Novel Bioplastic Based on PVA Functionalized with Anthocyanins: Synthesis, Biochemical Properties and Food Applications
Abstract
:1. Introduction
2. Results and Discussions
2.1. Response Surface Optimization and Verification of Predictive Model
2.2. Statistical Analysis and Model Fitting
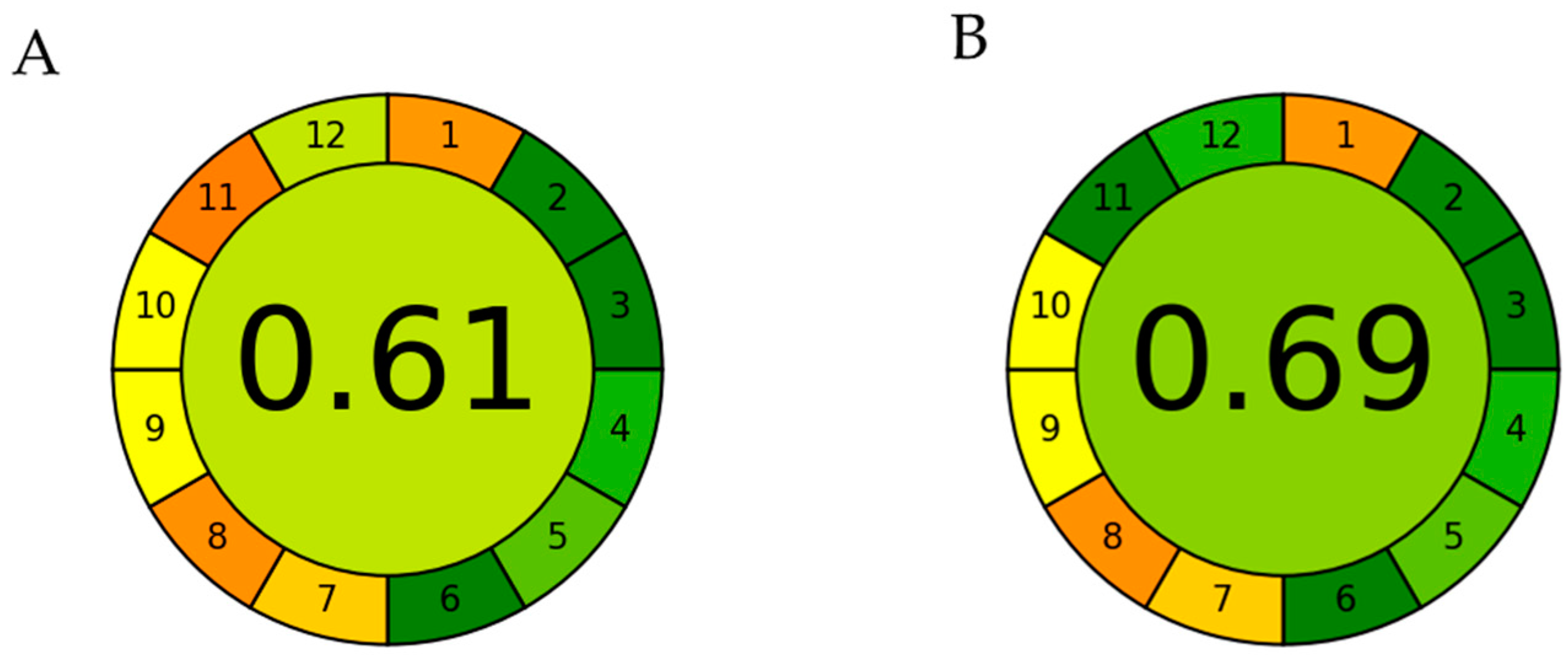
2.3. Optimization of MAE Conditions
2.4. RP-HPLC-DAD Identification of the Obtained Anthocyanins
2.5. Preparation and Characterization of PVA-Based Bioplastic with Anthocyanins
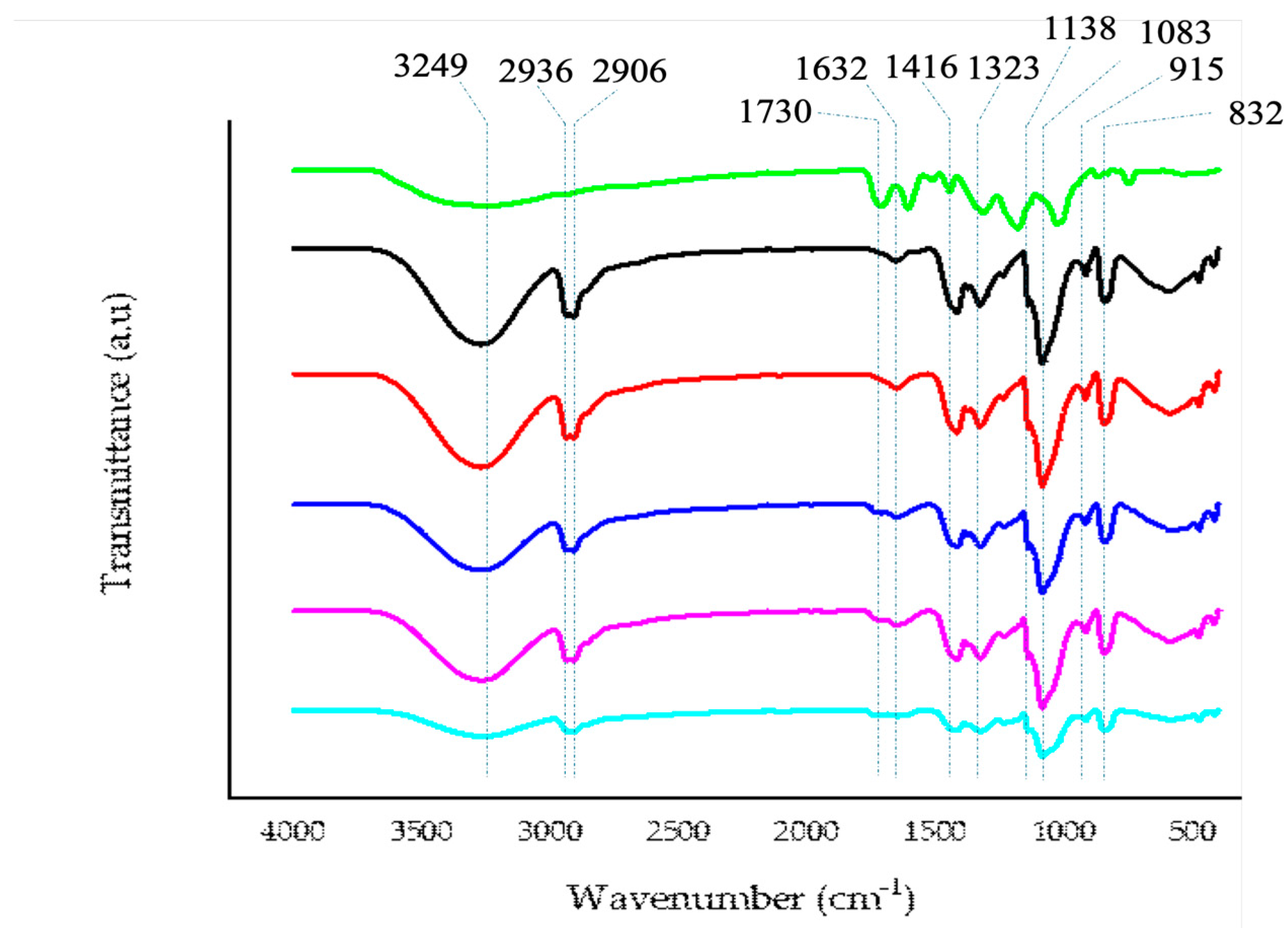
2.5.1. FTIR
2.5.2. Optical Properties
2.6. Mechanical Properties
2.7. Migration Test
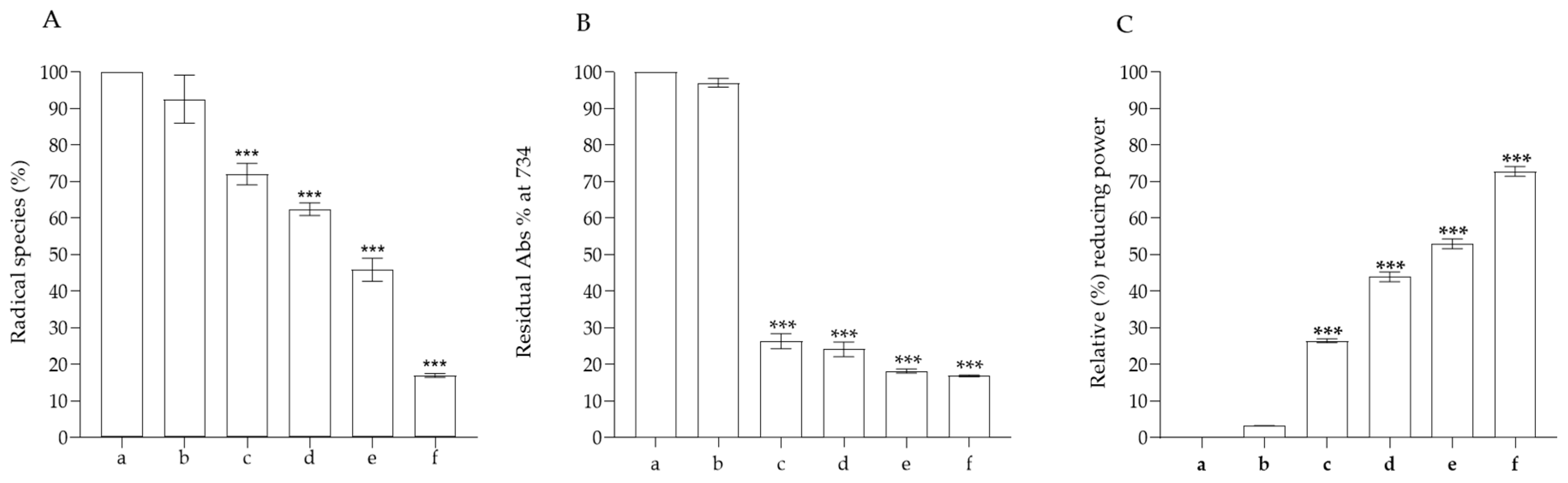
2.8. Antioxidant Activity
2.9. Antimicrobial Activity of Anthocyanins Obtained from Callistemon citrinus
2.10. Food Fresh-Keeping Test
3. Materials and Methods
3.1. Reagents and Standard Solutions
3.2. Optimization of the Extraction of Anthocyanins from C. citrinus and Production of PVA-Based Films
3.2.1. Sample Collection
3.2.2. Microwave Assisted Extraction (MAE) of Anthocyanins
3.2.3. Response Surface Methodology
3.2.4. Determination of Total Anthocyanin Content
3.2.5. Preparation of Anthocyanins and Identification of the Profile by RP-HPLC-DAD
3.2.6. Preparation of PVA-Based Bioplastics
3.3. Characterization of PVA-Based Bioplastics Functionalized with Anthocyanins
3.3.1. Fourier Transform Infrared Spectroscopy (FTIR-ATR)
3.3.2. Optical and Color Properties
3.3.3. Mechanical Properties and Thickness
3.4. Migration Test of Anthocyanins in Different Food-Simulants
3.5. Antioxidant Capacity
3.5.1. Ferric Reducing Power (FRAP) Assay
3.5.2. Quenching of the Stable ABTS Radical
3.5.3. Inhibition of the Stable 2,2-Diphenylpyrylhydrazyl Radical (DPPH)
3.6. Antimicrobial Assays
3.6.1. Microbial Strains and Culture Conditions
3.6.2. Antimicrobial Testing
3.6.3. Antibacterial Activity of PVA-Based Bioplastics
3.7. Food Fresh-Keeping Test
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Symeonides, D.; Loizia, P.; Zorpas, A.A. Tire waste management system in Cyprus in the framework of circular economy strategy. Environ. Sci. Pollut. Res. Int. 2019, 26, 35445–35460. [Google Scholar] [CrossRef] [PubMed]
- Armaghan Chizaryfard, P.T.C.N. The transformation to a cirular economy: Framing an evolutionary view. J. Evol. Econ. 2021, 31, 19. [Google Scholar] [CrossRef]
- Nordahl, S.L.; Scown, C.D. Recommendations for life-cycle assessment of recyclable plastics in a circular economy. Chem. Sci. 2024, 15, 9397–9407. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Facchin, M.; Brahimi, S.; Belhamdi, H.; Gatto, V.; Beghetto, V. Agri-Food Wastes for Bioplastics: European Prospective on Possible Applications in Their Second Life for a Circular Economy. Polymers 2022, 14, 2752. [Google Scholar] [CrossRef]
- Guicherd, M.; Ben Khaled, M.; Gueroult, M.; Nomme, J.; Dalibey, M.; Grimaud, F.; Alvarez, P.; Kamionka, E.; Gavalda, S.; Noel, M.; et al. An engineered enzyme embedded into PLA to make self-biodegradable plastic. Nature 2024, 631, 884–890. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Torrisi, A.; Torrisi, L. Diffusion of nitrogen gas through polyethylene based films. Polim. Cristallografia. 2021, 4, e10207. [Google Scholar] [CrossRef]
- Rhodes, C.J. Solving the plastic problem: From cradle to grave, to reincarnation. Sci. Prog. 2019, 102, 218–248. [Google Scholar] [CrossRef]
- Beena Unni, A.; Muringayil Joseph, T. Enhancing Polymer Sustainability: Eco-Conscious Strategies. Polymers 2024, 16, 1769. [Google Scholar] [CrossRef]
- Shaikh, S.; Yaqoob, M.; Aggarwal, P. An overview of biodegradable packaging in food industry. Curr. Res. Food Sci. 2021, 4, 503–520. [Google Scholar] [CrossRef]
- Beghetto, V.; Gatto, V.; Samiolo, R.; Scolaro, C.; Brahimi, S.; Facchin, M.; Visco, A. Plastics today: Key challenges and EU strategies towards carbon neutrality: A review. Environ. Pollut. 2023, 334, 122102. [Google Scholar] [CrossRef]
- Elsaeed, S.; Zaki, E.; Diab, A.; Tarek, M.A.; Omar, W.A.E. New polyvinyl alcohol/gellan gum-based bioplastics with guava and chickpea extracts for food packaging. Sci. Rep. 2023, 13, 22384. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Kalia, V.C.; Singh Patel, S.K.; Shanmugam, R.; Lee, J.K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef]
- Prajapati, R.A.; Jadeja, G.C. Red dragon fruit-soy protein isolate biofilm: UV-blocking, antioxidant & improved mechanical properties for sustainable food packaging. J. Food Sci. Technol. 2024, 61, 1686–1700. [Google Scholar] [CrossRef]
- Yildirim, S.; Rocker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef]
- Narayanasamy, A.; Patel, S.K.S.; Singh, N.; Rohit, M.V.; Lee, J.K. Valorization of Algal Biomass to Produce Microbial Polyhydroxyalkanoates: Recent Updates, Challenges, and Perspectives. Polymers 2024, 16, 2227. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Biodegradable and Water Resistant Poly(vinyl) Alcohol (PVA)/Starch (ST)/Glycerol (GL)/Halloysite Nanotube (HNT) Nanocomposite Films for Sustainable Food Packaging. Front. Mater. 2019, 6, 58. [Google Scholar] [CrossRef]
- Wu, H.F.; Yue, L.Z.; Jiang, S.L.; Lu, Y.Q.; Wu, Y.X.; Wan, Z.Y. Biodegradation of polyvinyl alcohol by different dominant degrading bacterial strains in a baffled anaerobic bioreactor. Water Sci. Technol. 2019, 79, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Kawai, F.; Hu, X. Biochemistry of microbial polyvinyl alcohol degradation. Appl. Microbiol. Biotechnol. 2009, 84, 227–237. [Google Scholar] [CrossRef]
- Alonso-Lopez, O.; Lopez-Ibanez, S.; Beiras, R. Assessment of Toxicity and Biodegradability of Poly(vinyl alcohol)-Based Materials in Marine Water. Polymers 2021, 13, 3742. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Lagana, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Roselli, V.; Pugliese, G.; Leuci, R.; Brunetti, L.; Gambacorta, L.; Tufarelli, V.; Piemontese, L. Green Methods to Recover Bioactive Compounds from Food Industry Waste: A Sustainable Practice from the Perspective of the Circular Economy. Molecules 2024, 29, 2682. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar] [PubMed]
- Lagana, G.; Barreca, D.; Smeriglio, A.; Germano, M.P.; D’Angelo, V.; Calderaro, A.; Bellocco, E.; Trombetta, D. Evaluation of Anthocyanin Profile, Antioxidant, Cytoprotective, and Anti-Angiogenic Properties of Callistemon citrinus Flowers. Plants 2020, 9, 1045. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Sun, Y.S.; Chen, L.; Han, L.K.; Zheng, Y.N. Application of response surface methodology to optimise ultrasonic-assisted extraction of four chromones in Radix Saposhnikoviae. Phytochem. Anal. 2011, 22, 313–321. [Google Scholar] [CrossRef]
- Zhao, L.C.; Liang, J.; Li, W.; Cheng, K.M.; Xia, X.; Deng, X.; Yang, G.L. The use of response surface methodology to optimize the ultrasound-assisted extraction of five anthraquinones from Rheum palmatum L. Molecules 2011, 16, 5928–5937. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, J.G.; Li, W.F.; Chen, J.; Wang, D.Y.; Zhu, L. Optimum extraction process of polyphenols from the bark of Phyllanthus emblica L. based on the response surface methodology. J. Sep. Sci. 2009, 32, 1437–1444. [Google Scholar] [CrossRef]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S.F. Optimizing the extraction of phenolic antioxidants from peanut skins using response surface methodology. J. Agric. Food Chem. 2009, 57, 3064–3072. [Google Scholar] [CrossRef]
- Mylonaki, S.; Kiassos, E.; Makris, D.P.; Kefalas, P. Optimisation of the extraction of olive (Olea europaea) leaf phenolics using water/ethanol-based solvent systems and response surface methodology. Anal. Bioanal. Chem. 2008, 392, 977–985. [Google Scholar] [CrossRef]
- Kwon, J.H.; Belanger, J.M.; Pare, J.R. Optimization of microwave-assisted extraction (MAP) for ginseng components by response surface methodology. J. Agric. Food Chem. 2003, 51, 1807–1810. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Xie, M.Y.; Shen, M.Y.; Nie, S.P.; Li, C.; Wang, Y.X. Optimisation of microwave-assisted extraction of polysaccharides from Cyclocarya paliurus (Batal.) Iljinskaja using response surface methodology. J. Sci. Food Agric. 2010, 90, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Puyol, S.; Benitez, J.J.; Heredia-Guerrero, J.A. Transparency of polymeric food packaging materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Azman, E.M.; Charalampopoulos, D.; Chatzifragkou, A. Acetic acid buffer as extraction medium for free and bound phenolics from dried blackcurrant (Ribes nigrum L.) skins. J. Food Sci. 2020, 85, 3745–3755. [Google Scholar] [CrossRef]
- Jatav, J.; Chinchkar, A.V.; Bhattacharya, B. Chitosan film with pineapple peel extract in the extension of shelf life of Indian cottage cheese: Release kinetics and bio-accessibility studies. Food Res. Int. 2023, 174, 113580. [Google Scholar] [CrossRef]
- Bajić, M.; Ročnik, T.; Oberlintner, A.; Scognamiglio, F.; Novak, U.; Likozar, B. Natural plant extracts as active components in chitosan-based films: A comparative study. Food Packag. Shelf Life 2019, 21, 100365. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Wu, N.; Zhu, C.; Jiang, X.; Yuan, K.; Li, Y.; Sun, J.; Bai, W. Anthocyanins Prevent AAPH-Induced Steroidogenesis Disorder in Leydig Cells by Counteracting Oxidative Stress and StAR Abnormal Expression in a Structure-Dependent Manner. Antioxidants 2023, 12, 508. [Google Scholar] [CrossRef]
- Feng, C.; Su, S.; Wang, L.; Wu, J.; Tang, Z.; Xu, Y.; Shu, Q.; Wang, L. Antioxidant capacities and anthocyanin characteristics of the black-red wild berries obtained in Northeast China. Food Chem. 2016, 204, 150–158. [Google Scholar] [CrossRef]
- Lacombe, A.; Tadepalli, S.; Hwang, C.A.; Wu, V.C. Phytochemicals in lowbush wild blueberry inactivate Escherichia coli O157:H7 by damaging its cell membrane. Foodborne Pathog. Dis. 2013, 10, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, C.; Zhong, W.; Shu, Y.; Zhang, Y.; Yang, D. Antibacterial effect and mechanism of anthocyanin from Lycium ruthenicum Murr. Front. Microbiol. 2022, 13, 974602. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Minuti, A.; La Camera, E.; Barreca, D.; Romeo, O.; Nostro, A. Antimicrobial Susceptibility of Staphylococcus aureus Strains and Effect of Phloretin on Biofilm Formation. Curr. Microbiol. 2023, 80, 303. [Google Scholar] [CrossRef]
- Sun, X.H.; Zhou, T.T.; Wei, C.H.; Lan, W.Q.; Zhao, Y.; Pan, Y.J.; Wu, V.C.H. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Majdoub, Y.O.E.; Ginestra, G.; Mandalari, G.; Dugo, P.; Mondello, L.; Cacciola, F. The Digestibility of Hibiscus sabdariffa L. Polyphenols Using an In Vitro Human Digestion Model and Evaluation of Their Antimicrobial Activity. Nutrients 2021, 13, 2360. [Google Scholar] [CrossRef] [PubMed]
- de Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 19. [Google Scholar] [CrossRef]
- Nohynek, L.J.; Alakomi, H.L.; Kahkonen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.M.; Puupponen-Pimia, R.H. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Alakomi, H.L.; Oksman-Caldentey, K.M. The action of berry phenolics against human intestinal pathogens. Biofactors 2005, 23, 243–251. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Olmos, D.; Gonzalez-Benito, J. Polymeric Materials with Antibacterial Activity: A Review. Polymers 2021, 13, 613. [Google Scholar] [CrossRef]
- Adams, A.; De Kimpe, N. Chemistry of 2-acetyl-1-pyrroline, 6-acetyl-1,2,3,4-tetrahydropyridine, 2-acetyl-2-thiazoline, and 5-acetyl-2,3-dihydro-4H-thiazine: Extraordinary Maillard flavor compounds. Chem. Rev. 2006, 106, 2299–2319. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.S. Effect of reaction pH on enolization and racemization reactions of glucose and fructose on heating with amino acid enantiomers and formation of melanoidins as result of the Maillard reaction. Food Chem. 2008, 108, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Rodríguez-Saona, L.E.; Baggett, J.R.; Reed, G.L.; Durst, R.W.; Wrolstad, R.E. Anthocyanin pigment composition of red radish cultivars as potential food colorants. J. Food Sci. 1998, 63, 219–224. [Google Scholar] [CrossRef]
- Le, X.T.; Huynh, M.T.; Pham, T.N.; Than, V.T.; Toan, T.Q.; Bach, L.G.; Trung, N.Q. Optimization of Total Anthocyanin Content, Stability and Antioxidant Evaluation of the Anthocyanin Extract from Vietnamese Carissa carandas L. Fruits. Processes 2019, 7, 468. [Google Scholar] [CrossRef]
- Song, D.; Ma, L.W.; Pang, B.; An, R.; Nie, J.H.; Guo, Y.R.; Li, S.J. An Active Bio-Based Food Packaging Material of ZnO@Plant Polyphenols/Cellulose/Polyvinyl Alcohol: DESIGN, Characterization and Application. Int. J. Mol. Sci. 2023, 24, 1577. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, J.V.; Silva, K.A.; Giuliangeli, V.C.; Mendes, A.L.D.; Piai, L.P.; Michels, R.N.; Dal Bosco, T.C.; Ströher, G.R.; Shirai, M.A. Starch-PVA based films with Clitoria ternatea flower extract: Characterization, phenolic compounds release and compostability. Int. J. Biol. Macromol. 2024, 255, 128232. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Patanè, G.T.; Corrado, I.; Giosafatto, C.V.L.; Ginestra, G.; Nostro, A.; Foti, A.; Gucciardi, P.G.; Mandalari, G.; Barreca, D.; et al. Functionalization of Polyhydroxyalkanoates (PHA)-Based Bioplastic with Phloretin for Active Food Packaging: Characterization of Its Mechanical, Antioxidant, and Antimicrobial Activities. Int. J. Mol. Sci. 2023, 24, 11628. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Ficarra, S.; Tellone, E.; Leuzzi, U.; Galtieri, A.; Bellocco, E. Evaluation of the antioxidant and cytoprotective properties of the exotic fruit Mill. (Annonaceae). Food Res. Int. 2011, 44, 2302–2310. [Google Scholar] [CrossRef]
- Papalia, T.; Barreca, D.; Panuccio, M.R. Assessment of Antioxidant and Cytoprotective Potential of Jatropha (Jatropha curcas) Grown in Southern Italy. Int. J. Mol. Sci. 2017, 18, 660. [Google Scholar] [CrossRef]
- Bisignano, C.; Filocamo, A.; Faulks, R.M.; Mandalari, G. In vitro antimicrobial activity of pistachio (Pistacia vera L.) polyphenols. Fems Microbiol. Lett. 2013, 341, 62–67. [Google Scholar] [CrossRef]
- Xu, Z.J.; Yang, Z.H.; Ji, J.F.; Mou, Y.; Chen, F.; Xiao, Z.Y.; Liao, X.J.; Hu, X.S.; Ma, L.J. Polyphenol mediated non-enzymatic browning and its inhibition in apple juice. Food Chem. 2023, 404, 134504. [Google Scholar] [CrossRef] [PubMed]





| Runs | Microwave Power (X1) | Minutes (X2) | EtOH % (X3) | TAC (mg Cyanidin-3,5-O-diglucoside/g) |
|---|---|---|---|---|
| 1 | 200 | 2 | 70 | 219.275 |
| 2 | 300 | 6 | 20 | 247.105 |
| 3 | 100 | 10 | 45 | 217.755 |
| 4 | 200 | 6 | 45 | 246.835 |
| 5 | 300 | 6 | 70 | 234.170 |
| 6 | 300 | 2 | 45 | 248.660 |
| 7 | 100 | 6 | 20 | 217.755 |
| 8 | 100 | 2 | 45 | 235.515 |
| 9 | 200 | 6 | 45 | 246.835 |
| 10 | 200 | 6 | 45 | 244.235 |
| 11 | 200 | 2 | 20 | 252.305 |
| 12 | 200 | 10 | 20 | 234.170 |
| 13 | 100 | 6 | 70 | 197.625 |
| 14 | 200 | 10 | 70 | 252.610 |
| 15 | 300 | 10 | 45 | 265.280 |
| Parameters | Regression Parameter Coefficients |
|---|---|
| TAC (%) | |
| MW | 15.82 *** |
| Minutes | 1.76 |
| EtOH | −5.96 ** |
| MW*MW | −9.80 ** |
| Minutes*Minutes | 5.83 * |
| EtOH*EtOH | −12.01 ** |
| MW*Minutes | 8.59 ** |
| MW*EtOH | 1.80 |
| Minutes*EtOH | 12.87 ** |
| F-value (model) | 28.45 |
| R2 | 0.98 |
| Adjusted R2 | 0.94 |
| Predicted R2 | 0.70 |
| PVA + Anthocyanins | T% | Opacity (T%/mm) |
|---|---|---|
| PVA | 89.24 ± 2.22 | 2.46 ± 0.90 |
| 0.1 | 85.67 ± 1.56 | 3.35 ± 0.83 |
| 0.25 | 82.03 ± 2.28 | 2.86 ± 1.10 |
| 0.5 | 79.40 ± 0.50 | 3.33 ± 1.40 |
| 1 | 78.91 ± 0.01 | 3.42 ± 0.91 |
| Bioplastics | L* | a* | b* | ΔE* | |
|---|---|---|---|---|---|
| PVA |  | 93.80 ± 0.12 | −0.41 ± 0.09 | 2.92 ± 0.36 | 2.49 ± 0.22 |
| PVA + 0.10% |  | 89.68 ± 1.28 | −0.24 ± 0.47 | 13.16 ± 1.95 | 12.63 ± 2.31 |
| PVA + 0.25% |  | 89.12 ± 1.29 | 0.53 ± 1.51 | 16.03 ± 2.28 | 15.52 ± 2.38 |
| PVA + 0.50% |  | 81.08 ± 3.12 | 4.25 ± 2.08 | 31.34 ± 6.70 | 33.02 ± 7.60 |
| PVA + 1.0% |  | 76.75 ± 2.69 | 6.78 ± 2.10 | 39.92 ± 5.69 | 42.90 ± 6.56 |
| PVA + Anthocyanins [wt%] | Thickness [mm] | E [MPa] | sb [MPa] | eb [%] | qw [°] |
|---|---|---|---|---|---|
| 0.00 | 29.92 ± 9.86 | 248.4 ± 8.5 | 23.76 ± 0.90 | 120.85 ± 6.34 | 14.8 ± 1.6 |
| 0.10 | 29.81 ± 8.04 | 502.7 ± 5.5 | 28.39 ± 0.83 | 192.04 ± 9.84 | 19.0 ± 1.1 |
| 0.25 | 30.57 ± 8.27 | 654.9 ± 7.9 | 17.26 ± 1.10 | 93.71 ± 7.12 | 30.2 ± 1.6 |
| 0.50 | 37.05 ± 9.91 | 800.7 ± 8.6 | 6.73 ± 1.40 | 10.67 ± 7.78 | 27.6 ± 1.0 |
| 1.00 | 36.74 ± 9.23 | 1060.2 ± 7.5 | 5.05 ± 0.91 | 4.09 ± 2.78 | 31.2 ± 2.5 |
| Strain | MIC | MBC/MFC |
|---|---|---|
| Staphylococcus aureus ATCC 6538 | 0.500–0.250 | >1.000 |
| Staphylococcus aureus ATCC 43300 | 0.062–0.031 | >1000 |
| Escherichia coli ATCC 10536 | >1.000 | |
| Pseudomonas aeruginosa ATCC 9027 | >1.000 | |
| Salmonella typhimurium ATCC 13311 | >1.000 | |
| Listeria monocitogenes ATCC 13932 | 0.500 | >1.000 |
| Listeria monocitogenes A240 (1/2b) | 1.000 | >1.000 |
| Listeria monocitogenes G282 (4b) | 1.000 | >1.000 |
| Listeria monocitogenes A256 (1/2a) | 0.25–0.125 | >1.000 |
| Listeria monocitogenes G197 (1/2c) | 0.250 | >1.000 |
| Candida albicans ATCC 10231 | >1.000 |
| Hours | L* | a* | b* | ΔE* | |
|---|---|---|---|---|---|
| Apple samples alone | 0 | −26.46 ± 2.96 | 1.76 ± 1.64 | 19.06 ± 0.50 | 32.73 ± 2.24 |
| Apple samples alone | 72 | −30.62 ± 0.27 | 12.79 ± 0.32 | 37.8 ± 0.76 | 50.30 ± 0.60 |
| Apple samples in PVA bags | −28.14 ± 0.14 | 7.40 ± 2.08 | 33.25 ± 0.2 | 44.23 ± 0.50 | |
| Apple samples in PVA +1.0% anthocyanins bags | −26.65 ± 2.23 | 5.41 ± 4.0 | 28.06 ± 2.21 | 39.11 ± 2.56 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patanè, G.T.; Calderaro, A.; Putaggio, S.; Ginestra, G.; Mandalari, G.; Cirmi, S.; Barreca, D.; Russo, A.; Gervasi, T.; Neri, G.; et al. Novel Bioplastic Based on PVA Functionalized with Anthocyanins: Synthesis, Biochemical Properties and Food Applications. Int. J. Mol. Sci. 2024, 25, 9929. https://doi.org/10.3390/ijms25189929
Patanè GT, Calderaro A, Putaggio S, Ginestra G, Mandalari G, Cirmi S, Barreca D, Russo A, Gervasi T, Neri G, et al. Novel Bioplastic Based on PVA Functionalized with Anthocyanins: Synthesis, Biochemical Properties and Food Applications. International Journal of Molecular Sciences. 2024; 25(18):9929. https://doi.org/10.3390/ijms25189929
Chicago/Turabian StylePatanè, Giuseppe Tancredi, Antonella Calderaro, Stefano Putaggio, Giovanna Ginestra, Giuseppina Mandalari, Santa Cirmi, Davide Barreca, Annamaria Russo, Teresa Gervasi, Giovanni Neri, and et al. 2024. "Novel Bioplastic Based on PVA Functionalized with Anthocyanins: Synthesis, Biochemical Properties and Food Applications" International Journal of Molecular Sciences 25, no. 18: 9929. https://doi.org/10.3390/ijms25189929














