X-ray Fluorescence Microscopy to Develop Elemental Classifiers and Investigate Elemental Signatures in BALB/c Mouse Intestine a Week after Exposure to 8 Gy of Gamma Rays
Abstract
:1. Introduction
2. Results
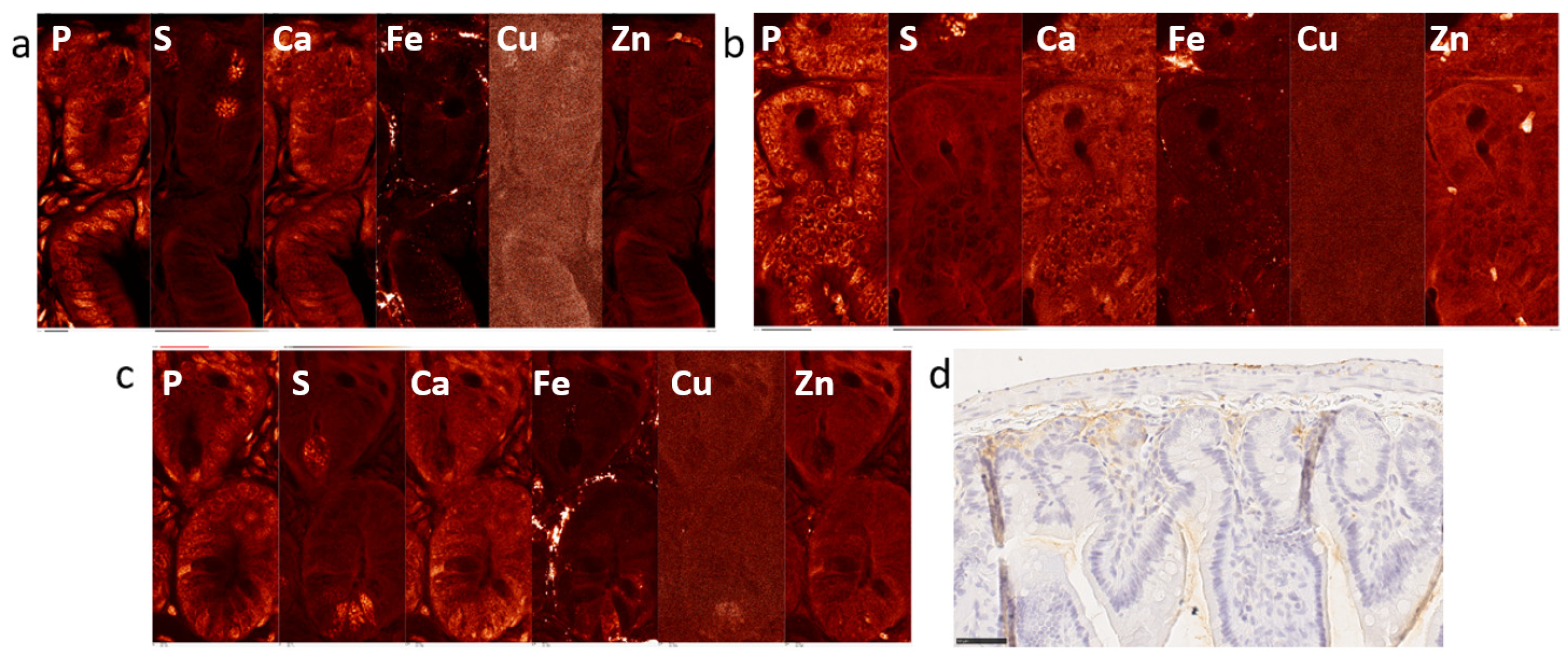
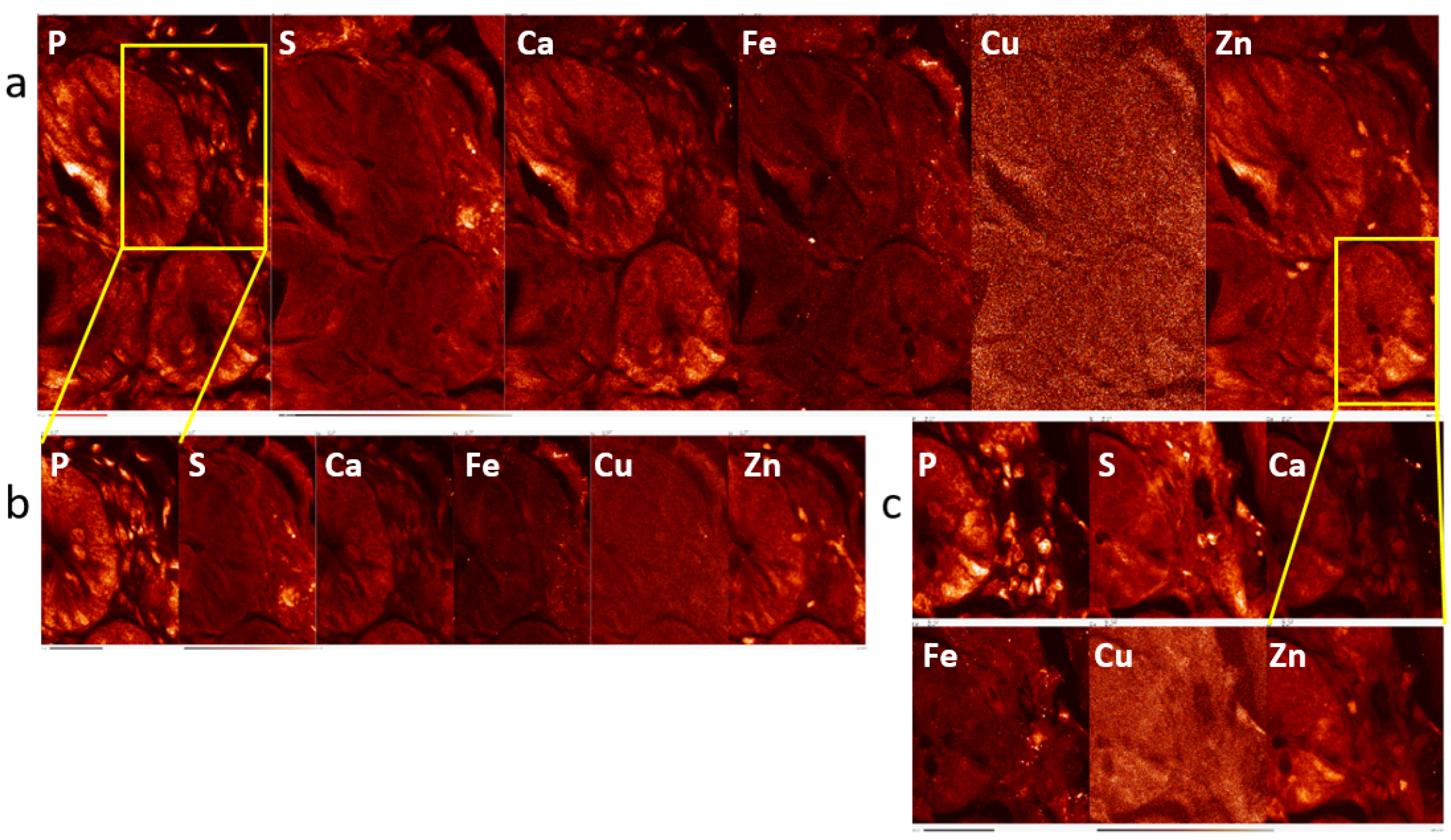
2.1. Elemental Maps of Control and Irradiated Mouse Intestine Samples
2.2. Statistical Analyses of ROI Data Extracted from XFM Images
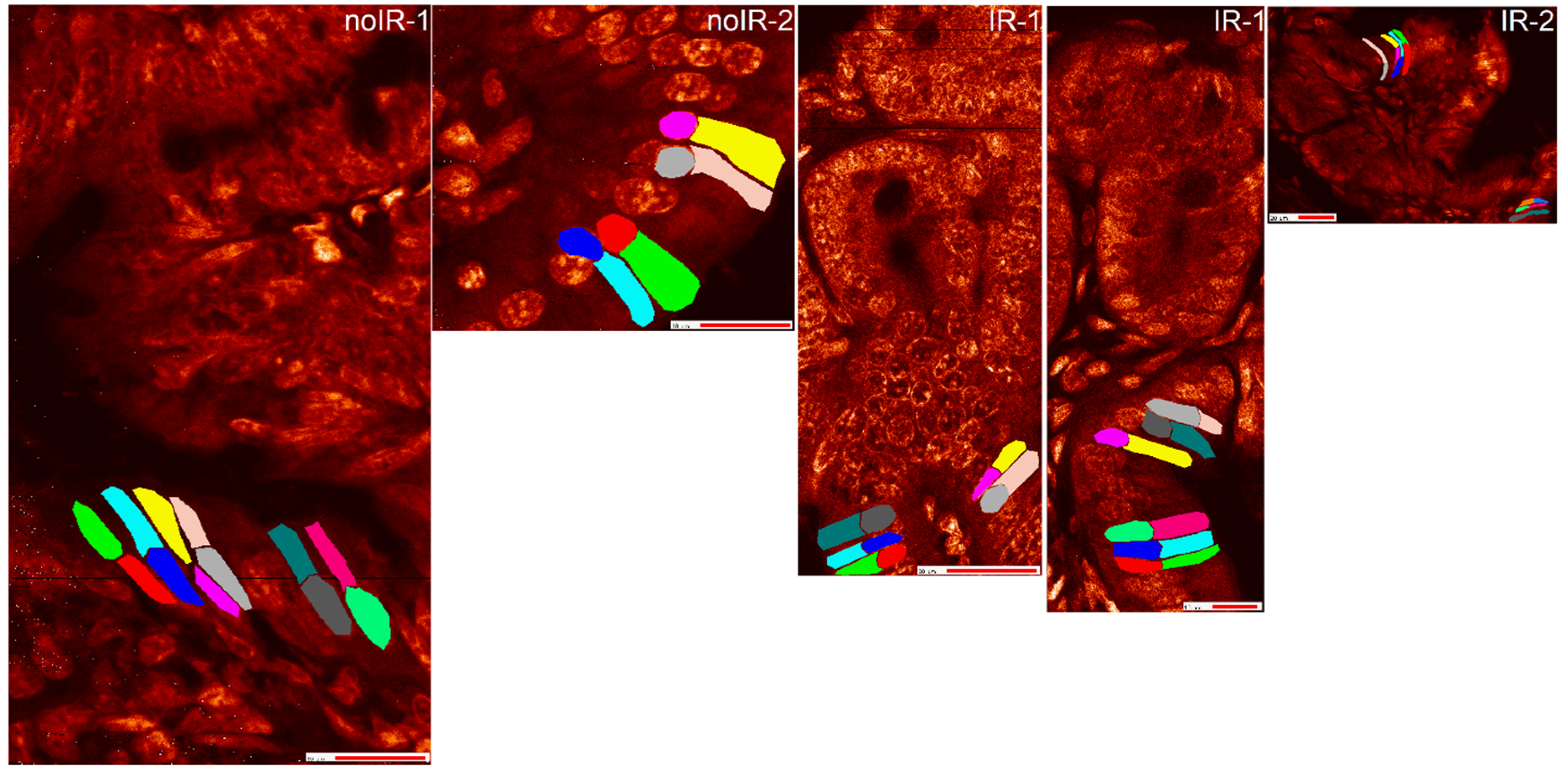
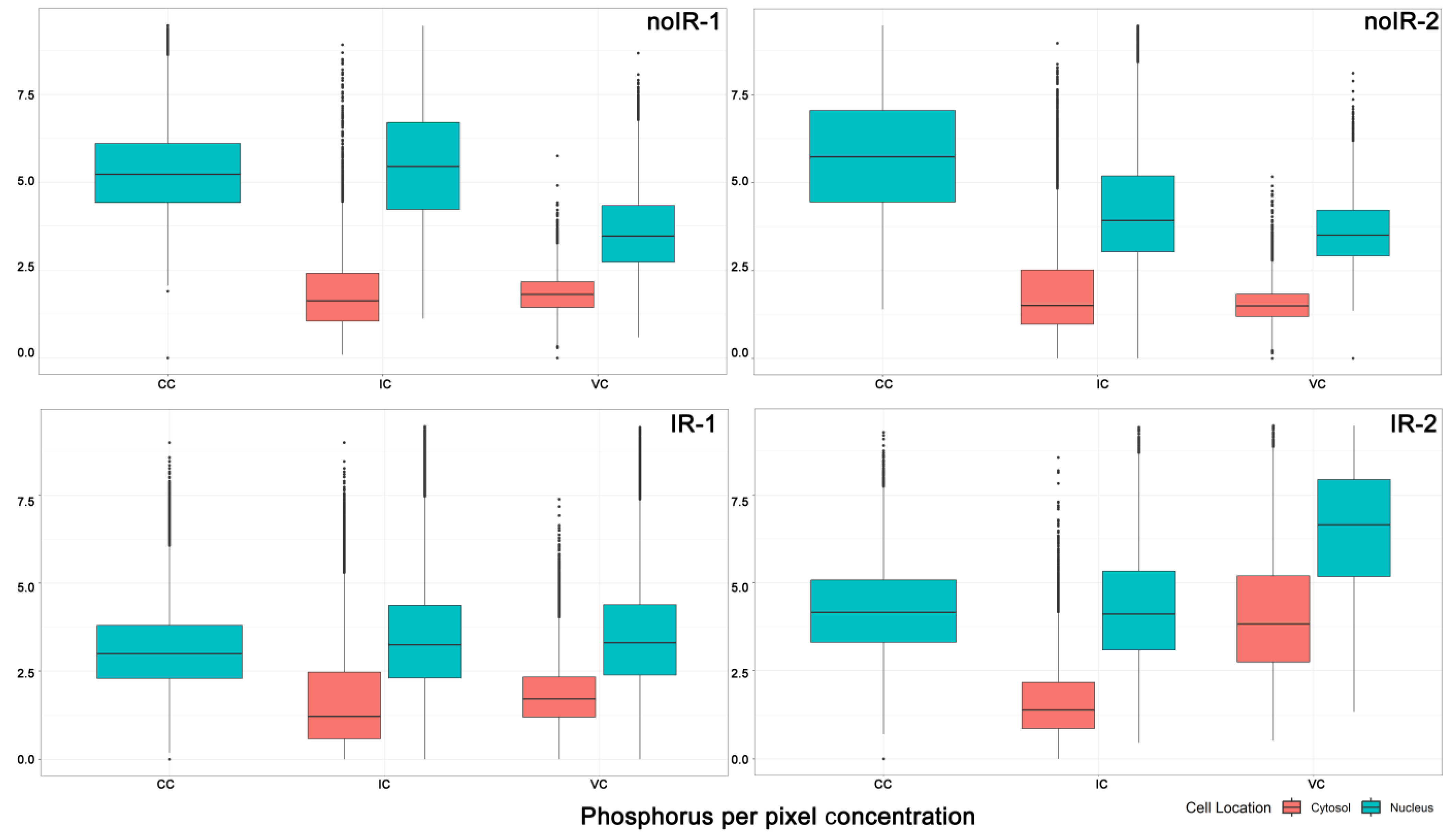
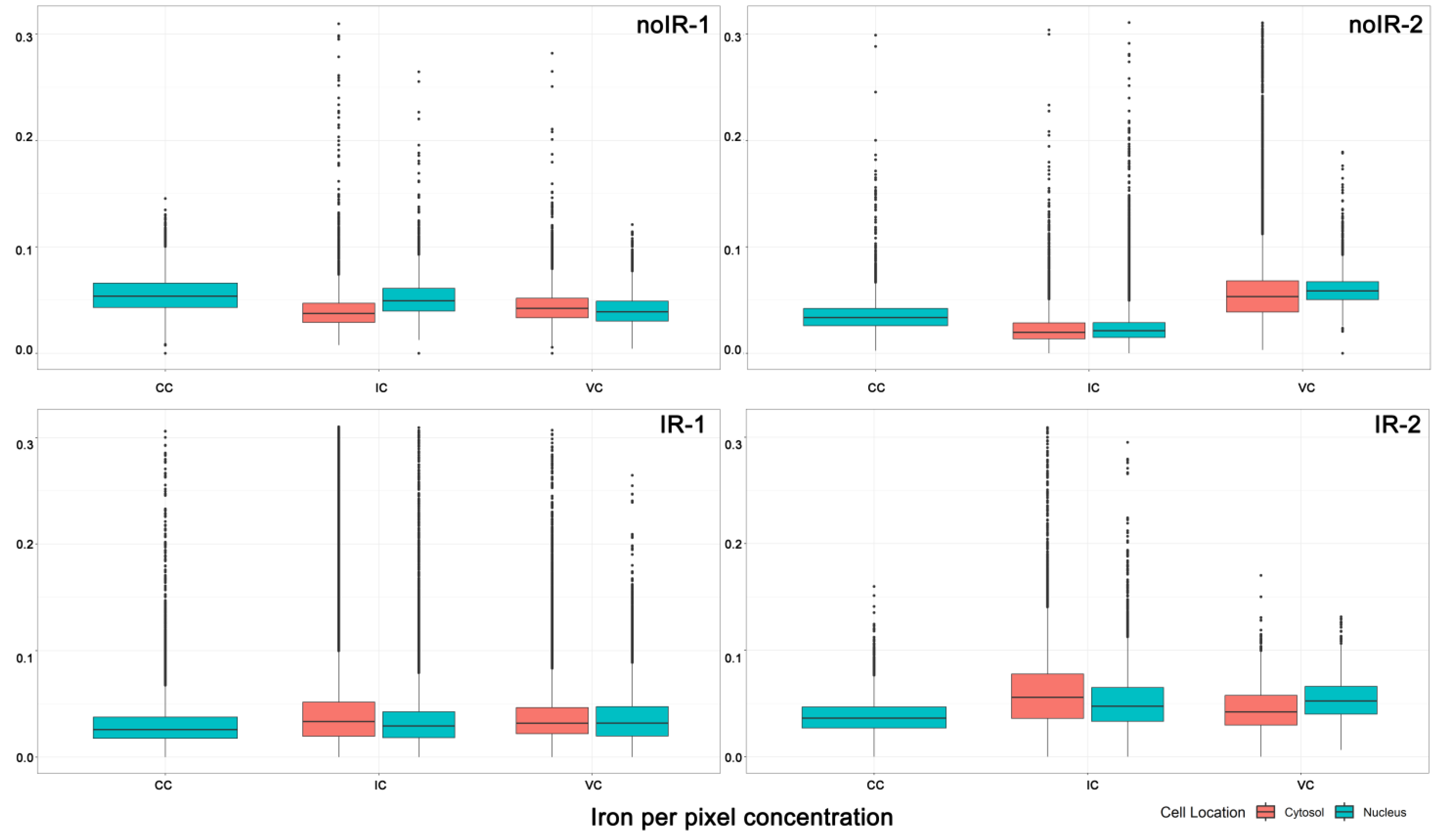
2.2.1. Mean Pixel Concentration Analysis—Cell Type Analyses
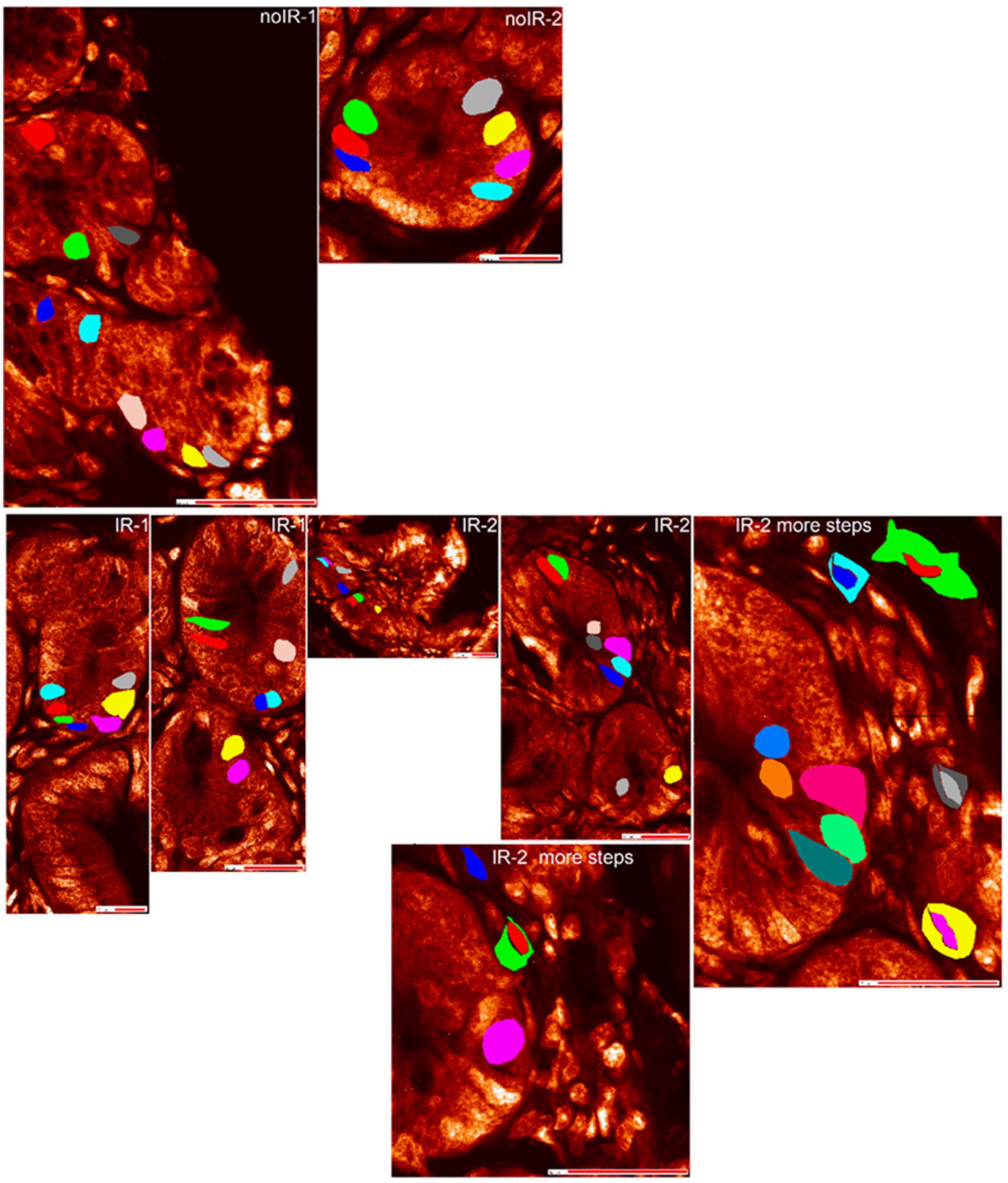
2.2.2. Mean Pixel Concentration Analysis—Individual Cell Analyses
2.2.3. ANOVA Evaluation of Cell-to-Cell Elemental Differences
2.2.4. T-Test Evaluation of Differences between Different Scans of the Same Cells
2.2.5. Tissue Region Analyses
3. Discussion
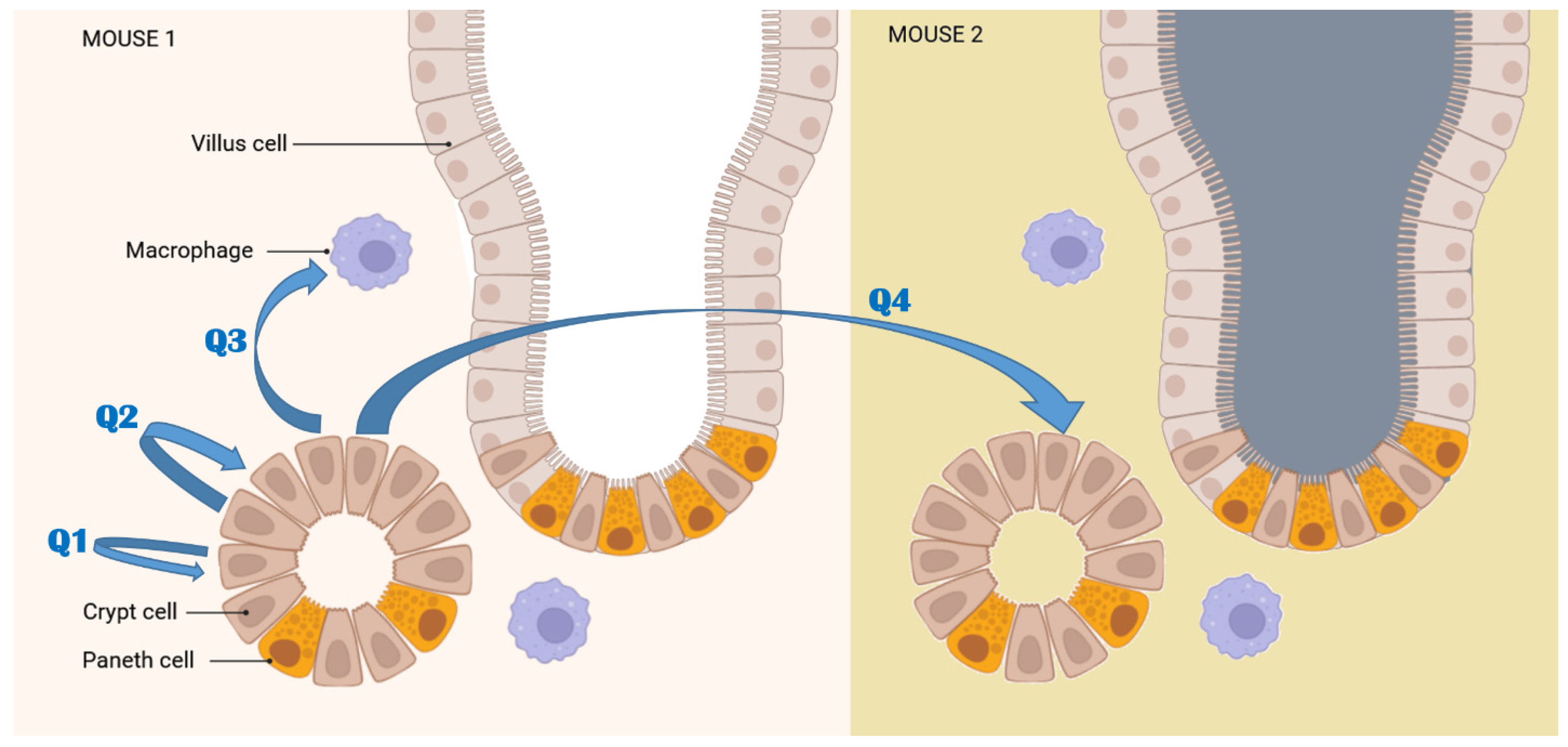
4. Materials and Methods
Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Booth, C.; Tudor, G.; Tudor, J.; Katz, B.P.; MacVittie, T.J. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012, 103, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, A.; Fu, Q.; Shi, Z.; Chen, X.; Wang, Y.; Xu, W.; Wang, T.; Zhang, S.; Hu, S. Ferroptosis plays an important role in promoting ionizing radiation-induced intestinal injuries. Biochem. Biophys. Res. Commun. 2022, 595, 7–13. [Google Scholar] [CrossRef]
- Mirzoeva, S.; Paunesku, T.; Wanzer, M.B.; Shirvan, A.; Kaempfer, R.; Woloschak, G.E.; Small, W., Jr. Single administration of p2TA (AB103), a CD28 antagonist peptide, prevents inflammatory and thrombotic reactions and protects against gastrointestinal injury in total-body irradiated mice. PLoS ONE 2014, 9, e101161. [Google Scholar] [CrossRef]
- Horseman, T.; Rittase, W.B.; Slaven, J.E.; Bradfield, D.T.; Frank, A.M.; Anderson, J.A.; Hays, E.C.; Ott, A.C.; Thomas, A.E.; Huppmann, A.R.; et al. Ferroptosis, Inflammation, and Microbiome Alterations in the Intestine in the Göttingen Minipig Model of Hematopoietic-Acute Radiation Syndrome. Int. J. Mol. Sci. 2024, 25, 4535. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, T.; Huang, H.-C.; Zhao, Y.-Y.; He, M.; Yuan, W.; Li, L.; Li, J.; Wu, D.-M.; Xu, Y. Activation of pyroptosis and ferroptosis is involved in radiation-induced intestinal injury in mice. Biochem. Biophys. Res. Commun. 2022, 631, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Rittase, W.B.; Slaven, J.E.; Suzuki, Y.J.; Muir, J.M.; Lee, S.-H.; Rusnak, M.; Brehm, G.V.; Bradfield, D.T.; Symes, A.J.; Day, R.M. Iron Deposition and Ferroptosis in the Spleen in a Murine Model of Acute Radiation Syndrome. Int. J. Mol. Sci. 2022, 23, 11029. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M.; Rittase, W.B.; Slaven, J.E.; Lee, S.-H.; Brehm, G.V.; Bradfield, D.T.; Muir, J.M.; Wise, S.Y.; Fatanmi, O.O.; Singh, V.K. Iron Deposition in the Bone Marrow and Spleen of Nonhuman Primates with Acute Radiation Syndrome. Radiat. Res. 2023, 200, 593–600. [Google Scholar] [CrossRef]
- Recalcati, S.; Locati, M.; Gammella, E.; Invernizzi, P.; Cairo, G. Iron levels in polarized macrophages: Regulation of immunity and autoimmunity. Autoimmun. Rev. 2012, 11, 883–889. [Google Scholar] [CrossRef]
- Glasco, A.D.; Snyder, L.A.; Paunesku, T.; Howard, S.C.; Hooper, D.A.; Golden, A.P.; Woloschak, G.E. Revisiting the Historic Strontium-90 Ingestion Beagle Study Conducted at the University of California-Davis: Opportunity in Archival Materials. Radiat. Res. 2024, 202, 289–308. [Google Scholar] [CrossRef]
- Zander, A.; Paunesku, T.; Woloschak, G. Radiation databases and archives—Examples and comparisons. Int. J. Radiat. Biol. 2019, 95, 1378–1389. [Google Scholar] [CrossRef]
- Zander, A.; Paunesku, T.; Woloschak, G.E. Analyses of cancer incidence and other morbidities in gamma irradiated B6CF1 mice. PLoS ONE 2020, 15, e0231510. [Google Scholar] [CrossRef] [PubMed]
- Zander, A.; Paunesku, T.; Woloschak, G.E. Analyses of cancer incidence and other morbidities in neutron irradiated B6CF1 mice. PLoS ONE 2021, 16, e0231511. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Daniel, A.R.; Driver, L.M.; Lee, C.L.; Kirsch, D.G. Histological assessment of intestinal injury by ionizing radiation. Methods Cell Biol. 2023, 180, 147–175. [Google Scholar] [PubMed]
- Vlachaki, E.; Venou, T.M. Iron overload: The achilles heel of β-thalassemia. Transfus. Clin. Biol. 2024, 31, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Gucký, A.; Hamuľaková, S. Targeting Biometals in Alzheimer’s Disease with Metal Chelating Agents Including Coumarin Derivatives. CNS Drugs 2024, 38, 507–532. [Google Scholar] [CrossRef]
- Pushie, M.J.; Sylvain, N.J.; Hou, H.; Hackett, M.J.; E Kelly, M.; Webb, S.M. X-ray fluorescence microscopy methods for biological tissues. Metallomics 2022, 14, mfac032. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Y.; Wang, L.; Zhang, K.; Guan, Y.; Guo, Y.; Chen, C. In situ label-free X-ray imaging for visualizing the localization of nanomedicines and subcellular architecture in intact single cells. Nat. Protoc. 2024, 19, 30–59. [Google Scholar] [CrossRef]
- Chen, S.; Deng, J.; Yuan, Y.; Flachenecker, C.; Mak, R.; Hornberger, B.; Jin, Q.; Shu, D.; Lai, B.; Maser, J.; et al. The Bionanoprobe: Hard X-ray fluorescence nanoprobe with cryogenic capabilities. J. Synchrotron Radiat. 2014, 21 Pt 1, 66–75. [Google Scholar] [CrossRef]
- Chen, S.; Paunesku, T.; Yuan, Y.; Jin, Q.; Finney, L.; Hornberger, B.; Flachenecker, C.; Lai, B.; Brister, K.; Jacobsen, C.; et al. The Bionanoprobe: Synchrotron-based Hard X-ray Fluorescence Microscopy for 2D/3D Trace Element Mapping. Micros. Today 2015, 23, 26–29. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, S.; Paunesku, T.; Gleber, S.C.; Liu, W.C.; Doty, C.B.; Mak, R.; Deng, J.; Jin, Q.; Lai, B.; et al. Epidermal growth factor receptor targeted nuclear delivery and high-resolution whole cell X-ray imaging of Fe3O4@TiO2 nanoparticles in cancer cells. ACS Nano 2013, 7, 10502–10517. [Google Scholar] [CrossRef]
- Copeland-Hardin, L.; Paunesku, T.; Murley, J.S.; Crentsil, J.; Antipova, O.; Li, L.; Maxey, E.; Jin, Q.; Hooper, D.; Lai, B.; et al. Proof of principle study: Synchrotron X-ray fluorescence microscopy for identification of previously radioactive microparticles and elemental mapping of FFPE tissues. Sci. Rep. 2023, 13, 7806. [Google Scholar] [CrossRef] [PubMed]
- Paunesku, T.; Gordon, A.C.; White, S.; Harris, K.; Antipova, O.; Maxey, E.; Vogt, S.; Smith, A.; Daddario, L.; Procissi, D.; et al. Use of X-Ray Fluorescence Microscopy for Studies on Research Models of Hepatocellular Carcinoma. Front. Public Health. 2021, 9, 711506. [Google Scholar] [CrossRef] [PubMed]
- Refaat, T.; West, D.; El Achy, S.; Parimi, V.; May, J.; Xin, L.; Harris, K.R.; Liu, W.; Wanzer, M.B.; Finney, L.; et al. Distribution of Iron Oxide Core-Titanium Dioxide Shell Nanoparticles in VX2 Tumor Bearing Rabbits Introduced by Two Different Delivery Modalities. Nanomaterials 2016, 6, 143. [Google Scholar] [CrossRef]
- Paunesku, T.; Rajh, T.; Wiederrecht, G.; Maser, J.; Vogt, S.; Stojićević, N.; Protić, M.; Lai, B.; Oryhon, J.; Thurnauer, M.; et al. Biology of TiO2-oligonucleotide nanocomposites. Nat. Mater. 2003, 2, 343–346. [Google Scholar] [CrossRef]
- Di, Z.W.; Chen, S.; Gursoy, D.; Paunesku, T.; Leyffer, S.; Wild, S.M.; Vogt, S. Optimization-based simultaneous alignment and reconstruction in multi-element tomography. Opt. Lett. 2019, 44, 4331–4334. [Google Scholar] [CrossRef] [PubMed]
- Poropatich, K.; Paunesku, T.; Zander, A.; Wray, B.; Schipma, M.; Dalal, P.; Agulnik, M.; Chen, S.; Lai, B.; Antipova, O.; et al. Elemental Zn and its Binding Protein Zinc-α2-Glycoprotein are Elevated in HPV-Positive Oropharyngeal Squamous Cell Carcinoma. Sci. Rep. 2019, 9, 16965. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef] [PubMed]
- Winters, T.A.; Marzella, L.; Molinar-Inglis, O.; Price, P.W.; Han, N.C.; Cohen, J.E.; Wang, S.-J.; Fotenos, A.F.; Sullivan, J.M.; Esker, J.I.; et al. Gastrointestinal Acute Radiation Syndrome: Mechanisms, Models, Markers, and Medical Countermeasures. Radiat. Res. 2024, 201, 628–646. [Google Scholar] [CrossRef]
- Costantini, I.; Veneranda, M.; Prieto-Taboada, N.; De Francesco, A.M.; Castro, K.; Madariaga, J.M.; Arana, G. Combined in situ XRF–LIBS analyses as a novel method to determine the provenance of central Mediterranean obsidians. Eur. Phys. J. Plus 2023, 138, 603. [Google Scholar] [CrossRef]
- Kreuzer, M.; Stamenković, S.; Chen, S.; Andjus, P.; Dučić, T. Lipids status and copper in a single astrocyte of the rat model for amyotrophic lateral sclerosis: Correlative synchrotron-based X-ray and infrared imaging. J. Biophotonics 2020, 13, e202000069. [Google Scholar] [CrossRef]
- Szczerbowska-Boruchowska, M.; Stec, P.; Czyzycki, M.; Szczerbowski, Z.; Simon, R.; Baumbach, T.; Ziomber-Lisiak, A. The applicability of Fourier transform infrared microspectroscopy for correction against matrix effects in X-ray fluorescence microimaging of tissues. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 293, 122468. [Google Scholar] [CrossRef] [PubMed]
- Suárez, V.T.; Gallet, B.; Chevallet, M.; Jouneau, P.H.; Tucoulou, R.; Veronesi, G.; Deniaud, A. Correlative transmission electron microscopy and high-resolution hard X-ray fluorescence microscopy of cell sections to measure trace element concentrations at the organelle level. J. Struct. Biol. 2021, 213, 107766. [Google Scholar]
- Sala, S.; Zhang, Y.; De La Rosa, N.; Dreier, T.; Kahnt, M.; Langer, M.; Dahlin, L.B.; Bech, M.; Villanueva-Perez, P.; Kalbfleisch, S. Dose-efficient multimodal microscopy of human tissue at a hard X-ray nanoprobe beamline. J. Synchrotron Radiat. 2022, 29 Pt 3, 807–815. [Google Scholar] [CrossRef]
- Abe, M.; Ishiguro, N.; Uematsu, H.; Takazawa, S.; Kaneko, F.; Takahashi, Y. X-ray ptychographic and fluorescence microscopy using virtual single-pixel imaging based deconvolution with accurate probe images. Opt. Express 2023, 31, 26027–26039. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, A.; Batey, D.; Cipiccia, S.; Shi, X.; Rau, C.; Botchway, S.; Yusuf, M.; Robinson, I.K. X-ray Ptychography Imaging of Human Chromosomes After Low-dose Irradiation. Chromosome Res. 2021, 29, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Kashiv, Y.; Austin, J.R., 2nd; Lai, B.; Rose, V.; Vogt, S.; El-Muayed, M. Imaging trace element distributions in single organelles and subcellular features. Sci. Rep. 2016, 6, 21437. [Google Scholar] [CrossRef]
- Ward, J.; Marvin, R.; O’Halloran, T.; Jacobsen, C.; Vogt, S. Rapid and accurate analysis of an X-ray fluorescence microscopy data set through gaussian mixture-based soft clustering methods. Microsc. Microanal. 2013, 5, 1281–1289. [Google Scholar] [CrossRef]
- Wang, S.; Ward, J.; Leyffer, S.; Wild, S.; Jacobsen, C.; Vogt, S. Unsupervised cell identification on multidimensional X-ray fluorescence datasets. J. Synchrotron Radiat. 2014, 21 Pt 3, 568–579. [Google Scholar] [CrossRef]
- Chowdhury, M.A.Z.; Ok, K.; Luo, Y.; Liu, Z.; Chen, S.; O’Halloran, T.V.; Kettimuthu, R.; Tekawade, A. ROI-Finder: Machine learning to guide region-of-interest scanning for X-ray fluorescence microscopy. J. Synchrotron Radiat. 2022, 29 Pt 6, 1495–1503. [Google Scholar] [CrossRef]
- Chen, S.; Lastra, R.O.; Paunesku, T.; Antipova, O.; Li, L.; Deng, J.; Luo, Y.; Wanzer, M.B.; Popovic, J.; Li, Y.; et al. Development of Multi-Scale X-ray Fluorescence Tomography for Examination of Nanocomposite-Treated Biological Samples. Cancers 2021, 13, 4497. [Google Scholar] [CrossRef]
- Storer, J.B. Acute Response to Ionizing Radiation. In Biology of the Laboratory Mouse, 2nd ed.; Dover Publications Inc.: New York, NY, USA, 1966. [Google Scholar]
- VanderWeele, T.J. On the distinction between interaction and effect modification. Epidemiology 2009, 20, 863–871. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, A.; Dobinda, K.; Chen, S.; Zieba, P.; Paunesku, T.; Sun, Z.; Woloschak, G.E. X-ray Fluorescence Microscopy to Develop Elemental Classifiers and Investigate Elemental Signatures in BALB/c Mouse Intestine a Week after Exposure to 8 Gy of Gamma Rays. Int. J. Mol. Sci. 2024, 25, 10256. https://doi.org/10.3390/ijms251910256
Smith A, Dobinda K, Chen S, Zieba P, Paunesku T, Sun Z, Woloschak GE. X-ray Fluorescence Microscopy to Develop Elemental Classifiers and Investigate Elemental Signatures in BALB/c Mouse Intestine a Week after Exposure to 8 Gy of Gamma Rays. International Journal of Molecular Sciences. 2024; 25(19):10256. https://doi.org/10.3390/ijms251910256
Chicago/Turabian StyleSmith, Anthony, Katrina Dobinda, Si Chen, Peter Zieba, Tatjana Paunesku, Zequn Sun, and Gayle E. Woloschak. 2024. "X-ray Fluorescence Microscopy to Develop Elemental Classifiers and Investigate Elemental Signatures in BALB/c Mouse Intestine a Week after Exposure to 8 Gy of Gamma Rays" International Journal of Molecular Sciences 25, no. 19: 10256. https://doi.org/10.3390/ijms251910256






