Influence of Intraocular Pressure on the Expression and Activity of Sodium–Potassium Pumps in the Corneal Endothelium
Abstract
:1. Introduction
2. Results
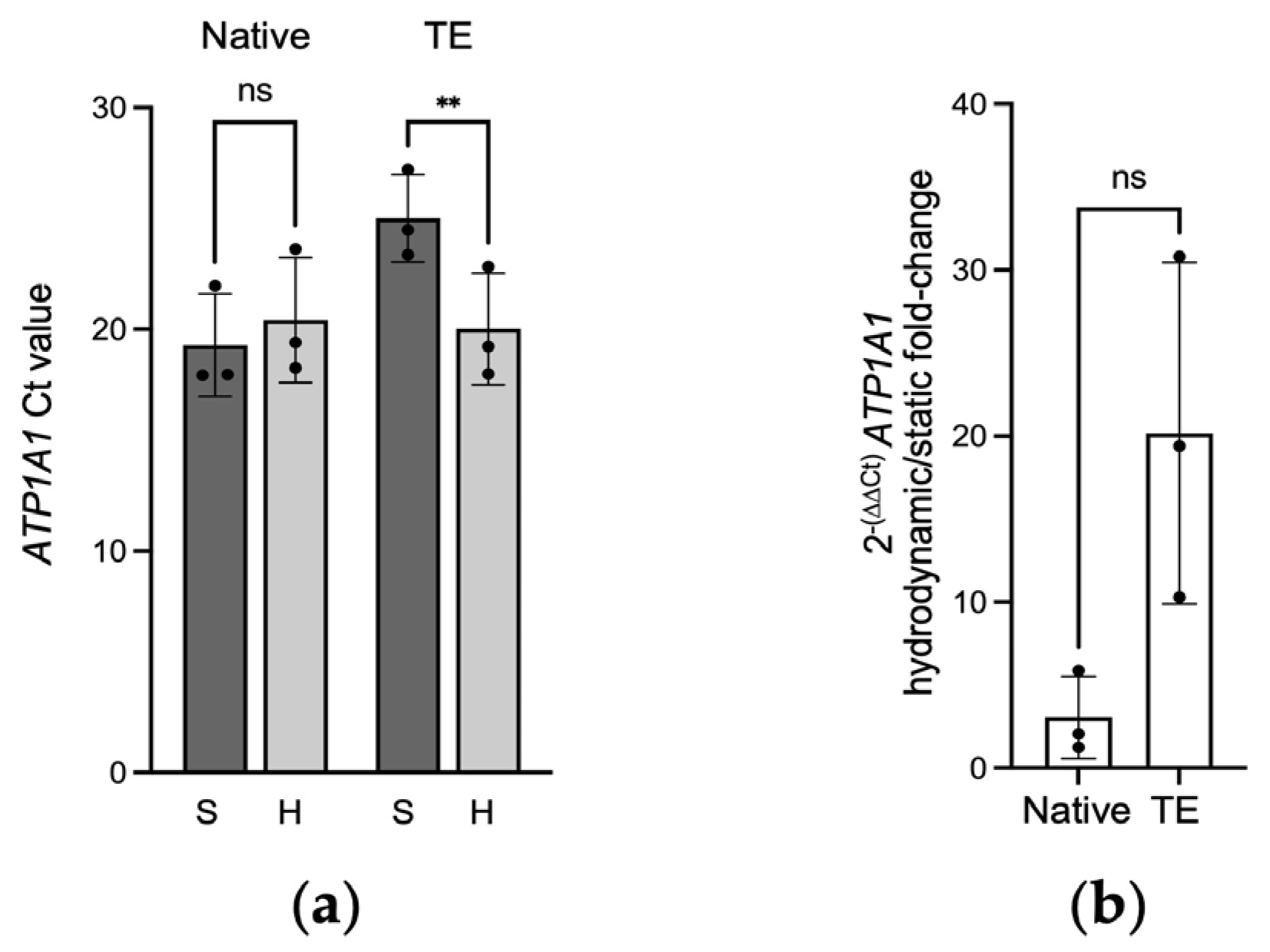
2.1. Gene Expression of ATP1A1 in Native and Tissue-Engineered Corneal Endothelium
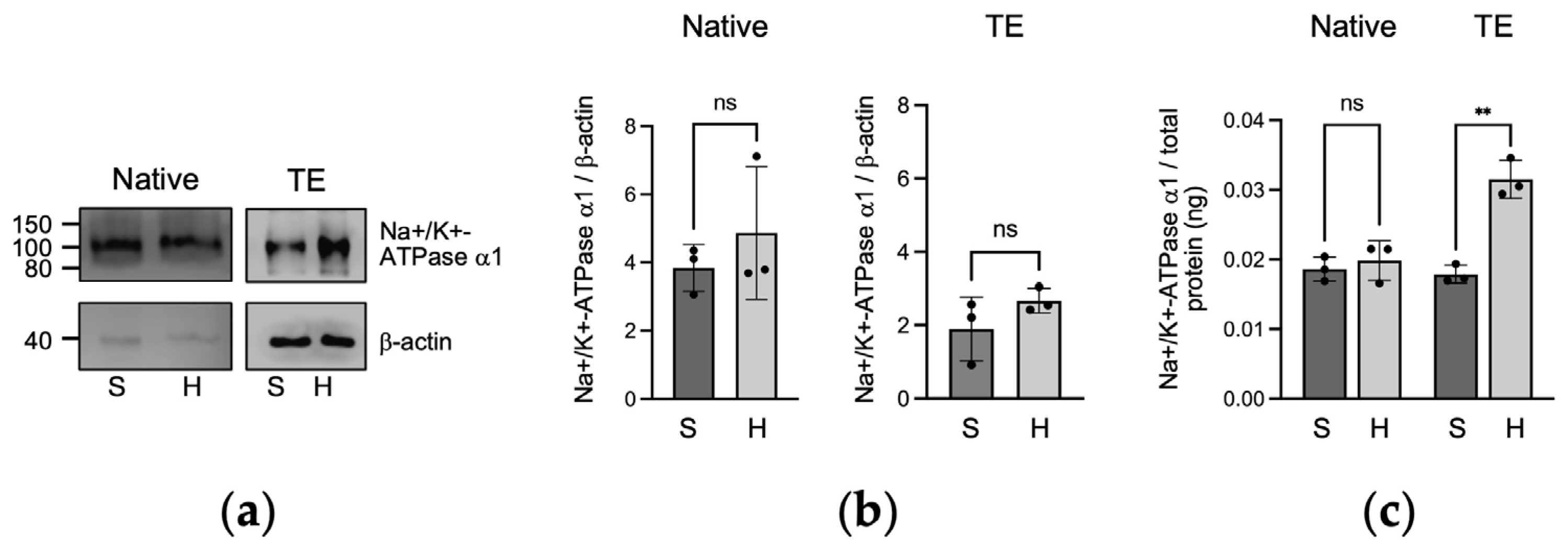
2.2. Protein Expression of Na+/K+-ATPase α1 in Native and Tissue-Engineered Corneal Endothelium
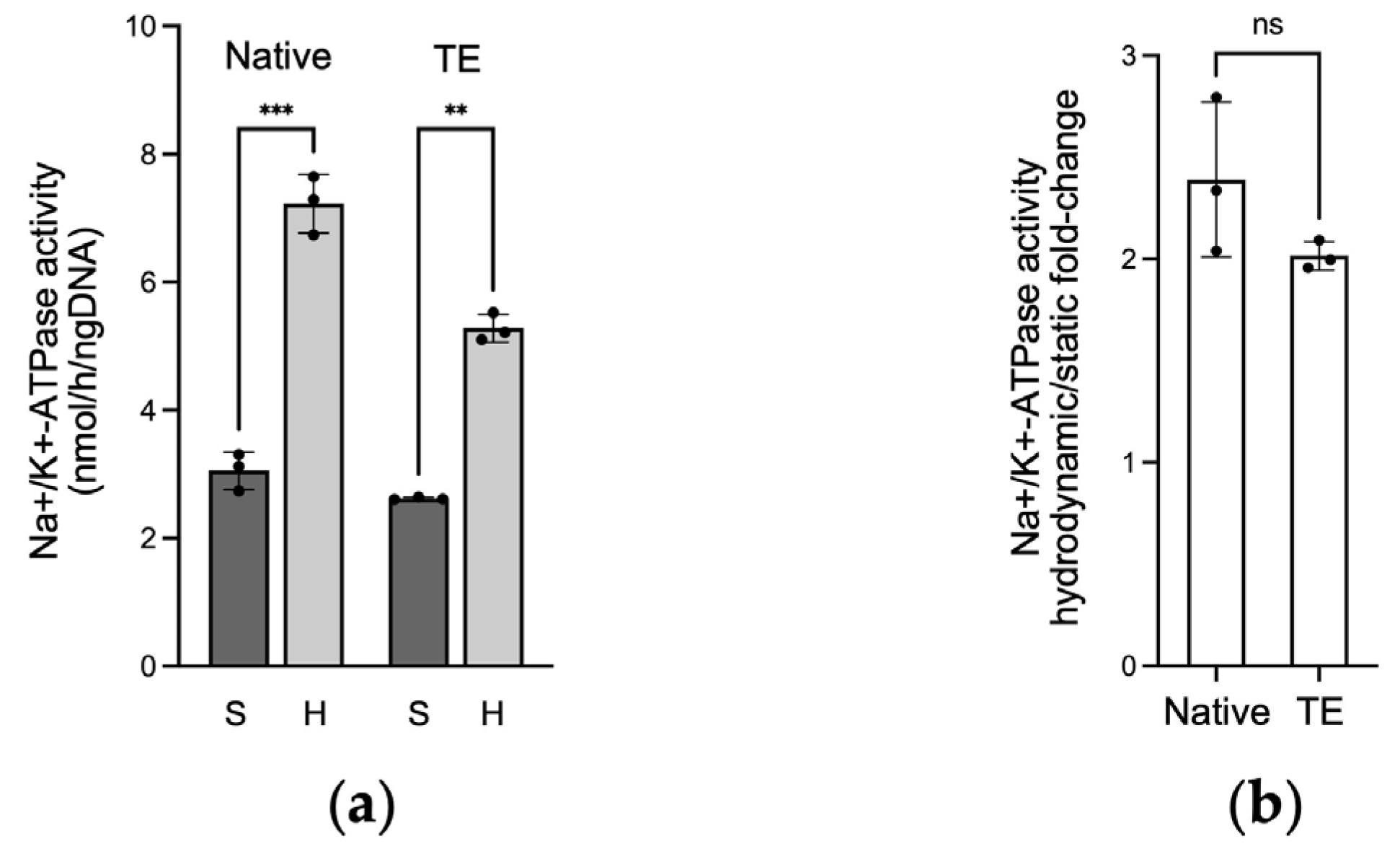
2.3. Na+/K+-ATPase Activity in Native and Tissue-Engineered Corneal Endothelium
3. Discussion
4. Materials and Methods
4.1. Corneal Specimens
4.2. Cell Culture and Tissue Engineering of the Corneal Endothelium
4.3. Culture under Hydrodynamic and Static Conditions
4.4. Quantitative PCR (qPCR)
4.5. Western Blotting Analysis
4.6. ELISA Analysis
4.7. ATPase Assay
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angulo-Urarte, A.; van der Wal, T.; Huveneers, S. Cell-cell junctions as sensors and transducers of mechanical forces. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183316. [Google Scholar] [CrossRef] [PubMed]
- Martinac, B. Mechanosensitive ion channels: Molecules of mechanotransduction. J. Cell Sci. 2004, 117, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Ridone, P.; Vassalli, M.; Martinac, B. Piezo1 mechanosensitive channels: What are they and why are they important. Biophys. Rev. 2019, 11, 795–805. [Google Scholar] [CrossRef]
- Driscoll, T.P.; Bidone, T.C.; Ahn, S.J.; Yu, A.; Groisman, A.; Voth, G.A.; Schwartz, M.A. Integrin-based mechanosensing through conformational deformation. Biophys. J. 2021, 120, 4349–4359. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Forest, F.; Gain, P.; Rageade, D.; Bernard, A.; Acquart, S.; Peoc’h, M.; Defoe, D.M.; Thuret, G. 3D map of the human corneal endothelial cell. Sci. Rep. 2016, 6, 29047. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef]
- Lai, J.Y.; Chen, K.H.; Hsiue, G.H. Tissue-engineered human corneal endothelial cell sheet transplantation in a rabbit model using functional biomaterials. Transplantation 2007, 84, 1222–1232. [Google Scholar] [CrossRef]
- Proulx, S.; Bensaoula, T.; Nada, O.; Audet, C.; d’Arc Uwamaliya, J.; Devaux, A.; Allaire, G.; Germain, L.; Brunette, I. Transplantation of a tissue-engineered corneal endothelium reconstructed on a devitalized carrier in the feline model. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2686–2694. [Google Scholar] [CrossRef]
- Okumura, N.; Matsumoto, D.; Fukui, Y.; Teramoto, M.; Imai, H.; Kurosawa, T.; Shimada, T.; Kruse, F.; Schlotzer-Schrehardt, U.; Kinoshita, S.; et al. Feasibility of cell-based therapy combined with descemetorhexis for treating Fuchs endothelial corneal dystrophy in rabbit model. PLoS ONE 2018, 13, e0191306. [Google Scholar] [CrossRef]
- Edelhauser, H.F. The balance between corneal transparency and edema: The Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1754–1767. [Google Scholar] [CrossRef]
- Blanco, G.; DeTomaso, A.W.; Koster, J.; Xie, Z.J.; Mercer, R.W. The alpha-subunit of the Na,K-ATPase has catalytic activity independent of the beta-subunit. J. Biol. Chem. 1994, 269, 23420–23425. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.J.; Alvarado, J.A.; Weddel, J.E. Histology of the Human Eye: An Atlas and Text-Book; W.B. Saunders: Philadelphia, PA, USA, 1971; p. 687. [Google Scholar]
- Hatou, S.; Higa, K.; Inagaki, E.; Yoshida, S.; Kimura, E.; Hayashi, R.; Tsujikawa, M.; Tsubota, K.; Nishida, K.; Shimmura, S. Validation of Na,K-ATPase pump function of corneal endothelial cells for corneal regenerative medicine. Tissue Eng. Part C Methods 2013, 19, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Millard, C.; Kaufman, P.L. Aqueous humor: Secretion and dynamics. In Duane’s Foundations of Clinical Ophthalmology; Tasman, W.J.E., Ed.; Lippincott-Raven: Philadelphia, PA, USA, 1995. [Google Scholar]
- Carlson, K.H.; Bourne, W.M.; McLaren, J.W.; Brubaker, R.F. Variations in human corneal endothelial cell morphology and permeability to fluorescein with age. Exp. Eye Res. 1988, 47, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Theriault, M.; Roy, O.; Brunette, I.; Proulx, S. Physiological pressure enhances the formation of tight junctions in engineered and native corneal endothelium. Exp. Eye Res. 2019, 179, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Anney, P.; Theriault, M.; Proulx, S. Hydrodynamic forces influence the gene transcription of mechanosensitive intercellular junction associated genes in corneal endothelial cells. Exp. Eye Res. 2021, 206, 108532. [Google Scholar] [CrossRef]
- Rajasekaran, S.A.; Beyenbach, K.W.; Rajasekaran, A.K. Interactions of tight junctions with membrane channels and transporters. Biochim. Biophys. Acta 2008, 1778, 757–769. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Atilano, S.R.; Garner, M.H.; Maguen, E.; Nesburn, A.B.; Kenney, M.C. Extracellular matrix and Na+,K+-ATPase in human corneas following cataract surgery: Comparison with bullous keratopathy and Fuchs’ dystrophy corneas. Cornea 2002, 21, 74–80. [Google Scholar] [CrossRef]
- Proulx, S.; Audet, C.; Uwamaliya, J.; Deschambeault, A.; Carrier, P.; Giasson, C.J.; Brunette, I.; Germain, L. Tissue engineering of feline corneal endothelium using a devitalized human cornea as carrier. Tissue Eng. Part A 2009, 15, 1709–1718. [Google Scholar] [CrossRef]
- Proulx, S.; d’Arc Uwamaliya, J.; Carrier, P.; Deschambeault, A.; Audet, C.; Giasson, C.J.; Guerin, S.L.; Auger, F.A.; Germain, L. Reconstruction of a human cornea by the self-assembly approach of tissue engineering using the three native cell types. Mol. Vis. 2010, 16, 2192–2201. [Google Scholar]
- Theriault, M.; Gendron, S.P.; Brunette, I.; Rochette, P.J.; Proulx, S. Function-Related Protein Expression in Fuchs Endothelial Corneal Dystrophy Cells and Tissue Models. Am. J. Pathol. 2018, 188, 1703–1712. [Google Scholar] [CrossRef]
- Garcin, T.; Gauthier, A.S.; Crouzet, E.; He, Z.; Herbepin, P.; Perrache, C.; Acquart, S.; Cognasse, F.; Forest, F.; Thuret, G.; et al. Innovative corneal active storage machine for long-term eye banking. Am. J. Transplant. 2019, 19, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Schmedt, T.; Chen, Y.; Nguyen, T.T.; Li, S.; Bonanno, J.A.; Jurkunas, U.V. Telomerase immortalization of human corneal endothelial cells yields functional hexagonal monolayers. PLoS ONE 2012, 7, e51427. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Blanco, G.; Mercer, R.W.; Fleming, T.; Pepose, J.S. Human corneal endothelial cell expression of Na+,K+-adenosine triphosphatase isoforms. Arch. Ophthalmol. 2003, 121, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, Y.; Lei, H.; Zeng, Y.; Cui, Z.; Zeng, Q.; Zhu, D.; Lian, R.; Zhang, J.; Chen, Z.; et al. In vitro biomimetic platforms featuring a perfusion system and 3D spheroid culture promote the construction of tissue-engineered corneal endothelial layers. Sci. Rep. 2017, 7, 777. [Google Scholar] [CrossRef]
- Jayaram, H.; Kolko, M.; Friedman, D.S.; Gazzard, G. Glaucoma: Now and beyond. Lancet 2023, 402, 1788–1801. [Google Scholar] [CrossRef]
- Turner, D.C.; Girkin, C.A.; Downs, J.C. The Magnitude of Intraocular Pressure Elevation Associated with Eye Rubbing. Ophthalmology 2019, 126, 171–172. [Google Scholar] [CrossRef]
- Pecora, L.; Sibony, P.; Fourman, S. Eye-rubbing optic neuropathy. Am. J. Ophthalmol. 2002, 134, 460–461. [Google Scholar] [CrossRef]
- Nagarsheth, M.; Singh, A.; Schmotzer, B.; Babineau, D.C.; Sugar, J.; Lee, W.B.; Iyengar, S.K.; Lass, J.H.; Fuchs’ Genetics Multi-Center Study, G. Relationship Between Fuchs Endothelial Corneal Dystrophy Severity and Glaucoma and/or Ocular Hypertension. Arch. Ophthalmol. 2012, 130, 1384–1388. [Google Scholar] [CrossRef]
- Yarishkin, O.; Phuong, T.T.T.; Baumann, J.M.; De Ieso, M.L.; Vazquez-Chona, F.; Rudzitis, C.N.; Sundberg, C.; Lakk, M.; Stamer, W.D.; Krizaj, D. Piezo1 channels mediate trabecular meshwork mechanotransduction and promote aqueous fluid outflow. J. Physiol. 2021, 599, 571–592. [Google Scholar] [CrossRef]
- Zhu, W.; Hou, F.; Fang, J.; Bahrani Fard, M.R.; Liu, Y.; Ren, S.; Wu, S.; Qi, Y.; Sui, S.; Read, A.T.; et al. The role of Piezo1 in conventional aqueous humor outflow dynamics. iScience 2021, 24, 102042. [Google Scholar] [CrossRef]
- Garcia-Sanchez, J.; Lin, D.; Liu, W.W. Mechanosensitive ion channels in glaucoma pathophysiology. Vision. Res. 2024, 223, 108473. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, A.; Mammoto, T.; Ingber, D.E. Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 2012, 125, 3061–3073. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Liu, K.; Wang, F.; Su, Y. Role of mechanically-sensitive cation channels Piezo1 and TRPV4 in trabecular meshwork cell mechanotransduction. Hum. Cell 2024, 37, 394–407. [Google Scholar] [CrossRef]
- Rayson, B.M. [Ca2+]i regulates transcription rate of the Na+/K+-ATPase alpha 1 subunit. J. Biol. Chem. 1991, 266, 21335–21338. [Google Scholar] [CrossRef] [PubMed]
- Shahidullah, M.; Rosales, J.L.; Delamere, N. Activation of Piezo1 Increases Na,K-ATPase-Mediated Ion Transport in Mouse Lens. Int. J. Mol. Sci. 2022, 23, 12870. [Google Scholar] [CrossRef] [PubMed]
- Haydari, M.N.; Perron, M.C.; Laprise, S.; Roy, O.; Cameron, J.D.; Proulx, S.; Brunette, I. A short-term in vivo experimental model for Fuchs endothelial corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6343–6354. [Google Scholar] [CrossRef] [PubMed]
- Goyer, B.; Theriault, M.; Gendron, S.P.; Brunette, I.; Rochette, P.J.; Proulx, S. Extracellular Matrix and Integrin Expression Profiles in Fuchs Endothelial Corneal Dystrophy Cells and Tissue Model. Tissue Eng. Part A 2018, 24, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Rajabi, S.; Pedrigi, R.M.; Overby, D.R.; Read, A.T.; Ethier, C.R. In vitro models for glaucoma research: Effects of hydrostatic pressure. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6329–6339. [Google Scholar] [CrossRef]
- Santerre, K.; Xu, I.; Theriault, M.; Proulx, S. In Vitro Expansion of Corneal Endothelial Cells for Transplantation. Methods Mol. Biol. 2020, 2145, 17–27. [Google Scholar] [CrossRef]

| No. | Age, Sex | Preservation | Delay between Death and Culture (days) | Passage | Figure |
|---|---|---|---|---|---|
| 1 | 65, F | Optisol, 4 °C | 30 | 4 | Figure 1—PCR |
| 2 | 58, F | Optisol, 4 °C | n/a | 9 | Figure 1—PCR |
| 3 | 80, M | Fresh | 2 | 6 | Figure 1—PCR |
| 4 | 64, M | Optisol, 4 °C | 41 | 1 | Figure 2—WB/ELISA |
| 5 | 53, F | Optisol, 4 °C | 41 | 1 | Figure 2—WB/ELISA |
| 6 | 85, F | Optisol, 4 °C | 41 | 1 | Figure 2—WB/ELISA |
| 7 | 79, M | Optisol, 4 °C | 26 | 3 | Figure 4—ATPase activity |
| 8 | 83, M | Fresh | 7 | 1 | Figure 4—ATPase activity |
| 9 | 48, M | Optisol, 4 °C | 5 | 2 | Figure 4—ATPase activity |
| No. | Age, Sex | Preservation | Time Preserved at −20 °C (days) | Figure |
|---|---|---|---|---|
| 10 | 72, F | Optisol, 4 °C | 373 | Figure 1—PCR |
| 11 | 83, M | Optisol, 4 °C | 113 | Figure 1—PCR |
| 12 | 77, F | Optisol, 4 °C | 35 | Figure 1—PCR |
| 13 | 66, F | Optisol, 4 °C | 106 | Figure 2—WB/ELISA |
| 14 | 65, M | Optisol, 4 °C | 113 | Figure 2—WB/ELISA |
| 15 | 67, F | Optisol, 4 °C | 91 | Figure 2—WB/ELISA |
| 16 | 76, M | Optisol, 4 °C | 43 | Figure 4—ATPase activity |
| 17 | 74, F | Optisol, 4 °C | 119 | Figure 4—ATPase activity |
| 18 | 70, M | Optisol, 4 °C | 55 | Figure 4—ATPase activity |
| No. | Age, Sex | Preservation | Delay between Death and Bioreactor (days) | Figure |
|---|---|---|---|---|
| 19 | 70, M | Fresh | 4 | Figure 1—PCR |
| 20 | 70, F | Fresh | 5 | Figure 1—PCR |
| 21 | 56, F | Fresh | 3 | Figure 1—PCR |
| 22 | 78, F | Optisol, 4 °C | 28 | Figure 2—WB/ELISA |
| 23 | 84, F | Optisol, 4 °C | 20 | Figure 2—WB/ELISA |
| 24 | 76, F | Optisol, 4 °C | 9 | Figure 2—WB/ELISA |
| 25 | 79, M | Fresh | 12 | Figure 4—ATPase activity |
| 26 | 64, F | Fresh | 10 | Figure 4—ATPase activity |
| 27 | 80, M | Fresh | 9 | Figure 4—ATPase activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anney, P.; Charpentier, P.; Proulx, S. Influence of Intraocular Pressure on the Expression and Activity of Sodium–Potassium Pumps in the Corneal Endothelium. Int. J. Mol. Sci. 2024, 25, 10227. https://doi.org/10.3390/ijms251810227
Anney P, Charpentier P, Proulx S. Influence of Intraocular Pressure on the Expression and Activity of Sodium–Potassium Pumps in the Corneal Endothelium. International Journal of Molecular Sciences. 2024; 25(18):10227. https://doi.org/10.3390/ijms251810227
Chicago/Turabian StyleAnney, Princia, Pascale Charpentier, and Stéphanie Proulx. 2024. "Influence of Intraocular Pressure on the Expression and Activity of Sodium–Potassium Pumps in the Corneal Endothelium" International Journal of Molecular Sciences 25, no. 18: 10227. https://doi.org/10.3390/ijms251810227







