Microalgae Flocculation: Assessment of Extraction Yields and Biological Activity
Abstract
:1. Introduction
2. Results
2.1. Flocculation
2.1.1. Chitosan
2.1.2. Calcium Chloride and Sodium Glutamate
2.1.3. pH-Mediated Flocculation
2.2. Pigment Extraction
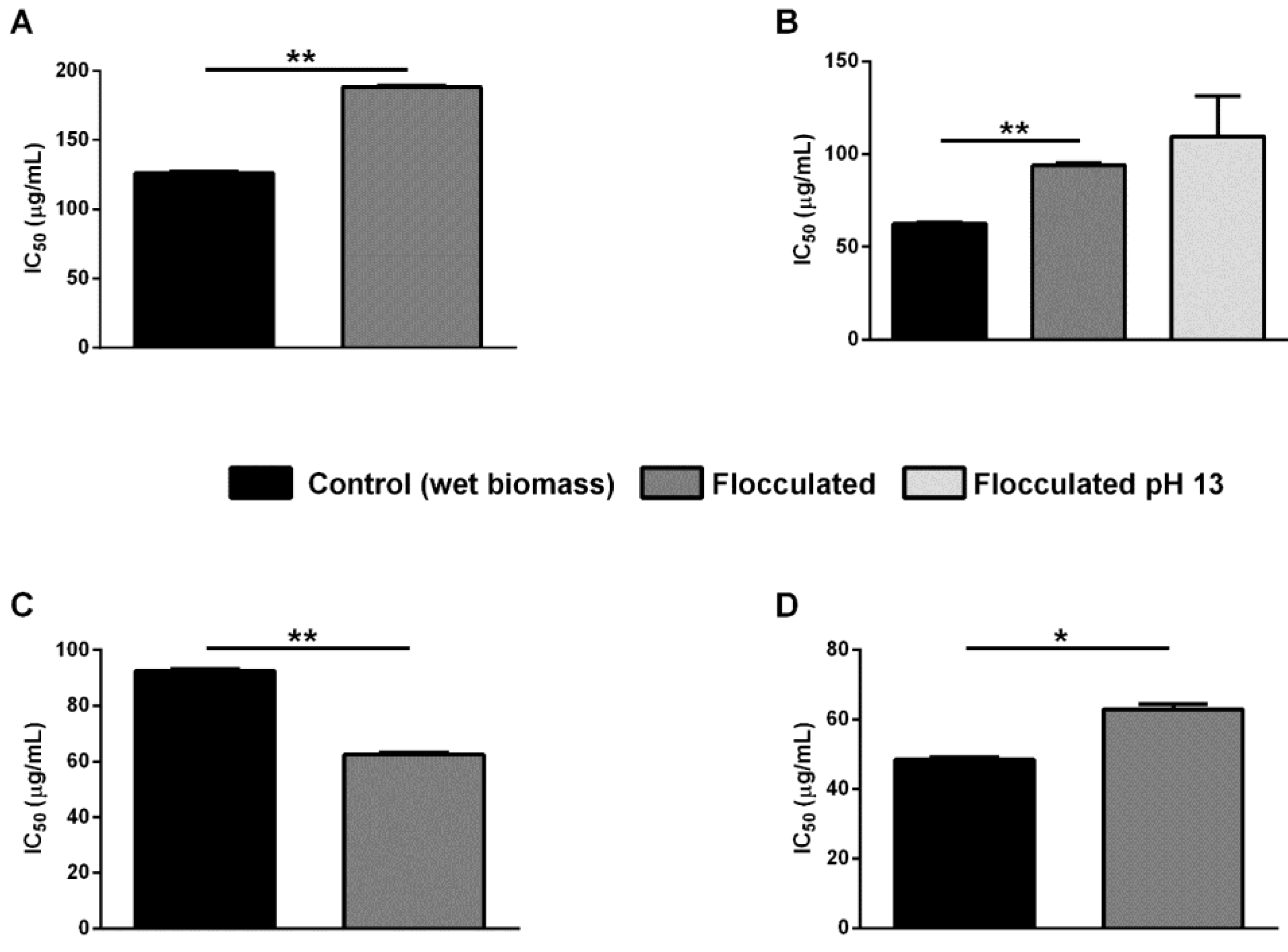
2.3. In Vitro Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Microalgae Strains and Culture Conditions
4.3. Flocculation
4.4. Pigments Extraction and Yields Determination
4.5. ABTS Assay
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddiqui, A.; Wei, Z.; Boehm, M.; Ahmad, N. Engineering Microalgae through Chloroplast Transformation to Produce High-Value Industrial Products. Biotechnol. Appl. Biochem. 2020, 67, 30–40. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A Review of High Value-Added Molecules Production by Microalgae in Light of the Classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Fernández Sevilla, J.M.; Molina Grima, E. Chapter 21—Costs Analysis of Microalgae Production. In Biomass, Biofuels, Biochemicals; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.-J., Chisti, Y.B.T.-B., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 551–566. ISBN 978-0-444-64192-2. [Google Scholar]
- Udayan, A.; Pandey, A.K.; Sharma, P.; Sreekumar, N.; Kumar, S. Emerging Industrial Applications of Microalgae: Challenges and Future Perspectives. Syst. Microbiol. Biomanuf. 2021, 1, 411–431. [Google Scholar] [CrossRef]
- Roy, M.; Mohanty, K. A Comprehensive Review on Microalgal Harvesting Strategies: Current Status and Future Prospects. Algal Res. 2019, 44, 101683. [Google Scholar] [CrossRef]
- Okoro, V.; Azimov, U.; Munoz, J.; Hernandez, H.H.; Phan, A.N. Microalgae Cultivation and Harvesting: Growth Performance and Use of Flocculants—A Review. Renew. Sustain. Energy Rev. 2019, 115, 109364. [Google Scholar] [CrossRef]
- Fuad, N.; Omar, R.; Kamarudin, S.; Harun, R.; Idris, A.; Wan, W.A. Mass Harvesting of Marine Microalgae Using Different Techniques. Food Bioprod. Process. 2018, 112, 169–184. [Google Scholar] [CrossRef]
- Hidayah, N.; Yasin, M.; Shafei, N.I.; Rushan, N.H.; Raihana, N.; Sepian, A.; Said, F.M. ScienceDirect the Effect of Microalgae Harvesting on Lipid for Biodiesel Production. Mater. Today Proc. 2019, 19, 1582–1590. [Google Scholar] [CrossRef]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Dual Role of Microalgae: Phycoremediation of Domestic Wastewater and Biomass Production for Sustainable Biofuels Production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting Techniques Applied to Microalgae: A Review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Najjar, Y.S.H.; Abu-Shamleh, A. Harvesting of Microalgae by Centrifugation for Biodiesel Production: A Review. Algal Res. 2020, 51, 102046. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Ki, M.R.; Pack, S.P. Recent Progress in Flocculation, Dewatering, and Drying Technologies for Microalgae Utilization: Scalable and Low-Cost Harvesting Process Development. Bioresour. Technol. 2022, 344, 126404. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Banerjee, C.; Negi, S.; Shukla, P. Microalgae Harvesting Techniques: Updates and Recent Technological Interventions. Crit. Rev. Biotechnol. 2023, 43, 342–368. [Google Scholar] [CrossRef]
- Ortiz, A.; García-Galán, M.J.; García, J.; Díez-Montero, R. Optimization and Operation of a Demonstrative Full Scale Microalgae Harvesting Unit Based on Coagulation, Flocculation and Sedimentation. Sep. Purif. Technol. 2021, 259, 118171. [Google Scholar] [CrossRef]
- Demir-Yilmaz, I.; Ftouhi, M.S.; Balayssac, S.; Guiraud, P.; Coudret, C.; Formosa-Dague, C. Bubble Functionalization in Flotation Process Improve Microalgae Harvesting. Chem. Eng. J. 2023, 452, 139349. [Google Scholar] [CrossRef]
- Xu, H.; Wang, H.; Liang, Z.; Chen, H.; Yang, D.; Tang, Z.; Dai, X. A Novel Biomineralization-Inspired Flocculation Approach for Harvesting High Quality Microalgal Biomass: Dual Action of Cationic Polyelectrolytes and Nanosilica. Bioresour. Technol. 2023, 388, 129739. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, B. A Novel Method to Harvest Microalgae via Co-Culture of Filamentous Fungi to Form Cell Pellets. Bioresour. Technol. 2012, 114, 529–535. [Google Scholar] [CrossRef]
- de Morais, E.G.; Sampaio, I.C.F.; Gonzalez-Flo, E.; Ferrer, I.; Uggetti, E.; García, J. Microalgae Harvesting for Wastewater Treatment and Resources Recovery: A Review. New Biotechnol. 2023, 78, 84–94. [Google Scholar] [CrossRef]
- Zhang, B.; Mao, B. Efficient Magnetic Flocculation for Microalgae Harvesting Using a Novel Composite Flocculant of Fe3O4-Based Starch-Grafted-Cationic Polyacrylamide. J. Water Process Eng. 2023, 55, 104121. [Google Scholar] [CrossRef]
- Gifuni, I.; Pollio, A.; Safi, C.; Marzocchella, A.; Olivieri, G. Current Bottlenecks and Challenges of the Microalgal Biorefinery. Trends Biotechnol. 2019, 37, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Mansour, E.A.; Abo El-Enin, S.A.; Hamouda, A.S.; Mahmoud, H.M. Efficacy of Extraction Techniques and Solvent Polarity on Lipid Recovery from Domestic Wastewater Microalgae. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100271. [Google Scholar] [CrossRef]
- Sati, H.; Mitra, M.; Mishra, S.; Baredar, P. Microalgal Lipid Extraction Strategies for Biodiesel Production: A Review. Algal Res. 2019, 38, 101413. [Google Scholar] [CrossRef]
- Sousa, V.; Pereira, R.N.; Vicente, A.A.; Dias, O.; Geada, P. Microalgae Biomass as an Alternative Source of Biocompounds: New Insights and Future Perspectives of Extraction Methodologies. Food Res. Int. 2023, 173, 113282. [Google Scholar] [CrossRef] [PubMed]
- Pocha, C.K.R.; Chia, W.Y.; Chew, K.W.; Munawaroh, H.S.H.; Show, P.L. Current Advances in Recovery and Biorefinery of Fucoxanthin from Phaeodactylum tricornutum. Algal Res. 2022, 65, 102735. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Tang, D.Y.Y.; Leong, H.Y.; Khoo, K.S.; Show, P.L.; Chew, K.W. Bridging Artificial Intelligence and Fucoxanthin for the Recovery and Quantification from Microalgae. Bioengineered 2023, 14, 2244232. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, K.; Alsulami, F.S.; Neyaz, L.A.; Abulreesh, H.H. Poly (γ) Glutamic Acid: A Unique Microbial Biopolymer with Diverse Commercial Applicability. Front. Microbiol. 2024, 15, 1348411. [Google Scholar] [CrossRef]
- Lama, S.; Muylaert, K.; Karki, T.B.; Foubert, I.; Henderson, R.K.; Vandamme, D. Flocculation Properties of Several Microalgae and a Cyanobacterium Species during Ferric Chloride, Chitosan and Alkaline Flocculation. Bioresour. Technol. 2016, 220, 464–470. [Google Scholar] [CrossRef]
- Liberti, D.; Imbimbo, P.; Giustino, E.; D’elia, L.; Ferraro, G.; Casillo, A.; Illiano, A.; Pinto, G.; Di Meo, M.C.; Alvarez-Rivera, G.; et al. Inside out Porphyridium cruentum: Beyond the Conventional Biorefinery Concept. ACS Sustain. Chem. Eng. 2022, 11, 381–389. [Google Scholar] [CrossRef]
- D’Elia, L.; Imbimbo, P.; Liberti, D.; Bolinesi, F.; Mangoni, O.; Pollio, A.; Olivieri, G.; Monti, D.M. Thermo Resistant Antioxidants from Photoautotrophic Microorganisms: Screening and Characterization. World J. Microbiol. Biotechnol. 2021, 37, 215. [Google Scholar] [CrossRef]
- Imbimbo, P.; Bueno, M.; D’Elia, L.; Pollio, A.; Ibañez, E.; Olivieri, G.; Monti, D.M. Green Compressed Fluid Technologies To Extract Antioxidants and Lipids from Galdieria phlegrea in a Biorefinery Approach. ACS Sustain. Chem. Eng. 2020, 8, 2939–2947. [Google Scholar] [CrossRef]
- Imbimbo, P.; Giustino, E.; Ferrara, A.; Alvarez, G.; Hassan, R.; Elena, A.; Chiara, M.; Meo, D.; Zarrelli, A.; Maria, D. Unveiling the Potential of Pseudococcomyxa simplex: A Stepwise Extraction for Cosmetic Applications. Appl. Microbiol. Biotechnol. 2024, 108, 390. [Google Scholar] [CrossRef]
- Rosmahadi, N.A.; Leong, W.H.; Rawindran, H.; Ho, Y.C.; Mohamad, M.; Ghani, N.A.; Bashir, M.J.K.; Usman, A.; Lam, M.K.; Lim, J.W. Assuaging Microalgal Harvesting Woes via Attached Growth: A Critical Review to Produce Sustainable Microalgal Feedstock. Sustainability 2021, 13, 11159. [Google Scholar] [CrossRef]
- Endrawati, H.; Widianingsih, W.; Nuraini, R.A.T.; Hartati, R.; Redjeki, S.; Riniatsih, I.; Mahendrajaya, R.T. The Effect of Chitosan Concentration on Flocculation Efficiency Microalgae Porphyridium cruentum (Rhodhophyta). IOP Conf. Ser. Earth Environ. Sci. 2021, 919, 012052. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Mat Yasin, N.H.; Derek, C.J.C.; Lim, J.K. Optimization of Microalgae Coagulation Process Using Chitosan. Chem. Eng. J. 2011, 173, 879–882. [Google Scholar] [CrossRef]
- Rossi, S.; Visigalli, S.; Castillo Cascino, F.; Mantovani, M.; Mezzanotte, V.; Parati, K.; Canziani, R.; Turolla, A.; Ficara, E. Metal-Based Flocculation to Harvest Microalgae: A Look beyond Separation Efficiency. Sci. Total Environ. 2021, 799, 149395. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Hu, T.; Li, S.; Nugroho, Y.K.; Li, B.; Cao, J.; Show, P.-L.; Hiltunen, E. Effects of Operating Parameters on Algae Chlorella vulgaris Biomass Harvesting and Lipid Extraction Using Metal Sulfates as Flocculants. Biomass Bioenergy 2020, 132, 105433. [Google Scholar] [CrossRef]
- Singh, H.M.; Tyagi, V.V.; Ahmad, S.; Kothari, R. Optimization of Flocculation Efficiency of Chlorella pyrenoidosa with CaCl2 Using the Box-Behnken Design of Response Surface Methodology: A Cost Effective Statistical Investigation. Biomass Convers. Biorefin. 2024, 14, 3261–3273. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Shobana, S.; Bakonyi, P.; Nemestóthy, N.; Xia, A.; Banu, J.R.; Kumar, G. A Review on Chemical Mechanism of Microalgae Flocculation via Polymers. Biotechnol. Rep. 2019, 21, e00302. [Google Scholar] [CrossRef]
- Babakhani, P.; Mahdavi, M.A.; Gheshlaghi, R.; Karimian, A. The Shift in Carbon Source Induces pH Increase and Autoflocculation in Microalgal Suspensions Facilitating Multi-Approach Biomass Harvesting. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Chen, C.Y.; Liu, C.H.; Lo, Y.C.; Chang, J.S. Perspectives on Cultivation Strategies and Photobioreactor Designs for Photo-Fermentative Hydrogen Production. Bioresour. Technol. 2011, 102, 8484–8492. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Fraeye, I.; Meesschaert, B.; Muylaert, K. Flocculation of Chlorella vulgaris Induced by High pH: Role of Magnesium and Calcium and Practical Implications. Bioresour. Technol. 2012, 105, 114–119. [Google Scholar] [CrossRef]
- Kuzhiumparambil, U.; Labeeuw, L.; Commault, A.; Vu, H.P.; Nguyen, L.N.; Ralph, P.J.; Nghiem, L.D. Effects of Harvesting on Morphological and Biochemical Characteristics of Microalgal Biomass Harvested by Polyacrylamide Addition, pH-Induced Flocculation, and Centrifugation. Bioresour. Technol. 2022, 359, 127433. [Google Scholar] [CrossRef] [PubMed]
- Taghavijeloudar, M.; Yaqoubnejad, P.; Ahangar, A.K.; Rezania, S. A Rapid, Efficient and Eco-Friendly Approach for Simultaneous Biomass Harvesting and Bioproducts Extraction from Microalgae: Dual Flocculation between Cationic Surfactants and Bio-Polymer. Sci. Total Environ. 2023, 854, 158717. [Google Scholar] [CrossRef] [PubMed]
- Imbimbo, P.; Romanucci, V.; Pollio, A.; Fontanarosa, C.; Amoresano, A.; Zarrelli, A.; Olivieri, G.; Monti, D.M. A Cascade Extraction of Active Phycocyanin and Fatty Acids from Galdieria phlegrea. Appl. Microbiol. Biotechnol. 2019, 103, 9455–9464. [Google Scholar] [CrossRef] [PubMed]
- Chua, E.T.; Shekh, A.Y.; Eltanahy, E.; Thomas-Hall, S.R.; Schenk, P.M. Effective Harvesting of Nannochloropsis Microalgae Using Mushroom Chitosan: A Pilot-Scale Study. Front. Bioeng. Biotechnol. 2020, 8, 771. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Molnár, Z.; Stirk, W.A.; Ördög, V.; Van Staden, J. Changes in Phytochemical Content and Pharmacological Activities of Three Chlorella Strains Grown in Different Nitrogen Conditions. J. Appl. Phycol. 2016, 28, 149–159. [Google Scholar] [CrossRef]
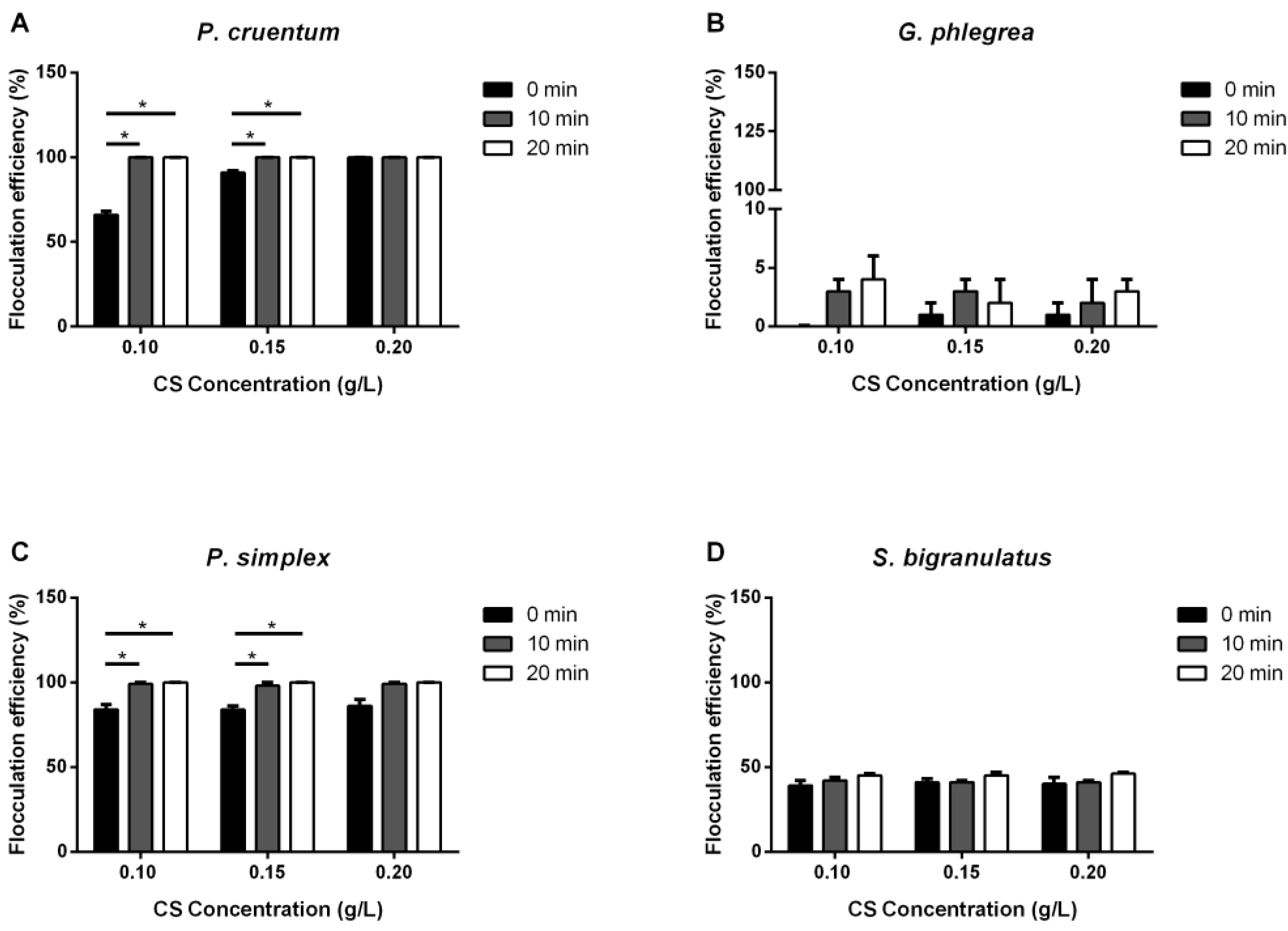
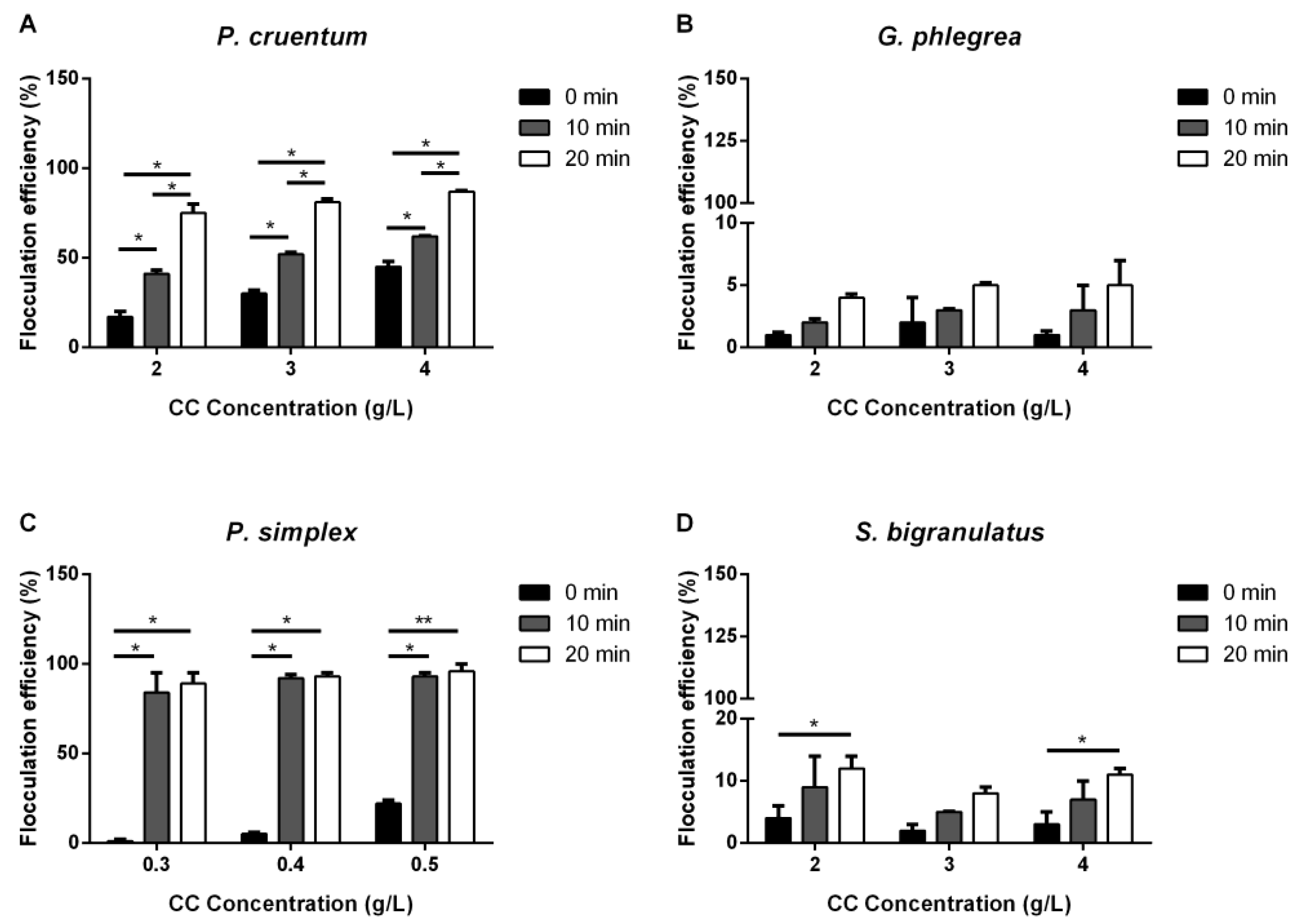
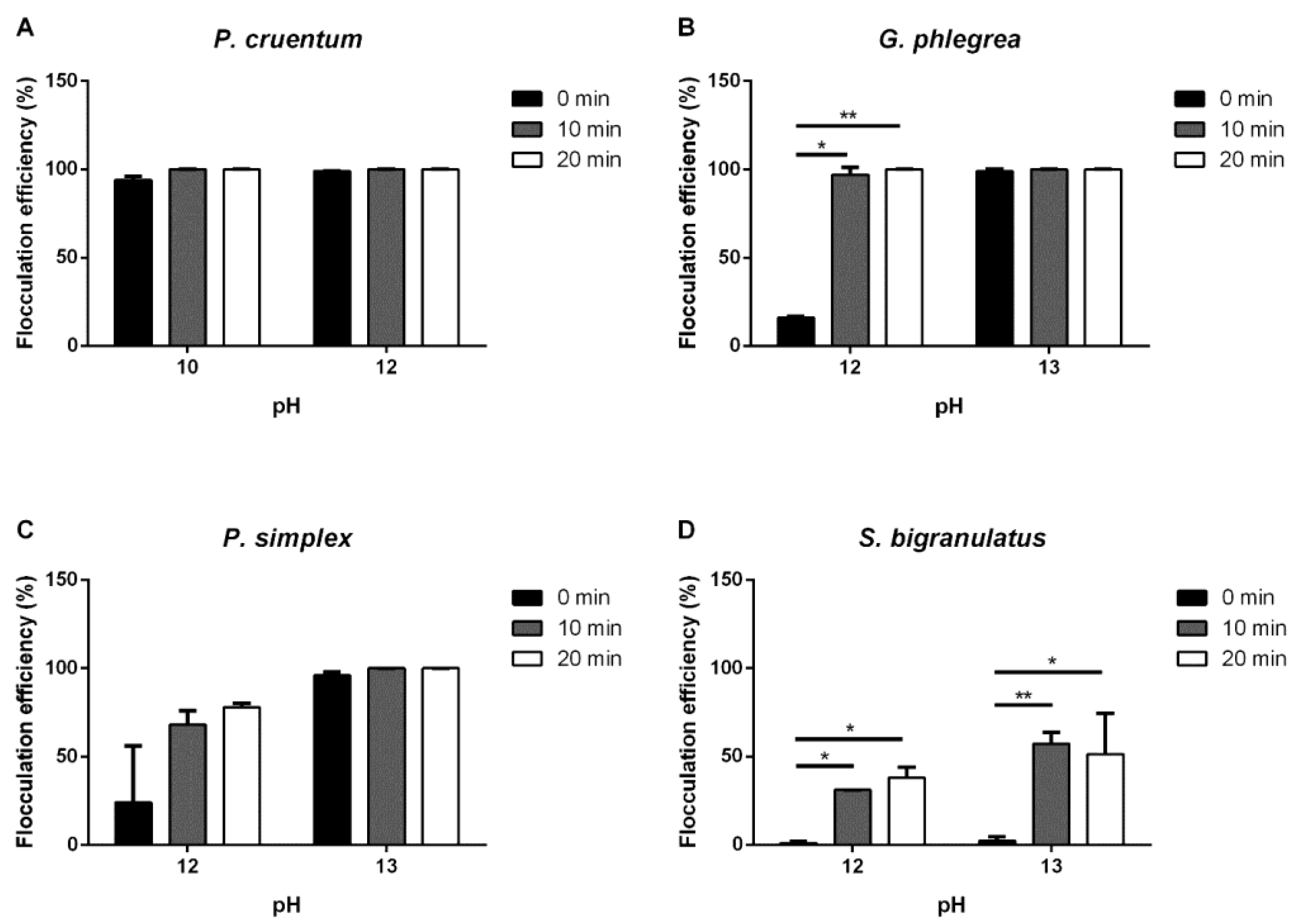
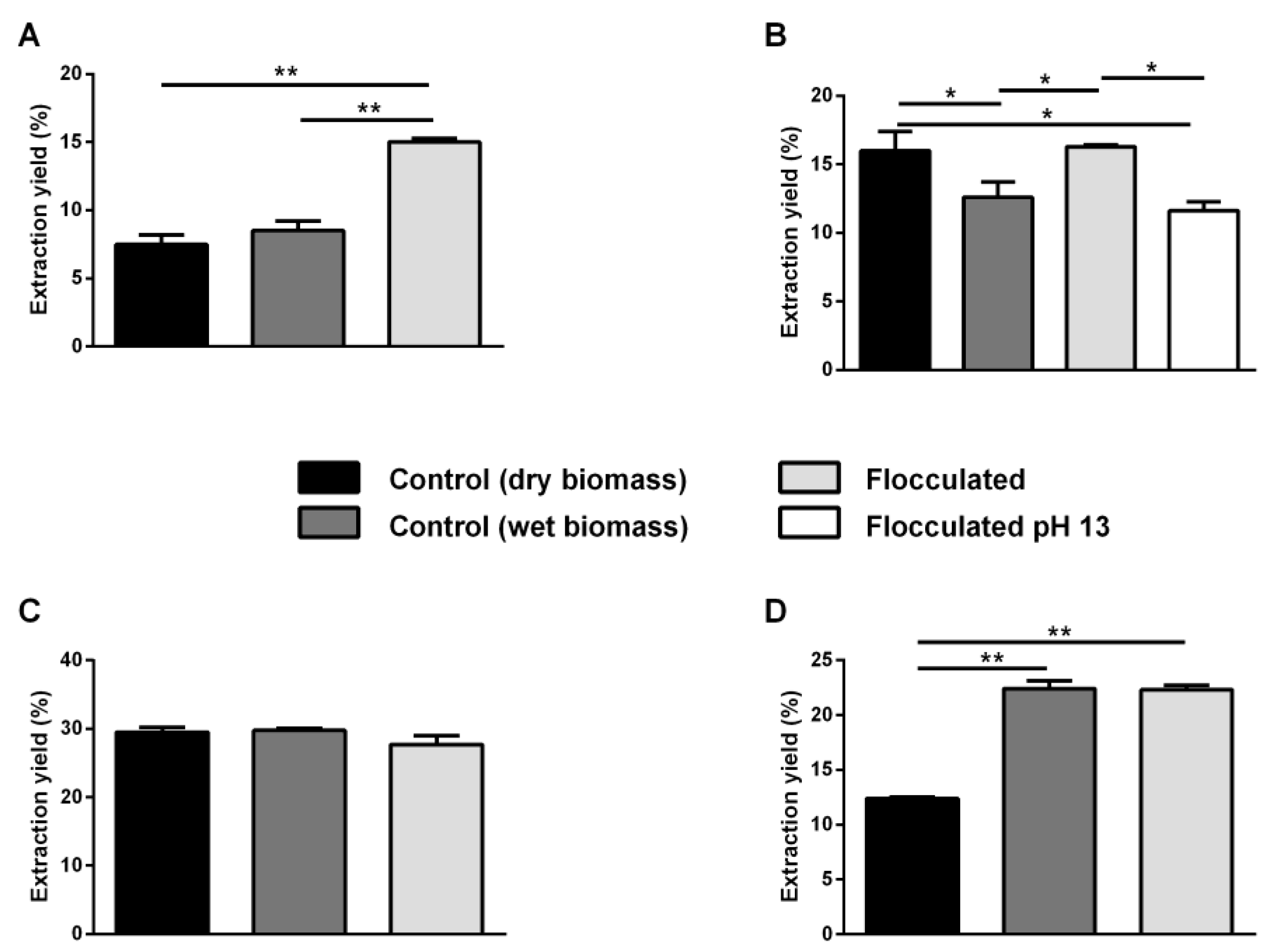
| Strain | Flocculation Condition | Flocculation Efficiency 20 Min |
|---|---|---|
| P. cruentum | 4.0 g/L CC | 87.0 ± 0.5 |
| G. phlegrea | pH 12 pH 13 | 100.0 ± 0.1 100.0 ± 0.1 |
| P. simplex | 0.5 g/L CC | 96 ± 4 |
| S. bigranulatus | 0.2 g/L CS | 46 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imbimbo, P.; Ferrara, A.; Giustino, E.; Liberti, D.; Monti, D.M. Microalgae Flocculation: Assessment of Extraction Yields and Biological Activity. Int. J. Mol. Sci. 2024, 25, 10238. https://doi.org/10.3390/ijms251910238
Imbimbo P, Ferrara A, Giustino E, Liberti D, Monti DM. Microalgae Flocculation: Assessment of Extraction Yields and Biological Activity. International Journal of Molecular Sciences. 2024; 25(19):10238. https://doi.org/10.3390/ijms251910238
Chicago/Turabian StyleImbimbo, Paola, Alfonso Ferrara, Enrica Giustino, Davide Liberti, and Daria Maria Monti. 2024. "Microalgae Flocculation: Assessment of Extraction Yields and Biological Activity" International Journal of Molecular Sciences 25, no. 19: 10238. https://doi.org/10.3390/ijms251910238








