- Karimi, S.; Zeyni, V.; Namazi, H. A fluorescent system based on graphene quantum dots-capped magnetic hydroxyapatite-MIL-100 metal-organic frameworks for pH-sensitive and controlled release of DOX. Diam. Relat. Mater. 2023, 140, 110502. [Google Scholar] [CrossRef]
- Ali, R.; Aziz, M.H.; Gao, S.; Khan, M.I.; Li, F.; Batool, T.; Shaheen, F.; Qiu, B. Graphene oxide/zinc ferrite nanocomposite loaded with doxorubicin as a potential theranostic mediu in cancer therapy and magnetic resonance imaging. Ceram. Int. 2022, 48, 10741–10750. [Google Scholar] [CrossRef]
- Haider, M.; Cagliani, R.; Jagal, J.; Jayakumar, M.N.; Fayed, B.; Shakartalla, S.B.; Pasricha, R.; Greish, K.; El-Awady, R. Peptide-functionalized graphene oxide quantum dots as colorectal cancer theranostics. J. Colloid Interface Sci. 2023, 630, 698–713. [Google Scholar] [CrossRef]
- Ghafary, S.M.; Rahimjazi, E.; Hamzehil, H.; Mousavi, S.M.M.; Nikkhah, M.; Hosseinkhani, S. Design and preparation of a theranostic peptideticle for targeted cancer therapy: Peptide-based codelivery of doxorubicin/curcumin and graphene quantum dots. Nanomed. -Nanotechnol. Biol. Med. 2022, 42, 102544. [Google Scholar] [CrossRef]
- Tehrani Fateh, S.; Ahmadi Kamalabadi, M.; Aliakbarniya, A.; Jafarinejad-Farsangi, S.; Koohi, M.; Jafari, E.; Miri Karam, Z.; Keyhanfar, F.; Shiralizadeh Dezfuli, A. Hydrophobic@amphiphilic hybrid nanostructure of iron-oxide and graphene quantum dot surfactant as a theranostic platform. OpenNano 2022, 6, 100037. [Google Scholar] [CrossRef]
- Saranya, J.; Saminathan, P.; Christabel Pravin, S.; Shaik, M.R.; Alwarthan, A.; Khan, M.; Shaik, B. Qualitative assessment on cisplatin loaded CeO2/Au/GO hybrid as theranostics platform in HeLa cell lines. Arab. J. Chem. 2023, 16, 105096. [Google Scholar] [CrossRef]
- Bugárová, N.; Annušová, A.; Bodík, M.; Šiffalovič, P.; Labudová, M.; Kajanová, I.; Zaťovičová, M.; Pastoreková, S.; Majková, E.; Omastová, M. Molecular targeting of bioconjugated graphene oxide nanocarriers revealed at a cellular level using label-free Raman imaging. Nanomed. Nanotechnol. Biol. Med. 2020, 30, 102280. [Google Scholar] [CrossRef] [PubMed]
- Ahina, K.M.; Kannan, K.; Vijayan, V.; Sreekumar, S.; Lakra, R.; Kiran, M.S. Zero dimensional Graphene Quantum Dots self-assembled collagen 3D bio-matrices for soft tissue regeneration. Mater. Today Commun. 2023, 37, 107244. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Contessotto, P.; Nedjari, S.; Martino, M.M.; Redenski, I.; Gabet, Y.; Speranza, G.; O’Brien, T.; Altankov, G.; Awaja, F. Clinical potential of plasma-functionalized graphene oxide ultrathin sheets for bone and blood vessel regeneration: Insights from cellular and animal models. Biomater. Adv. 2024, 161, 213867. [Google Scholar] [CrossRef]
- Ghosh, S.; Dhiman, M.; Gupta, S.; Roy, P.; Lahiri, D. Electro-conductive chitosan/graphene bio-nanocomposite scaffold for tissue engineering of the central nervous system. Biomater. Adv. 2023, 154, 213596. [Google Scholar] [CrossRef]
- Verstappen, K.; Klymov, A.; Cicuéndez, M.; da Silva, D.M.; Barroca, N.; Fernández-San-Argimiro, F.-J.; Madarieta, I.; Casarrubios, L.; Feito, M.J.; Diez-Orejas, R.; et al. Biocompatible adipose extracellular matrix and reduced graphene oxide nanocomposite for tissue engineering applications. Mater. Today Bio 2024, 26, 101059. [Google Scholar] [CrossRef]
- Motiee, E.-S.; Karbasi, S.; Bidram, E.; Sheikholeslam, M. Investigation of physical, mechanical and biological properties of polyhydroxybutyrate-chitosan/graphene oxide nanocomposite scaffolds for bone tissue engineering applications. Int. J. Biol. Macromol. 2023, 247, 125593. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Y.; Hou, Y.; Meng, D.; Pan, K.; Bartolo, P.; Li, L. Laser-induced fabrication of doped-graphene based on collagen for bone tissue engineering scaffold applications. CIRP Ann. 2024, 73, 165–168. [Google Scholar] [CrossRef]
- Zaman, M.S.; Khosravieh, Z.F.; Ahssan, M.; Salehiamin, M.; Ghoraishizadeh, S.; Darvishnia, F.; Rahmani, E.; Esmaeili, J. Fabrication of a conduit for future peripheral nerve regeneration using decellularized plant tissue modified with polyaniline/graphene oxide nanosheet. Mater. Today Commun. 2024, 39, 109204. [Google Scholar] [CrossRef]
- Sampath, V.; Krishnasamy, V. Synthesis and characterization of hydroxyapatite self-assembled nanocomposites on graphene oxide sheets from seashell waste: A green process for regenerative medicine. J. Mech. Behav. Biomed. Mater. 2024, 151, 106383. [Google Scholar] [CrossRef] [PubMed]
- Meira, R.M.; Ribeiro, S.; Irastorza, I.; Silván, U.; Lanceros-Mendez, S.; Ribeiro, C. Electroactive poly(vinylidene fluoride-trifluoroethylene)/graphene composites for cardiac tissue engineering applications. J. Colloid Interface Sci. 2024, 663, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, S.; Talebi, A.; Labbaf, S.; Karimzadeh, F. Conductive GelMA/alginate/polypyrrole/graphene hydrogel as a potential scaffold for cardiac tissue engineering; Physiochemical, mechanical, and biological evaluations. Int. J. Biol. Macromol. 2024, 259, 129276. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Tsai, P.-H.; Lin, Y.-H.; Huang, C.-Y.; Chung, J.H.Y.; Chen, G.-Y. Controllable graphene oxide-based biocompatible hybrid interface as an anti-fibrotic coating for metallic implants. Mater. Today Bio 2022, 15, 100326. [Google Scholar] [CrossRef]
- Zhang, J.; Singh, P.; Cao, Z.; Rahimi, S.; Pandit, S.; Mijakovic, I. Polydopamine/graphene oxide coatings loaded with tetracycline and green Ag nanoparticles for effective prevention of biofilms. Appl. Surf. Sci. 2023, 626, 157221. [Google Scholar] [CrossRef]
- Kaviya, M.; Ramakrishnan, P.; Mohamed, S.B.; Ramakrishnan, R.; Gimbun, J.; Veerabadran, K.M.; Kuppusamy, M.R.; Kaviyarasu, K.; Sridhar, T.M. Synthesis and characterization of nano-hydroxyapatite/graphene oxide composite materials for medical implant coating applications. Mater. Today Proc. 2021, 36, 204–207. [Google Scholar] [CrossRef]
- Romo-Rico, J.; Bright, R.; Krishna, S.M.; Vasilev, K.; Golledge, J.; Jacob, M.V. Antimicrobial graphene-based coatings for biomedical implant applications. Carbon Trends 2023, 12, 100282. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Zhou, B.; Jiang, X.; Zhang, D.; Luo, H. Graphene oxide/gallium nanoderivative as a multifunctional modulator of osteoblastogenesis and osteoclastogenesis for the synergistic therapy of implant-related bone infection. Bioact. Mater. 2023, 25, 594–614. [Google Scholar] [CrossRef]
- Qin, W.; Xing, T.; Tang, B.; Chen, W. Mechanical properties and osteogenesis of CFR-PEEK composite with interface strengthening by graphene oxide for implant application. J. Mech. Behav. Biomed. Mater. 2023, 148, 106222. [Google Scholar] [CrossRef]
- Menassol, G.; Dubois, L.; Nadolska, M.; Vadgama, P.; Martin, D.K.; Zebda, A. A biocompatible iron doped graphene based cathode for an implantable glucose biofuel cell. Electrochim. Acta 2023, 439, 141627. [Google Scholar] [CrossRef]
- Zhao, W.S.; Yang, S.; Zhang, D.X.; Zhou, T.X.; Huang, J.; Gao, M.; Jiang, Y.H.; Liu, Y.; Yang, J.H. Ultrasensitive dual-enhanced sandwich strategy for simultaneous detection of Escherichia coli and Staphylococcus aureus based on optimized aptamers-functionalized magnetic capture probes and graphene oxide-Au nanostars SERS tags. J. Colloid Interface Sci. 2023, 634, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Karasu, T.; İdil, N.; Özgür, E.; Uzun, L. Pseudomonas aeruginosa imprinted polydopamine@graphene-coated pencil graphite electrode for selective bacterial detection. Colloids Surf. A Physicochem. Eng. Asp. 2024, 681, 132788. [Google Scholar] [CrossRef]
- Chen, Y.; Xuan, X.; Li, M.; Jiang, D.; Li, H. Implantable antenna immunosensor based on Au-decorated graphene film for wireless CEA detection. Sens. Actuators B Chem. 2024, 416, 136037. [Google Scholar] [CrossRef]
- Tsou, K.-L.; Cheng, Y.-T. Miniaturized inkjet-printed flexible ion-selective sensing electrodes with the addition of graphene in PVC layer for fast response real-time monitoring applications. Talanta 2024, 275, 126107. [Google Scholar] [CrossRef]


| Sensor Surface Type | Biomolecule Detected | LOD | References |
|---|---|---|---|
| polystyrene | miRNA-21 | 1.74 pM | [113] |
| metal–organic frameworks (CuMOF, Fe@rGO) | microRNA 155 | 0.08 fM | [111] |
| micro-tapered long-period fiber grating functionalized by graphene oxide (GO-MTLPFG) | pepsin | 25.79 ng/mL | [69] |
| RGO, Au nanoparticles, and the aptamer conjugated on the polythiophene (PTP) modified electrode | H. pylori (Hsp60) | 0.0080 μM | [114] |
| printed electrochemical biosensor | glucose | 1.34 mM | [128] |
| six pairs of interdigital Cr/Au electrodes supported on Si/SiO2 substrate with an avidin immobilized | biotin | 90 fg/mL (0.37 pM) | [129] |
| aptamer and oligonucleotide–gold nanoparticles | insulin | 1.6 × 10−12 moL/L | [130] |
| thiophenol moieties | hepatitis C virus core protein | 90.9 aM | [131] |
| aptamers-functionalized graphene and magnetic β-cyclodextrin polymers | adenosine | 2.1 × 10−13 mol/L | [132] |
| (His)-tagged acetylcholinesterase (AChE) | paraoxon | 3 μM | [133] |
| graphene–peptide conjugates | leucine-rich alpha-2 glycoprotein-1 | 75 pg/mL | [134] |
| Complex Structure | Application | Specifications | References |
|---|---|---|---|
| MMC–Graphene@BODIPY–mPEG (MGBP) | fluorescence and photothermal imaging photo-chemotherapy | complementary has the ability for drug release | [162] |
| AuNPs/Gn/GQDs | fluorescence and SERS | minimal cytotoxic level in A549 cells | [163] |
| nitrogen-doped graphene quantum dots (N-GQDs) and gadolinium ions (Gd3+) | fluorescence and magnetic resonance imaging | biological imaging and diagnosis, drug release, good colloidal stability, hydrophilic | [164] |
| MNP–Herceptin–SK–BR3 cell-Herceptin–GQNP | fluorescence immunosensor | HER2+ breast cancer cell target | [103] |
| GQDs and Ag@AuNPs | antibody fluorescence immunoassay | good selectivity and sensitivity for 2019-nCoV mAb | [165] |
| graphene oxide–silver nanoparticles (GO-AgNPs) | fluorescence fluoresced to DNA | hydro solubility | [166] |
| Platform Structure | Specifications | References |
|---|---|---|
| graphene oxide encapsulated with folic acid-conjugated chitosan | pH sensitive, optimum value 5.3 | [172] |
| Hydroxypropyl-β-cyclodextrins (HP-β-CD) and carboxylated graphene nanomaterial | hydrophilic properties | [173] |
| GO nanocarrier | acidic environment activation | [174] |
| furan-containing copolymers embedded with rGO conjugated with maleimide-containing target molecules | photothermal activation | [139] |
| mGO@AL-g-PHPM@ICG/EP | light stability and photothermal conversion capacity | [175] |
| graphene oxide/carboxymethylated chitosan (GO/CMCh) nanocomposite hydrogel | minimal hemolysis ratio | [176] |
| quercetin/lurbinectedin-loaded GO NPs | toxic effect in neoplasm cell | [177] |
| hyaluronic acid (HA), graphene quantum dots (GQDs), and polyethyleneimine (PEI) | dual drug release system | [178] |
| graphene quantum dot | antimlaria system (Plasmodium falciparum) | [102] |
| SiO2@Fe3O4-HA-MIL100 and SiO2@Fe3O4-HA-MIL-100-GQDs nanocomposites | antioxidant properties | [179] |
| Sensor Surface Type | Biomolecule Detected | LOD | References |
|---|---|---|---|
| palladium-modified graphene oxide | zearalenone | 0.16 pg mL−1 | [3] |
| molecularly imprinted polydopamine films | Pseudomonas aeruginosa | 1.85 CFU/mL | [204] |
| sensitive film of Au-nanoparticles furnished with graphene | carcinoembryonic antigen | 2.46 MHz/log (ng/mL) | [205] |
| graphene PVC film | ion-selective | 0.1–100 mM | [206] |
| graphene oxide-Au surface-enhanced Raman scattering tag | Escherichia coli, Staphylococcus aureus | 10 cfu/mL | [203] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gostaviceanu, A.; Gavrilaş, S.; Copolovici, L.; Copolovici, D.M. Graphene-Oxide Peptide-Containing Materials for Biomedical Applications. Int. J. Mol. Sci. 2024, 25, 10174. https://doi.org/10.3390/ijms251810174
Gostaviceanu A, Gavrilaş S, Copolovici L, Copolovici DM. Graphene-Oxide Peptide-Containing Materials for Biomedical Applications. International Journal of Molecular Sciences. 2024; 25(18):10174. https://doi.org/10.3390/ijms251810174
Chicago/Turabian StyleGostaviceanu, Andreea, Simona Gavrilaş, Lucian Copolovici, and Dana Maria Copolovici. 2024. "Graphene-Oxide Peptide-Containing Materials for Biomedical Applications" International Journal of Molecular Sciences 25, no. 18: 10174. https://doi.org/10.3390/ijms251810174










