Cartilage Repair: Promise of Adhesive Orthopedic Hydrogels
Abstract
:1. Introduction
2. Existing Treatments and Challenges in Cartilage Repair
3. State of the Art in Adhesive Hydrogels
3.1. Hydrogel Composition
Clinical Impact
| Hydrogel Type | Examples (Source) | Pros | Cons | References |
|---|---|---|---|---|
| Natural Hydrogels | Alginate (Marine, Algae) Collagen (Animal) Hyaluronic Acid (Animal or Bacterial) Chitosan (Marine, Crustacean) Gelatin (Animal) Fibrin (Animal) Cellulose (Plant) | - Bioactivity and biocompatibility - Biodegradation - Supports cell adhesion, proliferation, and differentiation - Anti-inflammation and antioxidant | - Poor mechanical properties - Unpredictable degradation kinetics - Potential for immunogenicity | [33,39] |
| Synthetic Hydrogels | Polyethylene Glycol (PEG) Poly(N-isopropylacrylamide) (PNIPAAm) Poly(vinyl alcohol) (PVA) Poly(lactic-co-glycolic acid) (PLGA) Polycaprolactone (PCL) | - Precise control over mechanical and biochemical properties - Customizable scaffold design - Reproducible | - Risk of foreign body reaction - Poor biological activity - Uncertain long-term biocompatibility | [34,40] |
| Hybrid Hydrogels | Combinations of natural and synthetic components | - Synergizes advantages of both natural and synthetic materials - Balances bioactivity and mechanical strength | - Complexity in design and synthesis - Potential for uneven degradation or integration | [40,41,42] |
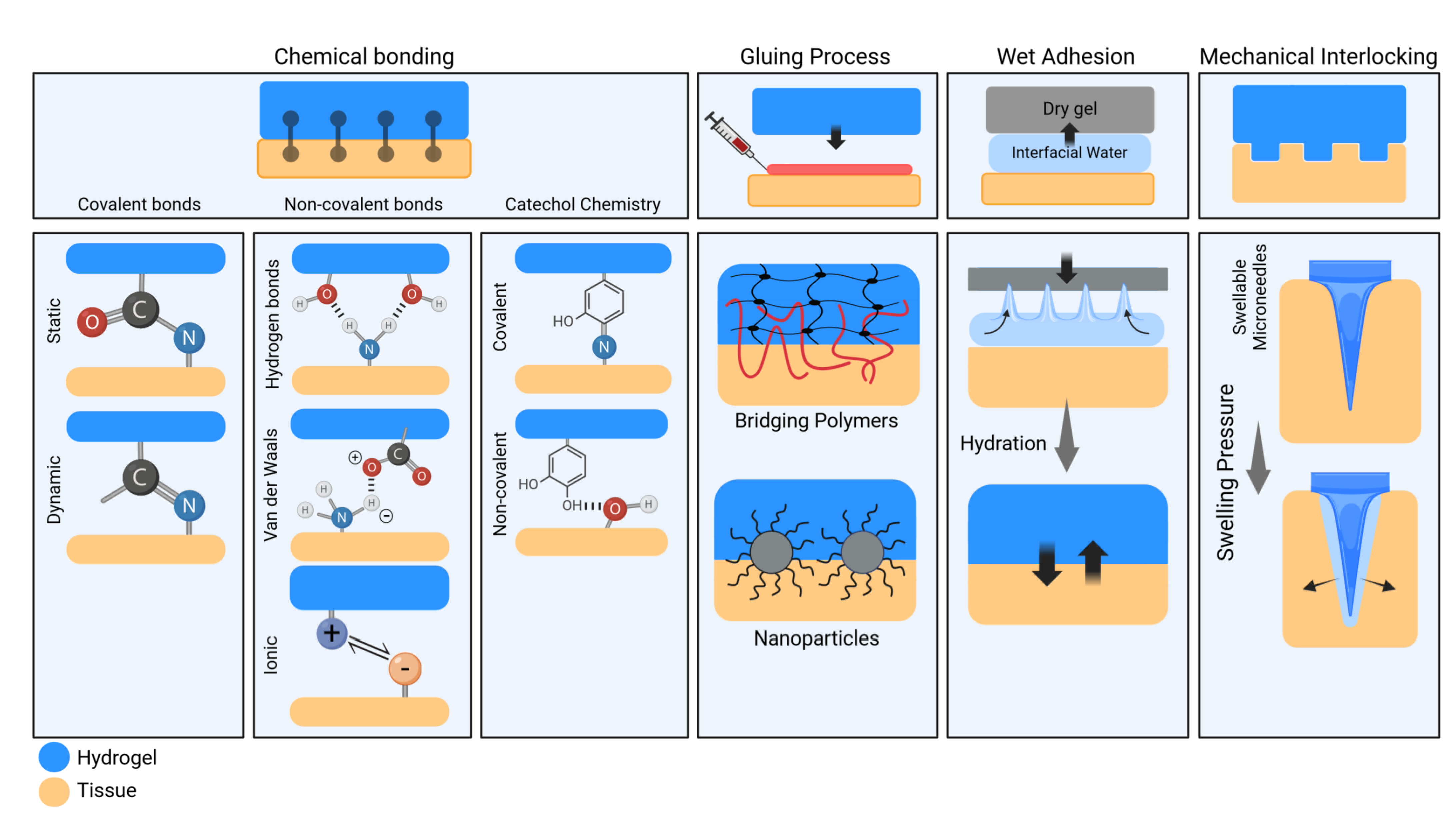
3.2. Adhesion Mechanisms of Hydrogels
3.2.1. Chemical Bonding
Covalent Bonding
Non-Covalent Interactions
Catechol Chemistry
3.2.2. Interfacial Gluing
3.2.3. Wet Adhesion
3.2.4. Mechanical Interlocking
3.2.5. Clinical Impact
3.3. Adhesive Hydrogels for Constituent Delivery
3.3.1. Hydrogels with Therapeutic Agent Incorporation
3.3.2. Hydrogels with Cellular Components and Various Cell Types
3.3.3. Gene Therapy and Exosome Therapeutics
| Component | Classification | Function | Examples/References |
|---|---|---|---|
| Therapeutic agents | NSAIDs | Alleviate pain and inflammation, reduce joint swelling, and inhibit osteoarthritis (OA) progression | Ibuprofen [75], Naproxen [76], Celecoxib [77], Methotrexate [78], and Hydroxychloroquine [79] |
| Corticosteroids | Alleviate pain and inflammation, reduce joint swelling, and inhibit OA progression | Prednisone [80], Dexamethasone [81], and Triamcinolone [82] | |
| Cellular sources and Components | Cells | Promote tissue regeneration, reduce inflammation, and enhance tissue repair | Articular chondrocytes, Nasal chondrocytes, Mesenchymal Stem Cells (MSCs), Adipose-derived Stem Cells (ASCs) [83], and Progenitor cells [84] |
| Cytokines | Promote cartilage regeneration | Fibroblast growth factor (FGF), TGF-β [85] | |
| Peptides | Promote cartilage regeneration | CK2.1 [86] | |
| Platelet-Rich Plasma | Promote cartilage regeneration, reduce inflammation, and enhance tissue repair | Concentrated platelets [87] | |
| Gene therapy and Exosome delivery | Transcription Factors | Enhance chondrocyte differentiation and promote tissue repair | Sox 9 [88] |
| Gene Vectors | Enhance chondrocyte differentiation and promote tissue repair | Lentiviral vectors, recombinant adeno-associated virus (rAAV) [89] | |
| MSC-Derived Exosomes | Modulate immune response and enhance tissue regeneration | MSC-derived exosomes [90] |
3.4. Hydrogel Delivery Modalities
3.4.1. Injectable Hydrogels
3.4.2. Granular Hydrogels
3.4.3. Preformed Hydrogels
4. Lateral Integration, an Unmet Need in Carriers for Cellular Therapy in Cartilage Defects
4.1. Mechanisms Leading to Problems in Lateral Integration
4.2. Strategies for Promoting Lateral Integration
Clinical Impact
5. Advantages and Disadvantages of Adhesive Hydrogel Scaffolds for Cartilage Repair
5.1. Enhanced Tissue Integration
5.2. Improved Cell Retention and Viability
5.3. Tunable Properties
5.4. Minimally Invasive Delivery
5.5. Biological Signaling
5.6. Disadvantages of Hydrogel Scaffolds
6. Adhesiveness Functionality and Quality Controls
6.1. Adhesiveness Assessment
6.1.1. Mechanical Testing
6.1.2. Physicochemical Characterization
6.2. In-Process Control Measures
6.2.1. Chemical Characterization
6.2.2. Crosslinking Efficiency
6.2.3. Sterility and Bioburden Control
6.2.4. Process Monitoring and Automation
7. Clinical Translation and Regulatory Considerations
7.1. Biocompatibility and Safety Assessment of Adhesive Hydrogels
7.2. Preclinical Efficacy for Adhesive Hydrogels
7.3. Clinical Trial Design for Adhesive Hydrogels
7.4. Regulatory Approval Pathway for Adhesive Hydrogels
8. Future Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomoll, A.H.; Minas, T. The quality of healing: Articular cartilage. Wound Repair Regen. 2014, 22, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Laurent, A.; Applegate, L.; Philippe, V. Grands défects chondraux et ostéochondraux du genou: Traitement par greffe chondrocytaire autologue. Rev. Med. Suisse 2022, 18, 2384–2390. [Google Scholar] [CrossRef]
- Martin, R.; Jakob, R.P. Review of KH Pridie (1959) on “A method of resurfacing osteoarthritic knee joints”. J. ISAKOS 2022, 7, 39–46. [Google Scholar] [CrossRef]
- Campbell, T.M.; Trudel, G. Protecting the regenerative environment: Selecting the optimal delivery vehicle for cartilage repair—A narrative review. Front. Bioeng. Biotechnol. 2024, 12, 1283752. [Google Scholar] [CrossRef]
- Philippe, V.; Laurent, A.; Abdel-Sayed, P.; Hirt-Burri, N.; Ann Applegate, L.; Martin, R. Human platelet lysate as an alternative to autologous serum for human chondrocyte clinical use. Cartilage 2021, 13, 509S–518S. [Google Scholar] [CrossRef] [PubMed]
- Philippe, V.; Laurent, A.; Hirt-Burri, N.; Abdel-Sayed, P.; Scaletta, C.; Schneebeli, V.; Michetti, M.; Brunet, J.-F.; Applegate, L.A.; Martin, R. Retrospective analysis of autologous chondrocyte-based cytotherapy production for clinical use: GMP process-based manufacturing optimization in a Swiss university hospital. Cells 2022, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Philippe, V.; Jeannerat, A.; Peneveyre, C.; Jaccoud, S.; Scaletta, C.; Hirt-Burri, N.; Abdel-Sayed, P.; Raffoul, W.; Darwiche, S.; Applegate, L.A. Autologous and Allogeneic Cytotherapies for Large Knee (Osteo) Chondral Defects: Manufacturing Process Benchmarking and Parallel Functional Qualification. Pharmaceutics 2023, 15, 2333. [Google Scholar] [CrossRef]
- Tuan, R.S. A second-generation autologous chondrocyte implantation approach to the treatment of focal articular cartilage defects. Arthritis Res. Ther. 2007, 9, 109. [Google Scholar] [CrossRef]
- Iwasa, J.; Engebretsen, L.; Shima, Y.; Ochi, M. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 561–577. [Google Scholar] [CrossRef]
- Jones, K.J.; Cash, B.M. Matrix-induced autologous chondrocyte implantation with autologous bone grafting for osteochondral lesions of the femoral trochlea. Arthrosc. Tech. 2019, 8, e259–e266. [Google Scholar] [CrossRef]
- Kutaish, H.; Tscholl, P.M.; Cosset, E.; Bengtsson, L.; Braunersreuther, V.; Mor, F.M.; Laedermann, J.; Furfaro, I.; Stafylakis, D.; Hannouche, D. Articular cartilage repair after implantation of hyaline cartilage beads engineered from adult dedifferentiated chondrocytes: Cartibeads preclinical efficacy study in a large animal model. Am. J. Sports Med. 2023, 51, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-N.; Yang, Z.; Hui, J.H.; Ouyang, H.-W.; Lee, E.H. Cartilaginous ECM component-modification of the micro-bead culture system for chondrogenic differentiation of mesenchymal stem cells. Biomaterials 2007, 28, 4056–4067. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Stampoultzis, T.; Karami, P.; Pioletti, D.P. Thoughts on cartilage tissue engineering: A 21st century perspective. Curr. Res. Transl. Med. 2021, 69, 103299. [Google Scholar] [CrossRef]
- Ngadimin, K.D.; Stokes, A.; Gentile, P.; Ferreira, A.M. Biomimetic hydrogels designed for cartilage tissue engineering. Biomater. Sci. 2021, 9, 4246–4259. [Google Scholar] [CrossRef]
- Kováč, J.; Priščáková, P.; Gbelcová, H.; Heydari, A.; Žiaran, S. Bioadhesive and Injectable Hydrogels and Their Correlation with Mesenchymal Stem Cells Differentiation for Cartilage Repair: A Mini-Review. Polymers 2023, 15, 4228. [Google Scholar] [CrossRef]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Betancourt, A.R. Microfractures or bone marrow stimulation (BMS): Evolution of the technique. Rev. Esp. Artrosc. Cir Articul. 2021, 28, 10–16. [Google Scholar]
- Russlies, M.; Rüther, P.; Köller, W.; Stomberg, P.; Behrens, P. Biomechanische Eigenschaften von Knorpelersatzgewebe nach verschiedenen Methoden der Knorpeldefektbehandlung beim Schaf. Z. Für Orthopädie Und Ihre Grenzgeb. 2003, 141, 465–471. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Brittberg, M. Clinical articular cartilage repair—An up to date review. Ann. Jt. 2018, 3. [Google Scholar] [CrossRef]
- Davies, R.L.; Kuiper, N.J. Regenerative medicine: A review of the evolution of autologous chondrocyte implantation (ACI) therapy. Bioengineering 2019, 6, 22. [Google Scholar] [CrossRef]
- Mirza, M.Z.; Swenson, R.D.; Lynch, S.A. Knee cartilage defect: Marrow stimulating techniques. Curr. Rev. Musculoskelet. Med. 2015, 8, 451–456. [Google Scholar] [CrossRef]
- Douleh, D.; Frank, R.M. Marrow stimulation: Microfracture, drilling, and abrasion. Oper. Tech. Sports Med. 2018, 26, 170–174. [Google Scholar] [CrossRef]
- Cong, B.; Sun, T.; Zhao, Y.; Chen, M. Current and novel therapeutics for articular cartilage repair and regeneration. Ther. Clin. Risk Manag. 2023, 19, 485–502. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of damaged articular cartilage: Current approaches and future directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef]
- Robinson, A.; Lindsay, A.; Vidal, A.; Frank, R.M. Osteochondral autograft transfer (OATS). Oper. Tech. Sports Med. 2020, 28, 150781. [Google Scholar] [CrossRef]
- Haikal, M.; Issac, R.T.; Snow, M. Osteochondral allograft transplantation of the knee: A review of indications, techniques, outcome and how to promote biology. Orthop. Trauma 2023, 37, 161–169. [Google Scholar] [CrossRef]
- Lai, W.C.; Bohlen, H.L.; Fackler, N.P.; Wang, D. Osteochondral allografts in knee surgery: Narrative review of evidence to date. Orthop. Res. Rev. 2022, 14, 263. [Google Scholar] [CrossRef]
- Gou, G.-H.; Tseng, F.-J.; Wang, S.-H.; Chen, P.-J.; Shyu, J.-F.; Weng, C.-F.; Pan, R.-Y. Autologous chondrocyte implantation versus microfracture in the knee: A meta-analysis and systematic review. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 289–303. [Google Scholar] [CrossRef]
- Dhillon, J.; Decilveo, A.P.; Kraeutler, M.J.; Belk, J.W.; McCulloch, P.C.; Scillia, A.J. Third-generation autologous chondrocyte implantation (cells cultured within collagen membrane) is superior to microfracture for focal chondral defects of the knee joint: Systematic review and meta-analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 2579–2586. [Google Scholar] [CrossRef]
- Liu, X.; Yu, H.; Wang, L.; Huang, Z.; Haq, F.; Teng, L.; Jin, M.; Ding, B. Recent advances on designs and applications of hydrogel adhesives. Adv. Mater. Interfaces 2022, 9, 2101038. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Mora-Boza, A.; García-Fernández, L. Emerging biofabrication techniques: A review on natural polymers for biomedical applications. Polymers 2021, 13, 1209. [Google Scholar] [CrossRef]
- Lin, X.; Wang, J.; Wu, X.; Luo, Y.; Wang, Y.; Zhao, Y. Marine-Derived Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2023, 33, 2211323. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.; Ryu, J.H. Adhesive catechol-conjugated hyaluronic acid for biomedical applications: A mini review. Appl. Sci. 2020, 11, 21. [Google Scholar] [CrossRef]
- Zahedi Tehrani, T.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. Natural based hydrogels promote chondrogenic differentiation of human mesenchymal stem cells. Front. Bioeng. Biotechnol. 2024, 12, 1363241. [Google Scholar] [CrossRef]
- Sánchez-Téllez, D.A.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Hydrogels for cartilage regeneration, from polysaccharides to hybrids. Polymers 2017, 9, 671. [Google Scholar] [CrossRef]
- Li, L.; Yu, F.; Zheng, L.; Wang, R.; Yan, W.; Wang, Z.; Xu, J.; Wu, J.; Shi, D.; Zhu, L. Natural hydrogels for cartilage regeneration: Modification, preparation and application. J. Orthop. Transl. 2019, 17, 26–41. [Google Scholar] [CrossRef]
- Wasyłeczko, M.; Sikorska, W.; Chwojnowski, A. Review of synthetic and hybrid scaffolds in cartilage tissue engineering. Membranes 2020, 10, 348. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016, 15, 190–196. [Google Scholar] [CrossRef]
- Karami, P.; Nasrollahzadeh, N.; Wyss, C.; O’Sullivan, A.; Broome, M.; Procter, P.; Bourban, P.E.; Moser, C.; Pioletti, D.P. An Intrinsically-Adhesive Family of Injectable and Photo-Curable Hydrogels with Functional Physicochemical Performance for Regenerative Medicine. Macromol. Rapid Commun. 2021, 42, 2000660. [Google Scholar] [CrossRef]
- Karami, P.; Wyss, C.S.; Khoushabi, A.; Schmocker, A.; Broome, M.; Moser, C.; Bourban, P.-E.; Pioletti, D.P. Composite double-network hydrogels to improve adhesion on biological surfaces. ACS Appl. Mater. Interfaces 2018, 10, 38692–38699. [Google Scholar] [CrossRef]
- Yang, J.; Bai, R.; Chen, B.; Suo, Z. Hydrogel adhesion: A supramolecular synergy of chemistry, topology, and mechanics. Adv. Funct. Mater. 2020, 30, 1901693. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, L.; Xiao, W.; Wang, Z.; Wang, S. Design of Adhesive Hemostatic Hydrogels Guided by the Interfacial Interactions with Tissue Surface. Adv. NanoBiomed Res. 2023, 3, 2200115. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering hydrogel adhesion for biomedical applications via chemical design of the junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef]
- Xu, K.; Wu, X.; Zhang, X.; Xing, M. Bridging wounds: Tissue adhesives’ essential mechanisms, synthesis and characterization, bioinspired adhesives and future perspectives. Burn. Trauma 2022, 10, tkac033. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Q.; Liu, H.; Zhao, Q.; Niu, Y.; Zhao, D. Adhesion mechanism and application progress of hydrogels. Eur. Polym. J. 2022, 173, 111277. [Google Scholar] [CrossRef]
- Ho, M.H.; van Hilst, Q.; Cui, X.; Ramaswamy, Y.; Woodfield, T.; Rnjak-Kovacina, J.; Wise, S.G.; Lim, K.S. From Adhesion to Detachment: Strategies to Design Tissue-Adhesive Hydrogels. Adv. NanoBiomed Res. 2024, 4, 2300090. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Zu, B.; Lei, D.; Guo, Y.; Wang, M.; Dou, X. Reversible adhesive hydrogel with enhanced sampling efficiency boosted by hydrogen bond and van der Waals force for visualized detection. Chem. Eng. J. 2023, 455, 140493. [Google Scholar] [CrossRef]
- Omar, J.; Ponsford, D.; Dreiss, C.A.; Lee, T.C.; Loh, X.J. Supramolecular hydrogels: Design strategies and contemporary biomedical applications. Chem. Asian J. 2022, 17, e202200081. [Google Scholar] [CrossRef]
- Zhou, S.; Bei, Z.; Wei, J.; Yan, X.; Wen, H.; Cao, Y.; Li, H. Mussel-inspired injectable chitosan hydrogel modified with catechol for cell adhesion and cartilage defect repair. J. Mater. Chem. B 2022, 10, 1019–1030. [Google Scholar] [CrossRef]
- Gowda, A.H.; Bu, Y.; Kudina, O.; Krishna, K.V.; Bohara, R.A.; Eglin, D.; Pandit, A. Design of tunable gelatin-dopamine based bioadhesives. Int. J. Biol. Macromol. 2020, 164, 1384–1391. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Zhong, W. Mussel-inspired hydrogel tissue adhesives for wound closure. RSC Adv. 2017, 7, 47380–47396. [Google Scholar] [CrossRef]
- Li, J.; Celiz, A.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.; Vasilyev, N.; Vlassak, J.; Suo, Z. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef]
- Rose, S.; Prevoteau, A.; Elzière, P.; Hourdet, D.; Marcellan, A.; Leibler, L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature 2014, 505, 382–385. [Google Scholar] [CrossRef]
- Rana, V.K.; Karami, P.; Nasrollahzadeh, N.; Pioletti, D.P. Nano Surface-Heterogeneities of Particles Modulate the Macroscopic Properties of Hydrogels. Adv. Mater. Interfaces 2023, 10, 2202248. [Google Scholar] [CrossRef]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry double-sided tape for adhesion of wet tissues and devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef]
- Yi, H.; Lee, S.H.; Seong, M.; Kwak, M.K.; Jeong, H.E. Bioinspired reversible hydrogel adhesives for wet and underwater surfaces. J. Mater. Chem. B 2018, 6, 8064–8070. [Google Scholar] [CrossRef]
- Yang, S.Y.; O’Cearbhaill, E.D.; Sisk, G.C.; Park, K.M.; Cho, W.K.; Villiger, M.; Bouma, B.E.; Pomahac, B.; Karp, J.M. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 2013, 4, 1702. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Lee, J.; Kim, B.J.; Joo, K.I.; Kim, K.H.; Lim, G.; Cha, H.J. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials 2019, 222, 119439. [Google Scholar] [CrossRef]
- Karami, P.; Rana, V.K.; Zhang, Q.; Boniface, A.; Guo, Y.; Moser, C.; Pioletti, D.P. NIR Light-Mediated Photocuring of Adhesive Hydrogels for Noninvasive Tissue Repair via Upconversion Optogenesis. Biomacromolecules 2022, 23, 5007–5017. [Google Scholar] [CrossRef]
- Duan, W.-L.; Zhang, L.-N.; Bohara, R.; Martin-Saldaña, S.; Yang, F.; Zhao, Y.-Y.; Xie, Y.; Bu, Y.-Z.; Pandit, A. Adhesive hydrogels in osteoarthritis: From design to application. Mil. Med. Res. 2023, 10, 4. [Google Scholar] [CrossRef]
- Gao, W.; Chen, K.; He, W.; Zhao, S.; Cui, D.; Tao, C.; Xu, Y.; Xiao, X.; Feng, Q.; Xia, H. Synergistic chondrogenesis promotion and arthroscopic articular cartilage restoration via injectable dual-drug-loaded sulfated hyaluronic acid hydrogel for stem cell therapy. Compos. Part B Eng. 2023, 263, 110857. [Google Scholar] [CrossRef]
- Cui, P.; Pan, P.; Qin, L.; Wang, X.; Chen, X.; Deng, Y.; Zhang, X. Nanoengineered hydrogels as 3D biomimetic extracellular matrix with injectable and sustained delivery capability for cartilage regeneration. Bioact. Mater. 2023, 19, 487–498. [Google Scholar] [CrossRef]
- Wei, J.; Ran, P.; Li, Q.; Lu, J.; Zhao, L.; Liu, Y.; Li, X. Hierarchically structured injectable hydrogels with loaded cell spheroids for cartilage repairing and osteoarthritis treatment. Chem. Eng. J. 2022, 430, 132211. [Google Scholar] [CrossRef]
- Roelofs, A.; Rocke, J.; De Bari, C. Cell-based approaches to joint surface repair: A research perspective. Osteoarthr. Cartil. 2013, 21, 892–900. [Google Scholar] [CrossRef]
- Mumme, M.; Barbero, A.; Miot, S.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Asnaghi, A.M.; Baumhoer, D.; Bieri, O.; Kretzschmar, M. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: An observational first-in-human trial. Lancet 2016, 388, 1985–1994. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, L.; Xu, Y.; Li, H.; Xu, W.; Wu, S.; Wang, Y.; Tang, Z.; Lv, Y.; Yang, L. Mechano growth factor (MGF) and transforming growth factor (TGF)-β3 functionalized silk scaffolds enhance articular hyaline cartilage regeneration in rabbit model. Biomaterials 2015, 52, 463–475. [Google Scholar] [CrossRef]
- Tischer, T.; Bode, G.; Buhs, M.; Marquass, B.; Nehrer, S.; Vogt, S.; Zinser, W.; Angele, P.; Spahn, G.; Welsch, G.H. Platelet-rich plasma (PRP) as therapy for cartilage, tendon and muscle damage–German working group position statement. J. Exp. Orthop. 2020, 7, 1–11. [Google Scholar]
- Liang, Y.; Li, J.; Wang, Y.; He, J.; Chen, L.; Chu, J.; Wu, H. Platelet rich plasma in the repair of articular cartilage injury: A narrative review. Cartilage 2022, 13, 19476035221118419. [Google Scholar] [CrossRef]
- Wan, J.; He, Z.; Peng, R.; Wu, X.; Zhu, Z.; Cui, J.; Hao, X.; Chen, A.; Zhang, J.; Cheng, P. Injectable photocrosslinking spherical hydrogel-encapsulated targeting peptide-modified engineered exosomes for osteoarthritis therapy. J. Nanobiotechnol. 2023, 21, 284. [Google Scholar] [CrossRef]
- Bédouet, L.; Moine, L.; Pascale, F.; Nguyen, V.-N.; Labarre, D.; Laurent, A. Synthesis of hydrophilic intra-articular microspheres conjugated to ibuprofen and evaluation of anti-inflammatory activity on articular explants. Int. J. Pharm. 2014, 459, 51–61. [Google Scholar] [CrossRef]
- García-Fernández, L.; Olmeda-Lozano, M.; Benito-Garzón, L.; Pérez-Caballer, A.; San Román, J.; Vázquez-Lasa, B. Injectable hydrogel-based drug delivery system for cartilage regeneration. Mater. Sci. Eng. C 2020, 110, 110702. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, C.; Xiong, Y.; Wang, Y.; Pu, C.; He, J.; Chen, L.; Jiang, K.; Zhao, W.; Yang, H. MMP13-responsive hydrogel microspheres for osteoarthritis treatment by precise delivery of celecoxib. Mater. Des. 2024, 241, 112966. [Google Scholar] [CrossRef]
- Yi, J.; Liu, Y.; Xie, H.; An, H.; Li, C.; Wang, X.; Chai, W. Hydrogels for the treatment of rheumatoid arthritis. Front. Bioeng. Biotechnol. 2022, 10, 1014543. [Google Scholar] [CrossRef]
- Oliveira, I.M.; Fernandes, D.C.; Cengiz, I.F.; Reis, R.L.; Oliveira, J.M. Hydrogels in the treatment of rheumatoid arthritis: Drug delivery systems and artificial matrices for dynamic in vitro models. J. Mater. Sci. Mater. Med. 2021, 32, 74. [Google Scholar] [CrossRef]
- Bao, W.; Li, M.; Yang, Y.; Wan, Y.; Wang, X.; Bi, N.; Li, C. Advancements and frontiers in the high performance of natural hydrogels for cartilage tissue engineering. Front. Chem. 2020, 8, 53. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Xu, B.-X.; Fan, K.-J.; Fan, Y.-S.; Teng, H.; Wang, T.-Y. Dexamethasone-loaded thermo-sensitive hydrogel attenuates osteoarthritis by protecting cartilage and providing effective pain relief. Ann. Transl. Med. 2021, 9, 1120. [Google Scholar] [CrossRef]
- Chen, K.; Li, S.; Yuan, F.; Sun, P.; Zhang, Y. GEL-MAN hydrogel loaded with triamcinolone acetonide for the treatment of osteoarthritis. Front. Bioeng. Biotechnol. 2020, 8, 872. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Eswaramoorthy, R.; Lin, T.-H.; Chen, C.-H.; Fu, Y.-C.; Wang, C.-K.; Wu, S.-C.; Wang, G.-J.; Chang, J.-K.; Ho, M.-L. Enhancement of chondrogenesis of adipose-derived stem cells in HA-PNIPAAm-CL hydrogel for cartilage regeneration in rabbits. Sci. Rep. 2018, 8, 10526. [Google Scholar] [CrossRef]
- Seidlits, S.K.; Khaing, Z.Z.; Petersen, R.R.; Nickels, J.D.; Vanscoy, J.E.; Shear, J.B.; Schmidt, C.E. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010, 31, 3930–3940. [Google Scholar] [CrossRef]
- Ju, X.; Liu, X.; Zhang, Y.; Chen, X.; Chen, M.; Shen, H.; Feng, Y.; Liu, J.; Wang, M.; Shi, Q. A photo-crosslinked proteinogenic hydrogel enabling self-recruitment of endogenous TGF-β1 for cartilage regeneration. Smart Mater. Med. 2022, 3, 85–93. [Google Scholar] [CrossRef]
- Akkiraju, H.; Bonor, J.; Nohe, A. CK2. 1, a novel peptide, induces articular cartilage formation in vivo. J. Orthop. Res. 2017, 35, 876–885. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Niu, X.; Lin, Q.; Zhao, B.; Wang, Y.; Zhu, L. An in situ photocrosslinkable platelet rich plasma–complexed hydrogel glue with growth factor controlled release ability to promote cartilage defect repair. Acta Biomater. 2017, 62, 179–187. [Google Scholar] [CrossRef]
- Haseeb, A.; Kc, R.; Angelozzi, M.; de Charleroy, C.; Rux, D.; Tower, R.J.; Yao, L.; Pellegrino da Silva, R.; Pacifici, M.; Qin, L. SOX9 keeps growth plates and articular cartilage healthy by inhibiting chondrocyte dedifferentiation/osteoblastic redifferentiation. Proc. Natl. Acad. Sci. USA 2021, 118, e2019152118. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Babicz, H.; Madry, H.; Concheiro, A.; Alvarez-Lorenzo, C.; Cucchiarini, M. Supramolecular polypseudorotaxane gels for controlled delivery of rAAV vectors in human mesenchymal stem cells for regenerative medicine. Int. J. Pharm. 2017, 531, 492–503. [Google Scholar] [CrossRef]
- Luo, D.; Zhu, H.; Li, S.; Wang, Z.; Xiao, J. Mesenchymal stem cell-derived exosomes as a promising cell-free therapy for knee osteoarthritis. Front. Bioeng. Biotechnol. 2024, 12, 1309946. [Google Scholar] [CrossRef]
- Atwal, A.; Dale, T.P.; Snow, M.; Forsyth, N.R.; Davoodi, P. Injectable hydrogels: An emerging therapeutic strategy for cartilage regeneration. Adv. Colloid Interface Sci. 2023, 321, 103030. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Y.; Rui, B.; Lin, J.; Shen, J.; Xiao, H.; Liu, X.; Chai, Y.; Xu, J.; Yang, Y. A photoannealed granular hydrogel facilitating hyaline cartilage regeneration via improving chondrogenic phenotype. ACS Appl. Mater. Interfaces 2022, 14, 40674–40687. [Google Scholar] [CrossRef]
- Wei, P.; Ma, Y.; Qin, K.; Fan, Z. A 3D printed biomimetic scaffold for cartilage regeneration with lubrication, load-bearing, and adhesive fixation properties. Tribol. Int. 2024, 192, 109328. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Xu, X.; Abdou, P.; Jiang, Q.; Shi, D.; Gu, Z. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regen. Biomater. 2019, 6, 129–140. [Google Scholar] [CrossRef]
- Sharma, B.; Fermanian, S.; Gibson, M.; Unterman, S.; Herzka, D.A.; Cascio, B.; Coburn, J.; Hui, A.Y.; Marcus, N.; Gold, G.E. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci. Transl. Med. 2013, 5, 167ra166. [Google Scholar] [CrossRef] [PubMed]
- Wyss, C.S.; Karami, P.; Demongeot, A.; Bourban, P.-E.; Pioletti, D.P. Silk granular hydrogels self-reinforced with regenerated silk fibroin fibers. Soft Matter 2021, 17, 7038–7046. [Google Scholar] [CrossRef] [PubMed]
- Wyss, C.S.; Karami, P.; Bourban, P.-E.; Pioletti, D.P. Hybrid granular hydrogels: Combining composites and microgels for extended ranges of material properties. Soft Matter 2020, 16, 3769–3778. [Google Scholar] [CrossRef]
- Irwin, R.M.; Gao, T.; Boys, A.J.; Ortved, K.; Cohen, I.; Bonassar, L.J. Microscale strain mapping demonstrates the importance of interface slope in the mechanics of cartilage repair. J. Biomech. 2021, 114, 110159. [Google Scholar] [CrossRef]
- Komeili, A.; Luqman, S.; Federico, S.; Herzog, W. Effect of cracks on the local deformations of articular cartilage. J. Biomech. 2020, 110, 109970. [Google Scholar] [CrossRef]
- Shapiro, F.; Koide, S.; Glimcher, M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. JBJS 1993, 75, 532–553. [Google Scholar] [CrossRef]
- Getgood, A.; Henson, F.; Skelton, C.; Brooks, R.; Guehring, H.; Fortier, L.A.; Rushton, N. Osteochondral tissue engineering using a biphasic collagen/GAG scaffold containing rhFGF18 or BMP-7 in an ovine model. J. Exp. Orthop. 2014, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pabbruwe, M.B.; Esfandiari, E.; Kafienah, W.; Tarlton, J.F.; Hollander, A.P. Induction of cartilage integration by a chondrocyte/collagen-scaffold implant. Biomaterials 2009, 30, 4277–4286. [Google Scholar] [CrossRef] [PubMed]
- Monaco, G.; El Haj, A.J.; Alini, M.; Stoddart, M.J. Ex vivo systems to study chondrogenic differentiation and cartilage integration. J. Funct. Morphol. Kinesiol. 2021, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Link, J.M.; Hu, J.C.; Athanasiou, K.A. Chondroitinase ABC enhances integration of self-assembled articular cartilage, but its dosage needs to be moderated based on neocartilage maturity. Cartilage 2021, 13, 672S–683S. [Google Scholar] [CrossRef]
- Gilbert, S.J.; Singhrao, S.K.; Khan, I.M.; Gonzalez, L.G.; Thomson, B.M.; Burdon, D.; Duance, V.C.; Archer, C.W. Enhanced tissue integration during cartilage repair in vitro can be achieved by inhibiting chondrocyte death at the wound edge. Tissue Eng. Part A 2009, 15, 1739–1749. [Google Scholar] [CrossRef]
- Sahar, J.; Amin, E.; Fatemeh, Z. New Insights into Cartilage Tissue Engineering: Improvement of Tissue-Scaffold Integration to Enhance Cartilage Regeneration. Biomed. Res. Int. 2022, 25, 7638245. [Google Scholar]
- Van de Breevaart Bravenboer, J.; In der Maur, C.D.; Bos, P.K.; Feenstra, L.; Verhaar, J.A.; Weinans, H.; van Osch, G.J. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res. Ther. 2004, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bos, P.; DeGroot, J.; Budde, M.; Verhaar, J.; Van Osch, G. Specific enzymatic treatment of bovine and human articular cartilage: Implications for integrative cartilage repair. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2002, 46, 976–985. [Google Scholar] [CrossRef]
- Silverman, R.P.; Bonasser, L.; Passaretti, D.; Randolph, M.A.; Yaremchuk, M.J. Adhesion of tissue-engineered cartilage to native cartilage. Plast. Reconstr. Surg. 2000, 105, 1393–1398. [Google Scholar]
- Kim, H.T.; Teng, M.S.; Dang, A.C. Chondrocyte apoptosis: Implications for osteochondral allograft transplantation. Clin. Orthop. Relat. Res. 2008, 466, 1819–1825. [Google Scholar] [CrossRef]
- Cohen, I.; Melamed, E.; Robinson, D.; Nevo, Z. Repair of articular cartilage lesions in aged chickens by allogeneic transplantation of fresh embryonic epiphyses. Arch. Orthop. Trauma Surg. 2007, 127, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Altan, E.; Aydin, K.; Erkocak, O.; Senaran, H.; Ugras, S. The effect of platelet-rich plasma on osteochondral defects treated with mosaicplasty. Int. Orthop. 2014, 38, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulos, J.S.; DeCroos, A.J.; Petrera, M.; Park, S.; Kandel, R.A. Mechanical stimulation enhances integration in an in vitro model of cartilage repair. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2055–2064. [Google Scholar] [CrossRef] [PubMed]
- DiMicco, M.; Waters, S.; Akeson, W.; Sah, R. Integrative articular cartilage repair: Dependence on developmental stage and collagen metabolism. Osteoarthr. Cartil. 2002, 10, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Kapfinger, E.; Müller, M. Removal of proteoglycans from the surface of defects in articular cartilage transiently enhances coverage by repair cells. J. Bone Jt. Surg. Br. Vol. 1998, 80, 144–150. [Google Scholar] [CrossRef]
- Wang, C.-C.; Yang, K.-C.; Lin, K.-H.; Liu, Y.-L.; Yang, Y.-T.; Kuo, T.-F.; Chen, I.-H. Expandable scaffold improves integration of tissue-engineered cartilage: An in vivo study in a rabbit model. Tissue Eng. Part A 2016, 22, 873–884. [Google Scholar] [CrossRef]
- Wang, D.-A.; Varghese, S.; Sharma, B.; Strehin, I.; Fermanian, S.; Gorham, J.; Fairbrother, D.H.; Cascio, B.; Elisseeff, J.H. Multifunctional chondroitin sulphate for cartilage tissue–biomaterial integration. Nat. Mater. 2007, 6, 385–392. [Google Scholar] [CrossRef]
- Khan, I.M.; Gilbert, S.J.; Singhrao, S.; Duance, V.C.; Archer, C.W. Cartilage integration: Evaluation of the reasons for failure of integration during cartilage repair. A review. Eur Cell Mater. 2008, 16, 26–39. [Google Scholar] [CrossRef]
- Teng, L.; Chen, Y.; Jia, Y.-G.; Ren, L. Supramolecular and dynamic covalent hydrogel scaffolds: From gelation chemistry to enhanced cell retention and cartilage regeneration. J. Mater. Chem. B 2019, 7, 6705–6736. [Google Scholar] [CrossRef]
- Dehne, T.; Zehbe, R.; Krüger, J.P.; Petrova, A.; Valbuena, R.; Sittinger, M.; Schubert, H.; Ringe, J. A method to screen and evaluate tissue adhesives for joint repair applications. BMC Musculoskelet. Disord. 2012, 13, 1–9. [Google Scholar] [CrossRef]
- Kazusa, H.; Nakasa, T.; Shibuya, H.; Ohkawa, S.; Kamei, G.; Adachi, N.; Deie, M.; Nakajima, N.; Hyon, S.-H.; Ochi, M. Strong adhesiveness of a new biodegradable hydrogel glue, LYDEX, for use on articular cartilage. J. Appl. Biomater. Funct. Mater. 2013, 11, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, X.; Wang, S.; Zhang, Y.; Yang, A.; Cheng, Y.; Chen, X. Ultra-durable cell-free bioactive hydrogel with fast shape memory and on-demand drug release for cartilage regeneration. Nat. Commun. 2023, 14, 7771. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, S.; Liu, X.; Qin, Z.; Yang, Y.; Zang, J.; Zhao, X. Fatigue-resistant adhesion of hydrogels. Nat. Commun. 2020, 11, 1071. [Google Scholar] [CrossRef]
- Wei, K.; Senturk, B.; Matter, M.T.; Wu, X.; Herrmann, I.K.; Rottmar, M.; Toncelli, C. Mussel-inspired injectable hydrogel adhesive formed under mild conditions features near-native tissue properties. ACS Appl. Mater. Interfaces 2019, 11, 47707–47719. [Google Scholar] [CrossRef]
- Qiu, H.; Deng, J.; Wei, R.; Wu, X.; Chen, S.; Yang, Y.; Gong, C.; Cui, L.; Si, Z.; Zhu, Y. A lubricant and adhesive hydrogel cross-linked from hyaluronic acid and chitosan for articular cartilage regeneration. Int. J. Biol. Macromol. 2023, 243, 125249. [Google Scholar] [CrossRef]
- ASTM F2255-05; Standard Test Method for Strength Properties of Tissue Adhesives in Lap-Shear by Tension Loading. ASTM: West Conshohocken, PA, USA, 2015.
- Karami, P.; Stampoultzis, T.; Guo, Y.; Pioletti, D.P. A guide to preclinical evaluation of hydrogel-based devices for treatment of cartilage lesions. Acta Biomater. 2023, 158, 12–31. [Google Scholar] [CrossRef]
- Øvrebø, Ø.; Perale, G.; Wojciechowski, J.P.; Echalier, C.; Jeffers, J.R.; Stevens, M.M.; Haugen, H.J.; Rossi, F. Design and clinical application of injectable hydrogels for musculoskeletal therapy. Bioeng. Transl. Med. 2022, 7, e10295. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.J.; Ramesh, A.; Brama, P.A.; O’Byrne, J.M.; O’Brien, F.J.; Levingstone, T.J. The benefits and limitations of animal models for translational research in cartilage repair. J. Exp. Orthop. 2016, 3, 1–12. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Risk-Based Approach According to Annex I, Part IV of Directive 2001/83/EC Applied to Advanced Therapy Medicinal Products; EMA: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Jagadeeswaran, I.; Palani, N.; Lakshmanan, G. FDA-CFR Title 21-Food and Drugs: Parts 800 to 1299. In Medical Device Guidelines and Regulations Handbook; Springer: Berlin/Heidelberg, Germany, 2022; pp. 189–236. [Google Scholar]
- European Medicines Agency. EMA Guideline on Human Cell-Based Medicinal Products, EMEA/CHMP/410869/2006; EMA: Amsterdam, The Netherlands, 2008. [Google Scholar]
- European Commission. Guidelines on good manufacturing practice specific to advanced therapy medicinal products. Eur. Comm. J. 2017, 4, 1–32. [Google Scholar]




| Treatment Method | Advantages | Limitations | Integration Mechanisms | Implications and Factors Contributing to Weak Integration in Early Phases | References |
|---|---|---|---|---|---|
| Bone Marrow Stimulation | - Minimally invasive procedure - Cost-effective - Suitable for small defects | - Fibrocartilage rather than hyaline cartilage - Limited durability and long-term efficacy - Not suitable for large defects | - Clot formation and recruitment of mesenchymal stem cells | - Immature tissue formation and limited matrix deposition - Fibrocartilage formation may compromise mechanical properties and long-term function | [3,23,24] |
| Osteochondral Autograft Transfer (OATS) and Mosaicplasty | - Utilizes patient’s own osteochondral tissue - Structural support and immediate stability | - Limited availability of donor tissue - Risk of donor site morbidity - Not suitable for larger defects | - Integration through precise graft matching - Promotion of chondrocyte migration and matrix production - Bone-to-bone fusion | - Challenges in achieving seamless integration between graft and host tissue - Insufficient graft-host tissue congruency - Inadequate cell migration and matrix production | [25,26,27] |
| Osteochondral Allograft Transplantation | - Provides mature, hyaline-like cartilage - Suitable for larger defects - Eliminates risk of donor site morbidity compared to autografts | - Limited availability of matching grafts - High cost - Requires matching of graft size and contour - Requirement to implant the graft within 28 days | - Integration through precise graft matching - Promotion of chondrocyte migration and matrix production - Bone-to-bone fusion | - Requires adequate host tissue preparation for successful integration - Insufficient graft-host tissue matching - Inadequate cell migration and matrix production | [28,29,30] |
| First- and second-generation ACI | - Potential for hyaline-like cartilage formation | - Limited availability of healthy chondrocytes for implantation - Risk of cell leakage - Further tissue damage by suturing the membrane | - Chondrocyte proliferation and matrix production - Gradual infiltration of native cells and matrix from surrounding tissue | - Limited cell retention and survival in the defect area - Inadequate cell migration and matrix production - Challenges in achieving uniform integration with surrounding tissue | [25,26] |
| Third-generation ACI | - Improved cell retention and distribution within cell carriers - Early cell differentiation using pre-seeded scaffolds | - Limited availability of healthy chondrocytes for implantation - Higher cost compared to traditional ACI | - Chondrocyte proliferation and matrix production within the scaffold - Gradual infiltration of native cells and matrix from surrounding tissues | - Inadequate cell migration and matrix production - Scaffold degradation may affect tissue integration - Suboptimal extracellular matrix production | [25,26,31] |
| Mechanism | Contributing Parameters/Attributes | Examples/Previous Evidence |
|---|---|---|
| Cellular factors | Chondrocyte viability: Cell death hinders integration between neo-cartilage and existing tissue. | - Significant cell death reported at the interface between host and repaired tissue in partial-thickness chondral defects. - In vitro wounding induces a zone of cell death characterized by necrosis and apoptosis. |
| Chondrocyte phenotype: Dedifferentiation during expansion compromises chondrocyte function. | - Dedifferentiated chondrocytes show limited redifferentiation capacity, affecting integration. - Incomplete redifferentiation can compromise normal chondrocyte function. | |
| Donor-related factors | Donor age: Age-related decline in chondrocyte function impedes integration. | - Age-related reductions in chondrocyte function affect repair outcomes. - Young tissue exhibits better repair and integration outcomes compared to aged tissue. |
| Developmental origins: Differences in tissue origin affect biosynthetic capacities and matrix production. | - Tissues from different developmental origins may have varied integration capacities. - Mixing tissues from different origins may or may not result in segregation. | |
| Extracellular matrix factors | Collagen network: Collagen deposition and crosslinking influence integration. | - Collagenase treatment enhances integration by promoting collagen deposition and chondrocyte migration. - Lysyl-oxidase-mediated crosslinking affects fusion between cartilages. |
| Proteoglycans: The presence of proteoglycans inhibits chondrocyte migration and integration. | - Enzymatic removal of proteoglycans increases chondrocyte mobility and enhances integration. - Loss of proteoglycans using chemical crosslinkers enhances adhesion of cartilage surfaces. | |
| Biomaterials and scaffold integration | Low adhesion performance: Inadequate scaffold adhesion affects tissue integration. | - Scaffolds with poor adhesion may fail to properly integrate with surrounding cartilage. - Low adhesion can result in delamination of the repaired tissue from the host cartilage. |
| Inappropriate mechanical properties: Scaffold properties may not match physiological requirements, impacting integration. | - Scaffolds with mismatched mechanical properties may lead to mechanical failure and hinder integration. - Biomechanically weak scaffolds may collapse under load, preventing integration. | |
| Inadequate biocompatibility: Scaffold materials may elicit immune responses or cytotoxic effects, impeding integration. | - Biocompatibility issues with scaffold materials can lead to inflammation and hinder tissue integration. - Cytotoxicity of scaffold components may impair chondrocyte function and integration. | |
| Insufficient porosity: Low porosity limits cell infiltration and nutrient exchange, affecting integration. | - Scaffolds with inadequate porosity may restrict cell migration and proliferation, hindering tissue integration. - Poor nutrient exchange due to low porosity can impair cell viability and integration. |
| Parameter | Potential Strategy | Examples/Previous Evidence |
|---|---|---|
| Cellular factors | Promote chondrocyte viability: Use of caspase inhibitors to inhibit apoptotic cell death | Inhibition of apoptotic cell death using caspase inhibitors such as ZVAD-fmk has shown partial rescue of cell death and enhancing lateral integration [110]. |
| Utilization of young tissues | Utilizing tissues from younger donors: Higher biosynthetic capacities and integration potential | Transplantation of embryonic tissues into defects in mature animals has shown improved restoration of surface continuity and lateral integration [111]. |
| External stimuli and treatments | Use of growth factors: Controlled release to promote chondrogenesis and tissue integration | Use of platelet-rich plasma (PRP) as a growth factor blend, induced better graft integration [112]. |
| Mechanical stimulation | Spinner bioreactor stimulation enhanced integration, boosting collagen content and gene expression related to integration. Early loading post-surgery could improve cartilage integration [113]. | |
| Extracellular matrix factors | Modulate collagen network: Use of collagen crosslinking inhibitors to enhance fusion. | Inhibition of lysyl-oxidase-mediated collagen crosslinking accelerated collagen maturation and increased adhesive strength, promoting integration [114]. |
| Manipulate proteoglycan content: Enzymatic removal of proteoglycans to promote chondrocyte mobility. | Enzymatic removal of proteoglycans increased chondrocyte mobility and enhanced integration [115]. | |
| Biomaterials and scaffold integration | Scaffold adhesion | An intrinsically adhesive hydrogel demonstrated tissue integration after two days of in vivo implantation in cartilage defects [44]. |
| Optimal porosity | Allowing better cell infiltration and nutrient exchange, enhancing integration [116]. | |
| Surface modification | Bioadhesive glues and bridging polymers (e.g., fibrin, etc.) | Employing chondroitin sulfate (CS) functionalized with methacrylate and aldehyde groups facilitated mechanical stability for tissue repair [117]. |
| Consideration | Device Category | Description | Evaluation Method | Standards/References |
|---|---|---|---|---|
| Biocompatibility Assessment | Cell-Based | - Assessment of cell viability, proliferation, and differentiation within the hydrogel scaffold in vitro. - Evaluation of host immune response and tissue integration post-implantation. | - Live/dead staining, MTT assay, Alamar Blue assay for cell viability. - Immunohistochemistry for cell-specific markers (e.g., collagen type II, aggrecan) for differentiation. - ELISA for evaluation of inflammatory cytokines (e.g., TNF-α, IL-6) post-implantation. | - ISO 10993 series for biocompatibility testing. - ASTM F1903-98 for evaluation of tissue-engineered cartilage constructs. |
| Non-Cell-Based | - Examination of tissue response and integration without cellular components. - Focus on minimizing inflammatory reactions and promoting tissue regeneration. | - Histological analysis (e.g., H and E staining) for tissue response and integration. - Immunohistochemistry for ECM components (e.g., collagen type II, glycosaminoglycans). | - ISO 10993 series for biocompatibility testing. - ASTM F2150-18 for standard guide for tissue-engineered medical products (TEMPs). | |
| Preclinical Efficacy Studies | Cell-Based | - Demonstration of chondrogenic potential and matrix synthesis by seeded cells. - Evaluation of scaffold degradation and tissue remodeling. | - Immunohistochemistry for chondrogenic markers (e.g., collagen type II, aggrecan). - Biochemical assays (e.g., GAG/DNA content, hydroxyproline assay) for matrix synthesis. - SEM and mechanical testing for scaffold degradation and mechanical properties. | - ASTM F2451-05 for testing the mechanical properties of hydrogels for cartilage repair. - ISO 10993 series for biocompatibility testing. |
| Non-Cell-Based | - Emphasis on scaffold stability, mechanical properties, and biodegradation characteristics. - Assessment of tissue ingrowth and integration with surrounding cartilage. | - Mechanical testing (e.g., tensile, compressive, shear) for scaffold stability and properties. - Histomorphometry for tissue ingrowth and integration. | - ASTM F2451-05 for testing the mechanical properties of hydrogels for cartilage repair. - ISO 10993 series for biocompatibility testing. | |
| Clinical Trial Design | Cell-Based | - Consideration of cell sourcing, expansion, and delivery methods. -Evaluation of cell retention, survival, and functionality post-implantation. | - In vivo imaging techniques (e.g., MRI, CT) for cell tracking and localization. - Biopsies for histological evaluation of cell survival and phenotype. - Functional assessments (e.g., joint function scores, pain scales) for therapeutic outcomes. | - FDA Guidance for Industry: Preclinical Assessment of Investigational Cellular and Gene Therapy Products (FDA-2012-D-1038). - EMA Guideline on Human Cell-Based Medicinal Products [132]. |
| Non-Cell-Based | - Simplified trial design without the complexity of cell handling and processing. - Focus on scaffold delivery, integration, and therapeutic outcomes. | - In vivo imaging techniques (e.g., MRI, CT) for scaffold localization and integration. - Functional assessments (e.g., joint function scores, pain scales) for therapeutic outcomes. | - FDA Guidance for Industry: Considerations for the Design of Early-Phase Clinical Trials of Cellular and Gene Therapy Products. - Regulation (EU) 2017/745: New Medical Device Regulation (MDR). | |
| Regulatory Approval Pathway | Cell-Based | - Additional regulatory scrutiny for cell sourcing, processing, and manipulation. - Compliance with Good Manufacturing Practice (GMP) standards for cell-based therapies. | - Adherence to GMP regulations for cell isolation, expansion, and manipulation. - Documentation of cell identity, purity, and potency. - Validation of manufacturing processes and quality control measures. | - FDA Guidance for Industry: CGMP for Phase 1 Investigational Drug and Biological Products. - EMA Guidelines on Good Manufacturing Practice Specific to Advanced Therapy Medicinal Products [133]. |
| Non-Cell-Based | - Streamlined regulatory pathway focusing on scaffold composition, manufacturing, and performance. - Emphasis on biocompatibility, safety, and efficacy of the scaffold material. | - Compliance with regulatory guidelines for medical devices (e.g., ISO 13485). - Documentation of material characterization, sterilization, and biocompatibility testing. | - FDA Guidance for Premarket Approval (PMA) or Premarket Notification 510(k) depending on device classification. - ISO 13485:2016 for quality management systems for medical devices. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karami, P.; Laurent, A.; Philippe, V.; Applegate, L.A.; Pioletti, D.P.; Martin, R. Cartilage Repair: Promise of Adhesive Orthopedic Hydrogels. Int. J. Mol. Sci. 2024, 25, 9984. https://doi.org/10.3390/ijms25189984
Karami P, Laurent A, Philippe V, Applegate LA, Pioletti DP, Martin R. Cartilage Repair: Promise of Adhesive Orthopedic Hydrogels. International Journal of Molecular Sciences. 2024; 25(18):9984. https://doi.org/10.3390/ijms25189984
Chicago/Turabian StyleKarami, Peyman, Alexis Laurent, Virginie Philippe, Lee Ann Applegate, Dominique P. Pioletti, and Robin Martin. 2024. "Cartilage Repair: Promise of Adhesive Orthopedic Hydrogels" International Journal of Molecular Sciences 25, no. 18: 9984. https://doi.org/10.3390/ijms25189984








