Decursin Induces G1 Cell Cycle Arrest and Apoptosis through Reactive Oxygen Species-Mediated Endoplasmic Reticulum Stress in Human Colorectal Cancer Cells in In Vitro and Xenograft Models
Abstract
:1. Introduction
2. Results
2.1. Decursin Inhibits Crc Cell Growth
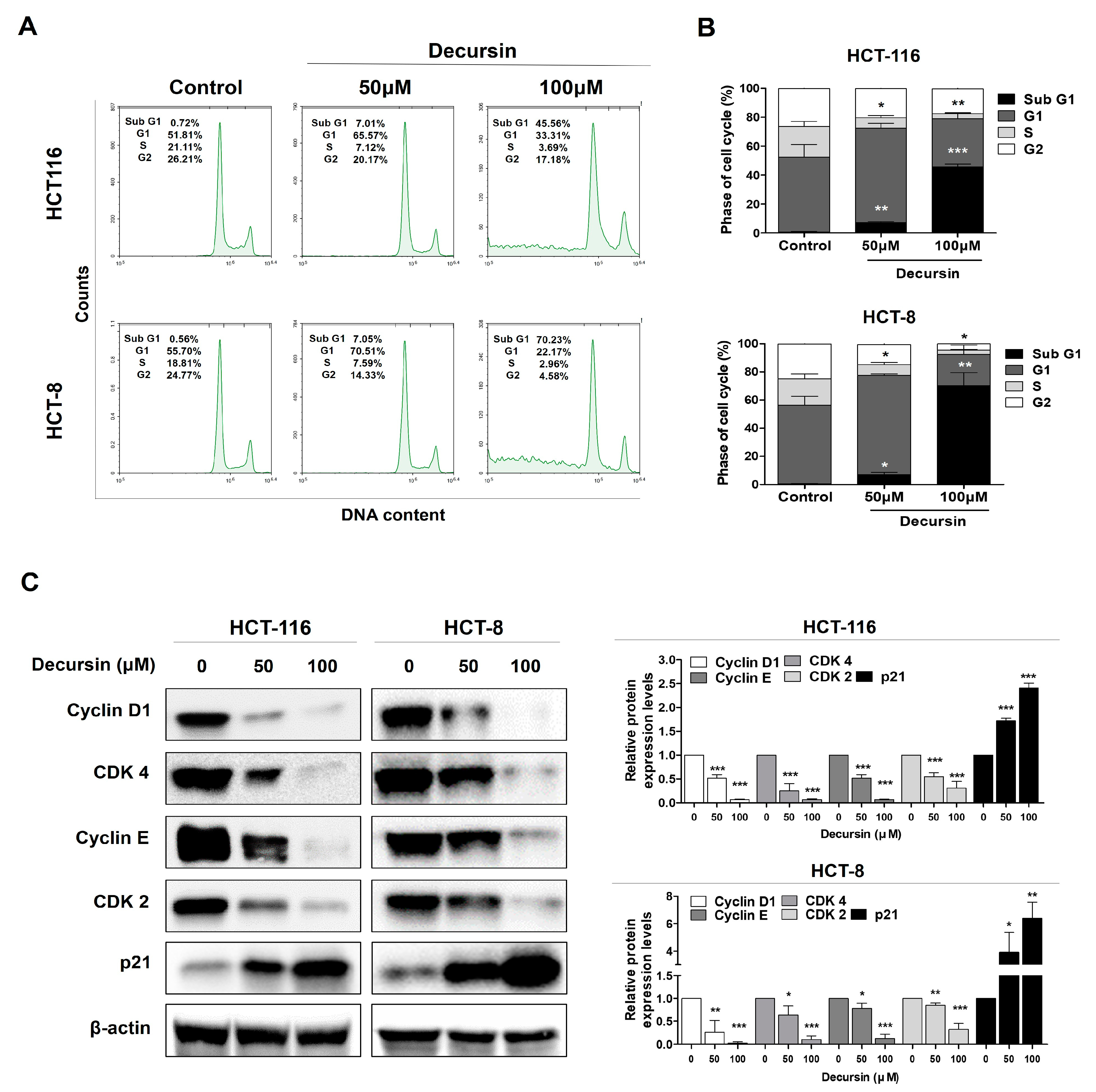
2.2. Decursin Triggers Cell Cycle Arrest at the G1 Phase in Crc Cells
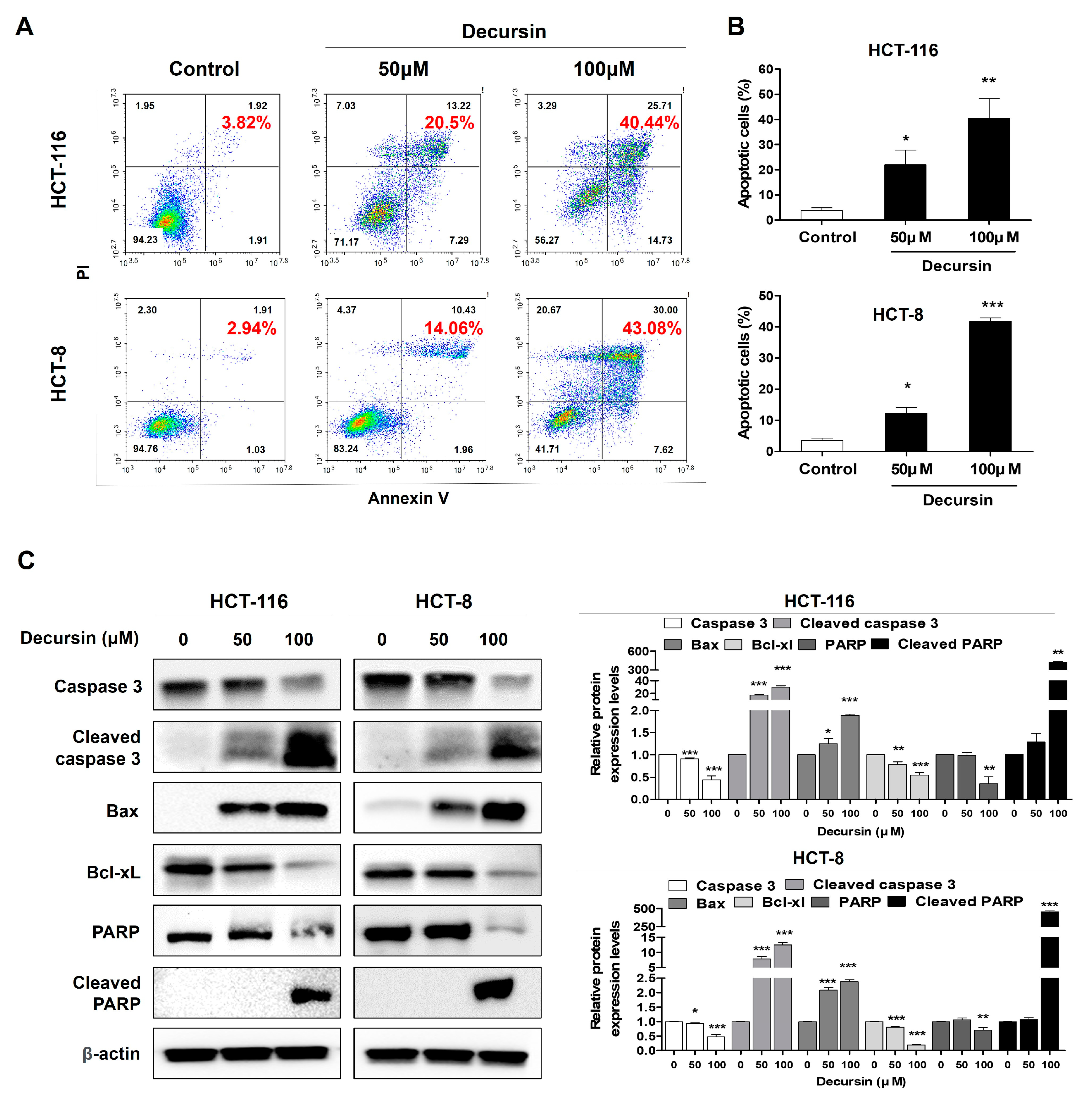
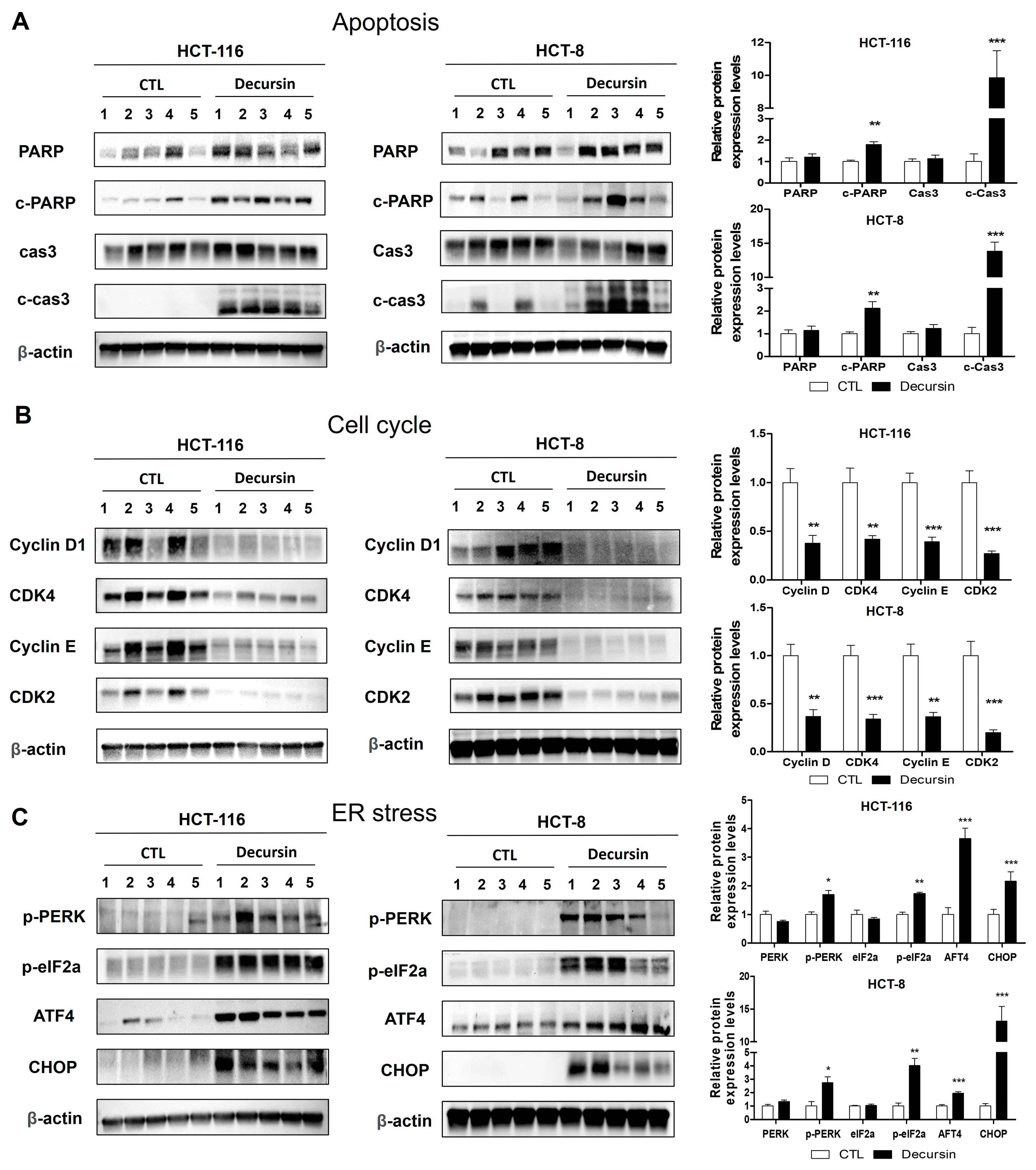
2.3. Decursin Induces Apoptosis in Crc Cells
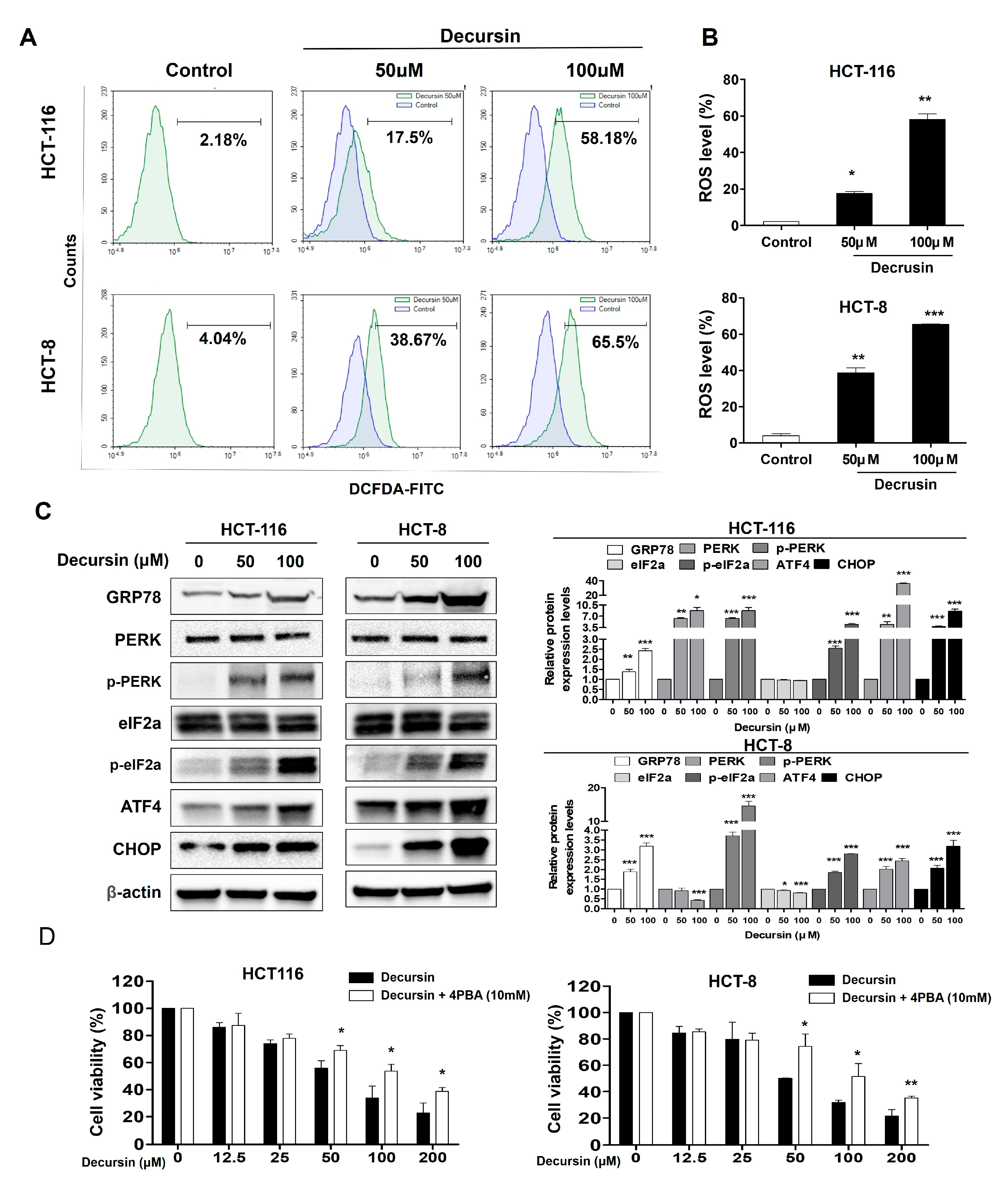
2.4. Decursin Causes Reactive Oxygen Species Accumulation and Endoplasmic Reticulum Stress in Crc Cells
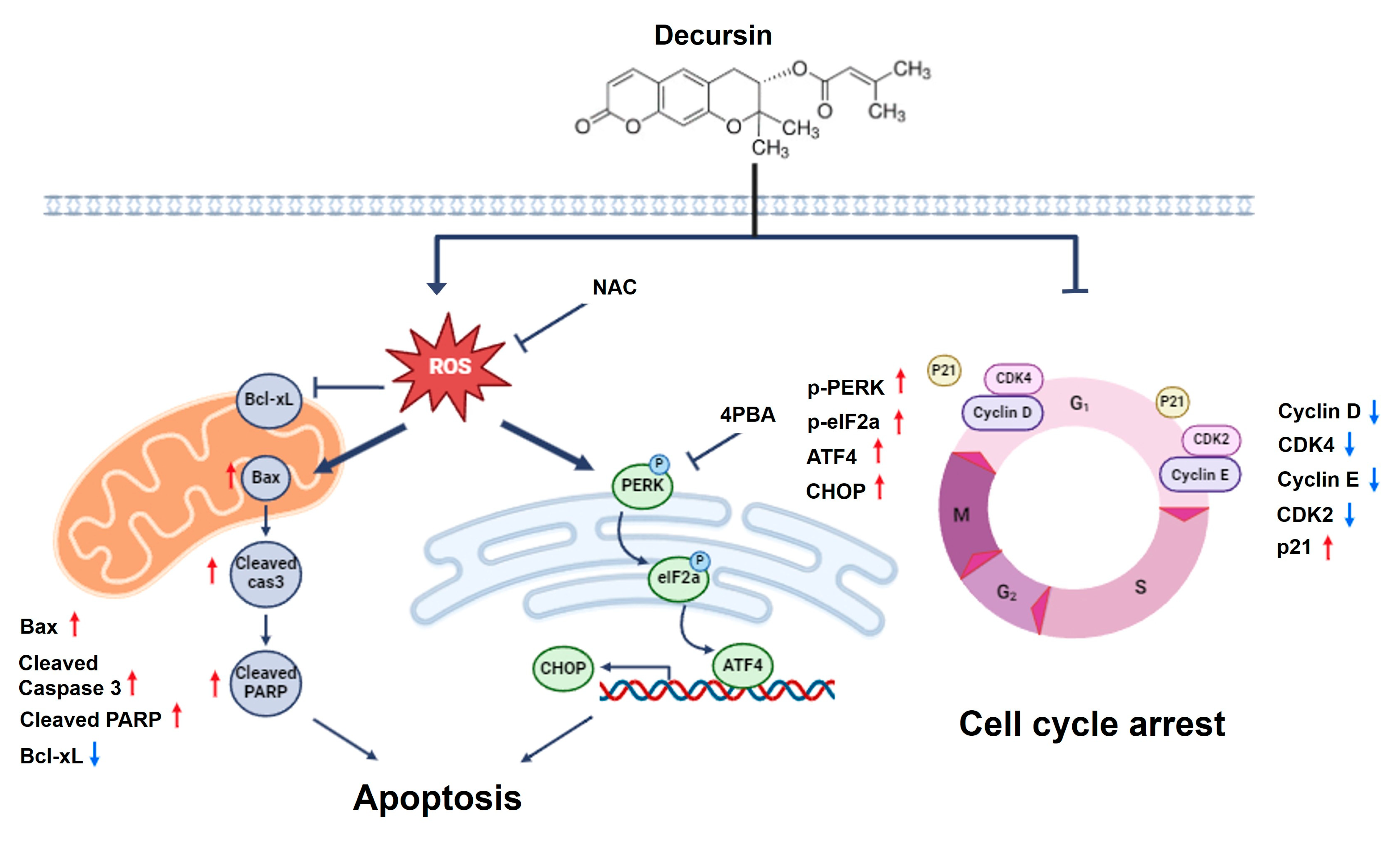
2.5. Decursin Induces Ros-Mediated Cell Cycle Arrest and Apoptosis via ER Stress in Crc Cells
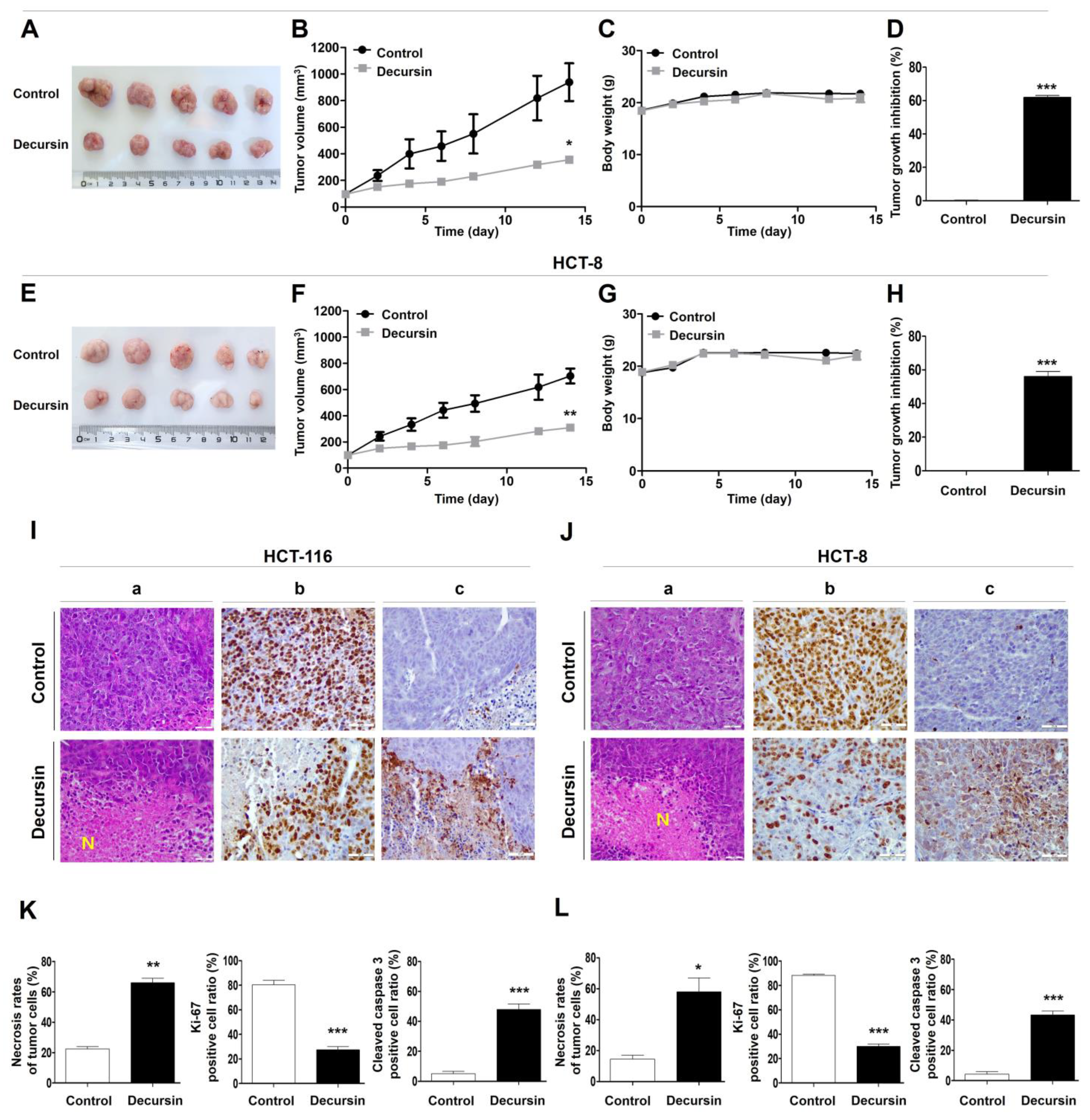
2.6. Decursin Suppresses Tumor Growth of Crc Cells In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Decursin Solution Preparation
4.2. Cell Viability Assay
4.3. Cell Cycle Analysis
4.4. Cell Apoptosis Assay
4.5. Intracellular ROS Measurement
4.6. Western Blotting
4.7. Animal Studies
4.8. H&E Staining and Immunohistochemistry
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chakedis, J.; Schmidt, C.R. Surgical Treatment of Metastatic Colorectal Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Townsend, A.; Price, T.; Karapetis, C. Selective internal radiation therapy for liver metastases from colorectal cancer. Cochrane Database Syst. Rev. 2009, 2009, CD007045. [Google Scholar] [CrossRef] [PubMed]
- Mojtahedi, Z.; Koo, J.S.; Yoo, J.W.; Kim, P.; Kang, H.-T.; Hwang, J.; Joo, M.K.; Shen, J.J. Palliative Care and Life-Sustaining/Local Procedures in Colorectal Cancer in the United States Hospitals: A Ten-Year Perspective. Cancer Manag. Res. 2021, 13, 7569–7577. [Google Scholar] [CrossRef] [PubMed]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef]
- Xian, Z.; Hu, B.; Wang, T.; Zeng, J.; Cai, J.; Zou, Q.; Zhu, P. lncRNA UCA1 Contributes to 5-Fluorouracil Resistance of Colorectal Cancer Cells Through miR-23b-3p/ZNF281 Axis. OncoTargets Ther. 2020, 13, 7571–7583. [Google Scholar] [CrossRef]
- Pardini, B.; Kumar, R.; Naccarati, A.; Novotny, J.; Prasad, R.B.; Forsti, A.; Hemminki, K.; Vodicka, P.; Bermejo, J.L. 5-Fluorouracil-based chemotherapy for colorectal cancer and MTHFR/MTRR genotypes. Br. J. Clin. Pharmacol. 2010, 72, 162–163. [Google Scholar] [CrossRef]
- Tournigand, C.; André, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. FOLFIRI Followed by FOLFOX6 or the Reverse Sequence in Advanced Colorectal Cancer: A Randomized GERCOR Study. J. Clin. Oncol 2004, 22, 229–237. [Google Scholar] [CrossRef]
- de Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; et al. Leucovorin and Fluorouracil With or Without Oxaliplatin as First-Line Treatment in Advanced Colorectal Cancer. J. Clin. Oncol 2000, 18, 2938–2947. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Aiello, P.; Sharghi, M.; Mansourkhani, S.M.; Ardekan, A.P.; Jouybari, L.; Daraei, N.; Peiro, K.; Mohamadian, S.; Rezaei, M.; Heidari, M.; et al. Medicinal Plants in the Prevention and Treatment of Colon Cancer. Oxidative Med. Cell. Longev. 2019, 2019, 1–51. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients 2023, 15, 1896. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Madu, C.O.; Lu, Y. Phytochemicals in Chemoprevention: A Cost-Effective Complementary Approach. J. Cancer 2021, 12, 3686–3700. [Google Scholar] [CrossRef]
- Dubey, N.; Dubey, N.; Bhadoria, U.; Shah, K.; Chauhan, N.S. Targeting colorectal cancer using dietary flavonols. Cancer Innov. 2023, 3, e99. [Google Scholar] [CrossRef]
- Yang, C.; Wu, L.; Jin, X.; Liu, A.; Jing, Z.; Feng, C.; Guo, Z.; Zhang, Y.; Ma, Y.; Li, F.; et al. Decrease in GPSM2 mediated by the natural product luteolin contributes to colon adenocarcinoma treatment and increases the sensitivity to fluorouracil. Biomed. Pharmacother. 2024, 176, 116847. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-O.; Lee, I.; Paik, S.-Y.; Kim, D.E.; Lim, J.D.; Kang, W.-S.; Ko, S. Ultrafine Angelica gigas Powder Normalizes Ovarian Hormone Levels and Has Antiosteoporosis Properties in Ovariectomized Rats: Particle Size Effect. J. Med. Food 2012, 15, 863–872. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Lee, M.K.; Kim, Y.C.; Sung, S.H. The simultaneous determination of coumarins in Angelica gigas root by high performance liquid chromatography–diode array detector coupled with electrospray ionization/mass spectrometry. J. Pharm. Biomed. Anal. 2007, 46, 258–266. [Google Scholar] [CrossRef]
- Lee, G.-H.; Lee, H.-Y.; Lim, Y.-J.; Kim, J.-H.; Jung, S.-J.; Jung, E.-S.; Chae, S.-W.; Lee, J.; Lim, J.; Rashid, M.M.U.; et al. Angelica gigas extract inhibits acetylation of eNOS via IRE1α sulfonation/RIDD-SIRT1-mediated posttranslational modification in vascular dysfunction. Aging 2023, 15, 13608–13627. [Google Scholar] [CrossRef]
- Bhat, T.A.; Dheeraj, A.; Nambiar, D.K.; Singh, S.P.; Yim, D.S.; Singh, R.P. Decursin inhibits EGFR-ERK1/2 signaling axis in advanced human prostate carcinoma cells. Prostate 2023, 83, 534–546. [Google Scholar] [CrossRef]
- Kim, B.S.; Seo, H.; Kim, H.-J.; Bae, S.M.; Son, H.-N.; Lee, Y.J.; Ryu, S.; Park, R.-W.; Nam, J.-O. Decursin from Angelica gigas Nakai Inhibits B16F10 Melanoma Growth Through Induction of Apoptosis. J. Med. Food 2015, 18, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Lee, H.-J.; Li, G.-X.; Guo, J.; Malewicz, B.; Zhao, Y.; Lee, E.-O.; Lee, J.-H.; Kim, M.-S.; Kim, S.-H.; et al. Potent Antiandrogen and Androgen Receptor Activities of anAngelica gigas–Containing Herbal Formulation: Identification of Decursin as a Novel and Active Compound with Implications for Prevention and Treatment of Prostate Cancer. Cancer Res. 2006, 66, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yun, M.; Kim, E.; Jung, D.; Won, G.; Kim, B.; Jung, J.H.; Kim, S. Decursin enhances TRAIL-induced apoptosis through oxidative stress mediated- endoplasmic reticulum stress signalling in non-small cell lung cancers. Br. J. Pharmacol. 2016, 173, 1033–1044. [Google Scholar] [CrossRef]
- Kim, J.; Jung, J.H.; Kim, S.; Jeong, S. Decursin Exerts Anti-cancer Activity in MDA-MB-231 Breast Cancer Cells Via Inhibition of the Pin1 Activity and Enhancement of the Pin1/p53 Association. Phytother. Res. 2013, 28, 238–244. [Google Scholar] [CrossRef]
- Moon, S.-K.; Kim, W.-J.; Lee, S.-J.; Choi, Y.D.; Moon, S.-K. Decursin inhibits growth of human bladder and colon cancer cells via apoptosis, G1-phase cell cycle arrest and extracellular signal-regulated kinase activation. Int. J. Mol. Med. 2010, 25, 635–641. [Google Scholar] [CrossRef]
- Son, S.H.; Park, K.; Park, S.K.; Kim, Y.C.; Kim, Y.S.; Lee, S.; Chung, W. Decursin and Decursinol from Angelica gigas Inhibit the Lung Metastasis of Murine Colon Carcinoma. Phytother. Res. 2010, 25, 959–964. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Y.-E.; Zhao, M.-Y.; Jiang, Y.-F.; Fu, X.; You, F.-M. [Decursin affects proliferation, apoptosis, and migration of colorectal cancer cells through PI3K/Akt signaling pathway]. Zhongguo Zhong Yao Za Zhi 2023, 48, 2334–2342. [Google Scholar] [CrossRef] [PubMed]
- Reijonen, P.; Österlund, P.; Isoniemi, H.; Arola, J.; Nordin, A. Histologically Verified Biliary Invasion was Associated with Impaired Liver Recurrence-Free Survival in Resected Colorectal Cancer Liver Metastases. Scand. J. Surg. 2018, 108, 201–209. [Google Scholar] [CrossRef]
- Kaspers, G.J. 20 years of Expert Review of Anticancer Therapy. Expert Rev. Anticancer. Ther. 2021, 22, 1–2. [Google Scholar] [CrossRef]
- Alese, O.B.; Wu, C.; Chapin, W.J.; Ulanja, M.B.; Zheng-Lin, B.; Amankwah, M.; Eads, J. Update on Emerging Therapies for Advanced Colorectal Cancer. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389574. [Google Scholar] [CrossRef]
- Millimouno, F.M.; Dong, J.; Yang, L.; Li, J.; Li, X. Targeting Apoptosis Pathways in Cancer and Perspectives with Natural Compounds from Mother Nature. Cancer Prev. Res. 2014, 7, 1081–1107. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Zhu, M.-L.; Li, J.-C.; Wang, L.; Zhong, X.; Zhang, Y.-W.; Tan, R.-Z.; Wang, H.-L.; Fan, J.-M.; Wang, L. Decursin inhibits the growth of HeLa cervical cancer cells through PI3K/Akt signaling. J. Asian Nat. Prod. Res. 2020, 23, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.-E.; Kim, N.; Joo, M.; Lee, M.-W.; Jeon, H.; Ryu, H.; Song, I.-C.; Song, G.-Y.; Lee, H.J. Decursin inhibits tumor growth, migration, and invasion in gastric cancer by down-regulating CXCR7 expression. Am J Cancer Res. 2019, 9, 2007–2008. [Google Scholar]
- Al Bitar, S.; Gali-Muhtasib, H. The role of the cyclin dependent kinase inhibitor p21(cip1/waf1) in targeting cancer: Molecular mechanisms and novel therapeutics. Cancers 2019, 11, 1475. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, C.; Skotheim, J.M.; de Bruin, R.A.M. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.I.; Kim, N.; Joo, M.; Lee, K.H.; Lee, M.W.; Jeon, H.J.; Ryu, H.; Kim, J.M.; Sul, J.Y.; et al. Decursin inhibits cell growth and autophagic flux in gastric cancer via suppression of cathepsin C. Am J Cancer Res 2021, 11, 1304–1320. [Google Scholar]
- Jang, J.; Jeong, S.-J.; Kwon, H.-Y.; Jung, J.H.; Sohn, E.J.; Lee, H.-J.; Kim, J.H.; Kim, S.-H. Decursin and Doxorubicin Are in Synergy for the Induction of Apoptosis via STAT3 and/or mTOR Pathways in Human Multiple Myeloma Cells. Evid. -Based Complement. Altern. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Steven, A.J.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef]
- Demény, M.A.; Virág, L. The PARP Enzyme Family and the Hallmarks of Cancer Part 1. Cell Intrinsic Hallmarks. Cancers 2021, 13, 2042. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Zhou, F.; Yang, Y.; Xing, D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2010, 278, 403–413. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J. Cell Biol 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Yao, N.; Li, Y.-J.; Zhang, D.-M.; Liu, D.-L.; Tang, M.-K.; Yiu, A.; Li, Y.; Chen, W.-M.; Lan, P.; Yao, Z.; et al. B4G2 Induces Mitochondrial Apoptosis by the ROS-Mediated Opening of Ca2+-Dependent Permeability Transition Pores. Cell. Physiol. Biochem. 2015, 37, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Verfaillie, T.; Rubio, N.; Garg, A.D.; Bultynck, G.; Rizzuto, R.; Decuypere, J.-P.; Piette, J.; Linehan, C.; Gupta, S.; Samali, A.; et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012, 19, 1880–1891. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, T.H.; Choi, W.G.; Chung, Y.H.; Ko, S.-G.; Cheon, C.; Cho, S.-G. SH003 Causes ER Stress-mediated Apoptosis of Breast Cancer Cells via Intracellular ROS Production. Cancer Genom. Proteom. 2023, 20, 88–116. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, X.; Sun, J.; Lei, Q.; Wang, Q.; Pan, D.; Ding, M.; Ding, Y. Reactive oxygen species mediate anlotinib-induced apoptosis via activation of endoplasmic reticulum stress in pancreatic cancer. Cell Death Dis. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, X.; Niu, G.; He, S.; Zhang, T.; Bai, Y.; Li, Y.; Hao, H.; Chen, C.; Shou, Z.; et al. Shikonin induces apoptosis and prosurvival autophagy in human melanoma A375 cells via ROS-mediated ER stress and p38 pathways. Artif. Cells Nanomed. Biotechnol. 2019, 47, 626–635. [Google Scholar] [CrossRef]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef] [PubMed]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef] [PubMed]
- Kweon, B.; Han, Y.-H.; Kee, J.-Y.; Mun, J.-G.; Jeon, H.D.; Yoon, D.H.; Choi, B.-M.; Hong, S.-H. Effect of Angelica gigas Nakai Ethanol Extract and Decursin on Human Pancreatic Cancer Cells. Molecules 2020, 25, 2028. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Q.; Jeong, S.-J.; Kwon, H.-Y.; Han, I.; Kim, H.S.; Lee, H.-J.; Lee, E.-O.; Ahn, K.S.; Jung, M.-H.; Zhu, S.; et al. Inhibition of cyclooxygenase-2-dependent survivin mediates decursin-induced apoptosis in human KBM-5 myeloid leukemia cells. Cancer Lett. 2010, 298, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Yim, D.; Singh, R.P.; Agarwal, C.; Lee, S.; Chi, H.; Agarwal, R. A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Cancer Res. 2005, 65, 1035–1044. [Google Scholar] [CrossRef]
- Kim, K.-M.; Lee, Y.-J.; Hong, Y.-G.; Kang, J.-S. Oral Acute and Subacute Toxicity Studies of Decursin and Decursinol Angelate of Angelica gigas Nakai. Mol. Cell. Toxicol. 2009, 5, 153–159. [Google Scholar]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef]
- Talib, W.H.; Awajan, D.; Hamed, R.A.; Azzam, A.O.; Mahmod, A.I.; Al-Yasari, I.H. Combination Anticancer Therapies Using Selected Phytochemicals. Molecules 2022, 27, 5452. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Go, S.-H.; Song, Y.; Lee, D.-K.; Park, J.-R. Decursin Induces G1 Cell Cycle Arrest and Apoptosis through Reactive Oxygen Species-Mediated Endoplasmic Reticulum Stress in Human Colorectal Cancer Cells in In Vitro and Xenograft Models. Int. J. Mol. Sci. 2024, 25, 9939. https://doi.org/10.3390/ijms25189939
Kim D, Go S-H, Song Y, Lee D-K, Park J-R. Decursin Induces G1 Cell Cycle Arrest and Apoptosis through Reactive Oxygen Species-Mediated Endoplasmic Reticulum Stress in Human Colorectal Cancer Cells in In Vitro and Xenograft Models. International Journal of Molecular Sciences. 2024; 25(18):9939. https://doi.org/10.3390/ijms25189939
Chicago/Turabian StyleKim, Danbee, Seok-Ho Go, Yeeun Song, Dong-Keon Lee, and Jeong-Ran Park. 2024. "Decursin Induces G1 Cell Cycle Arrest and Apoptosis through Reactive Oxygen Species-Mediated Endoplasmic Reticulum Stress in Human Colorectal Cancer Cells in In Vitro and Xenograft Models" International Journal of Molecular Sciences 25, no. 18: 9939. https://doi.org/10.3390/ijms25189939





