Molecular Characteristics and Expression Patterns of Carotenoid Cleavage Oxygenase Family Genes in Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Results and Analysis
2.1. Family Identification, Physicochemical Properties, and Subcellular Localization Prediction
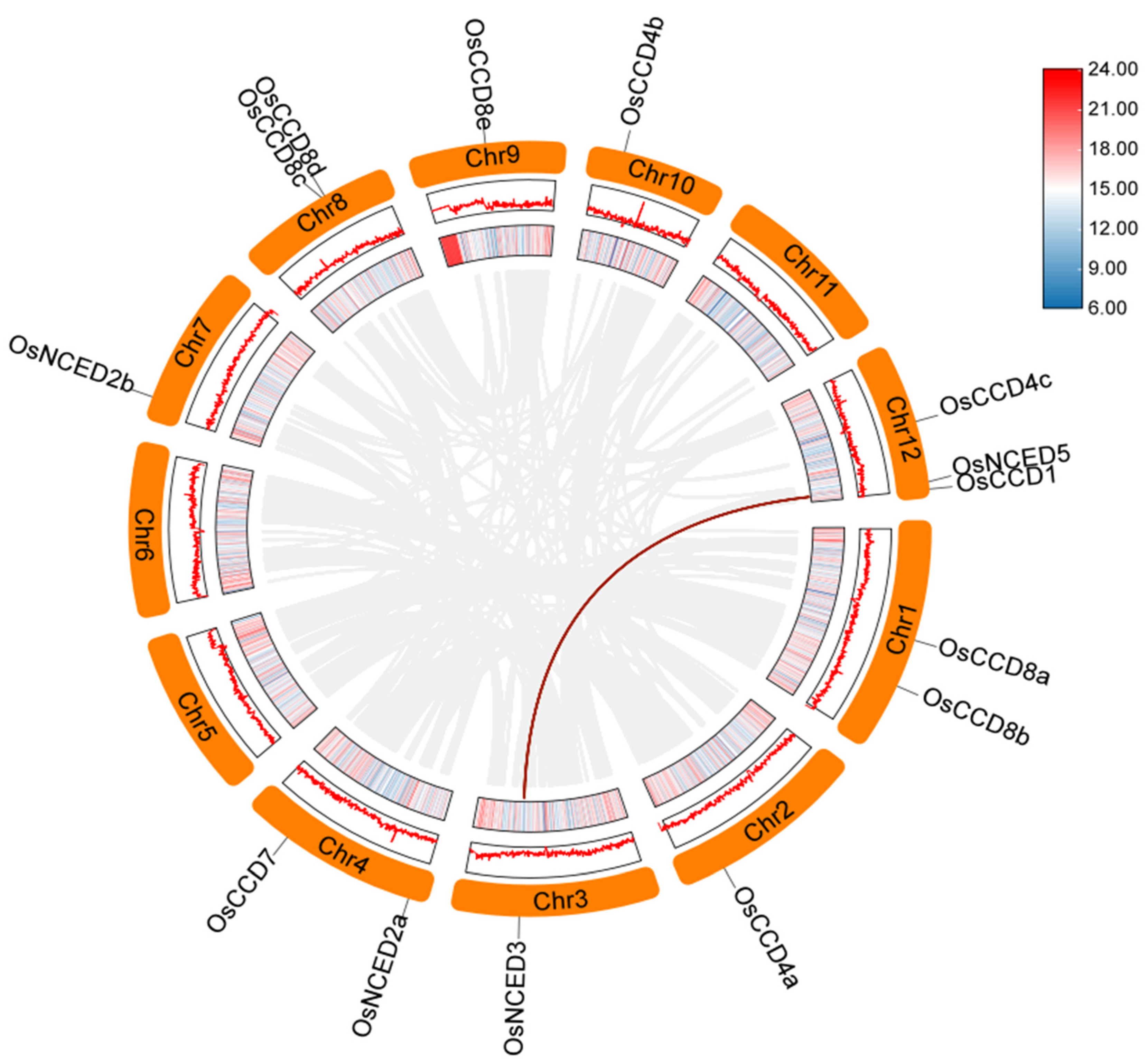
2.2. Chromosomal Localization of the OsCCO Family
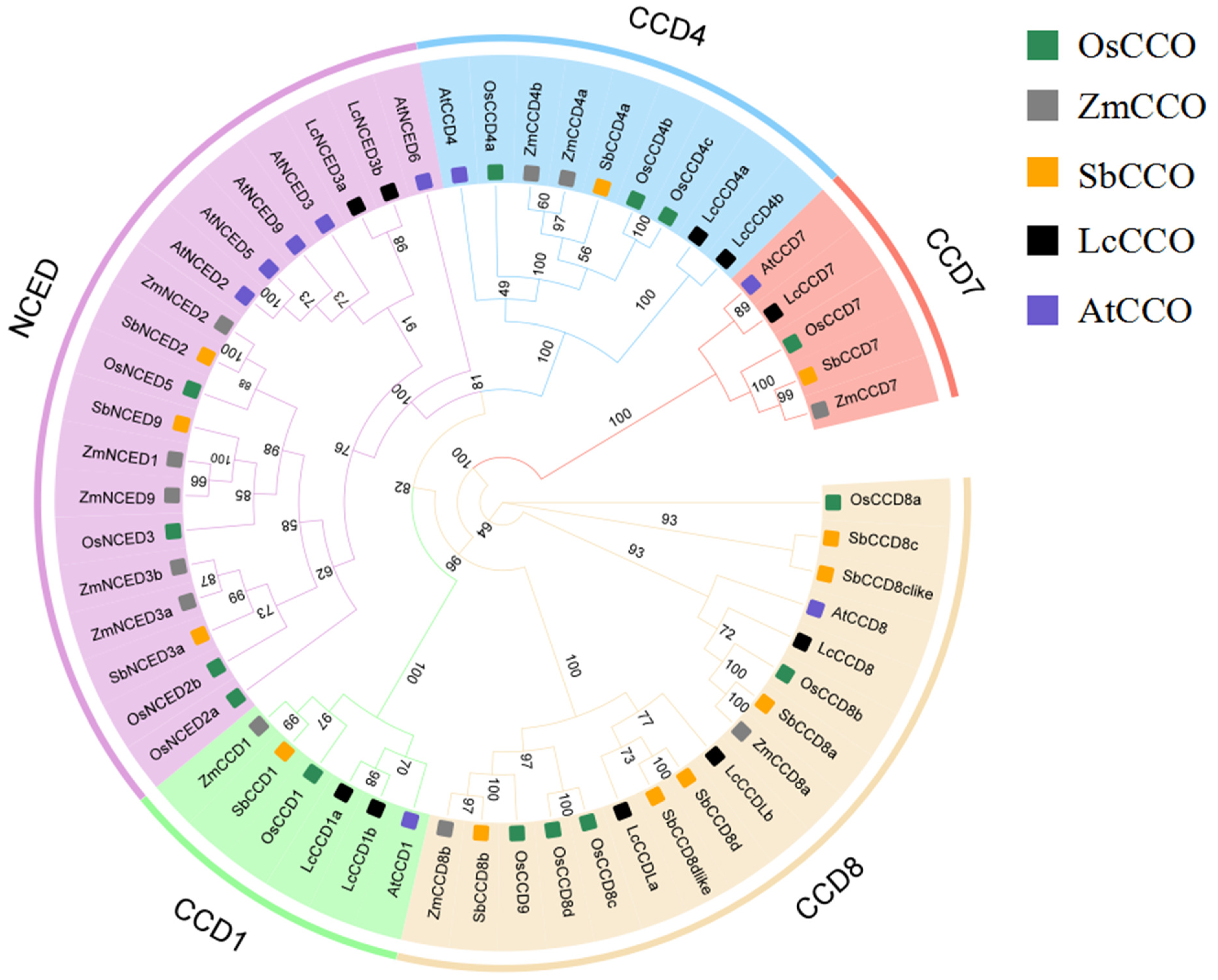
2.3. Phylogenetic Analysis of the OsCCO Family Proteins
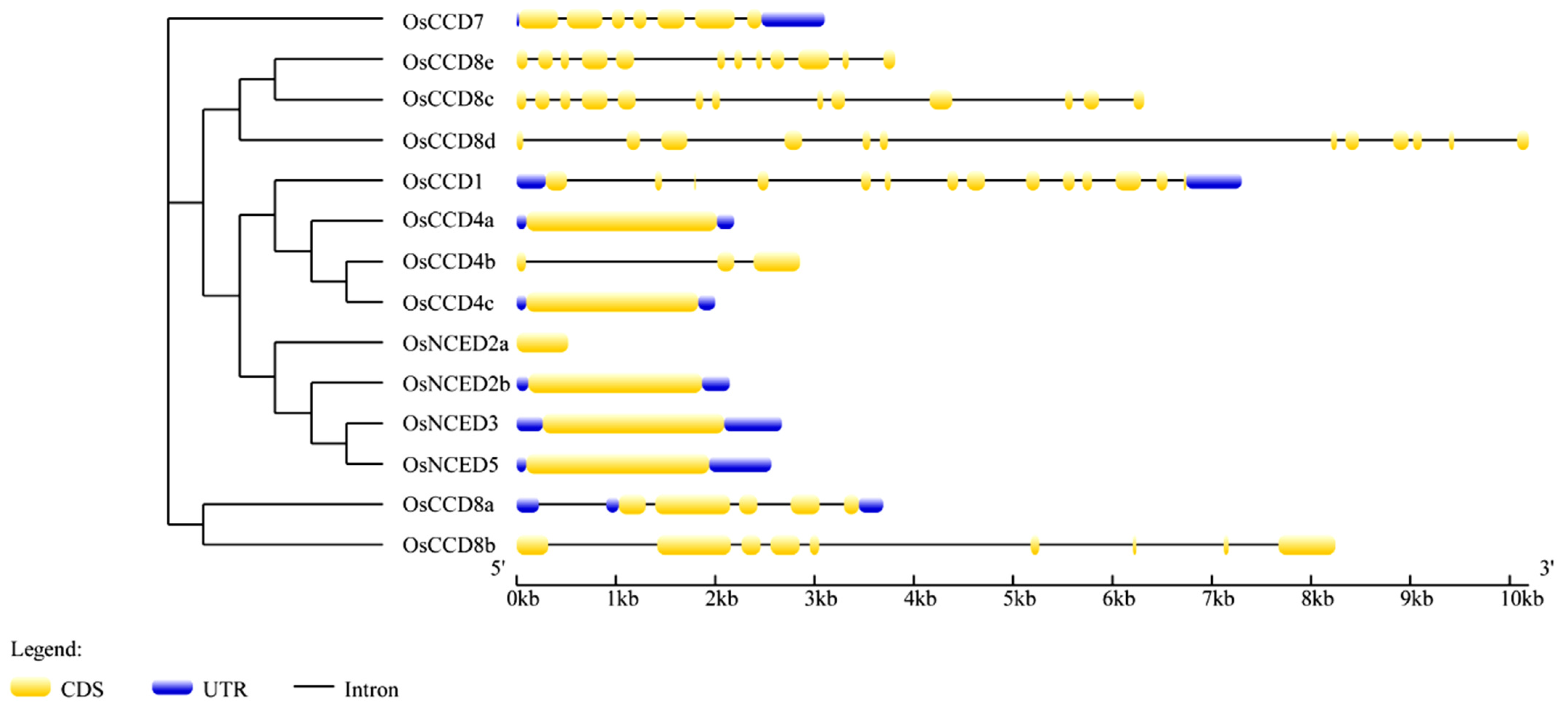
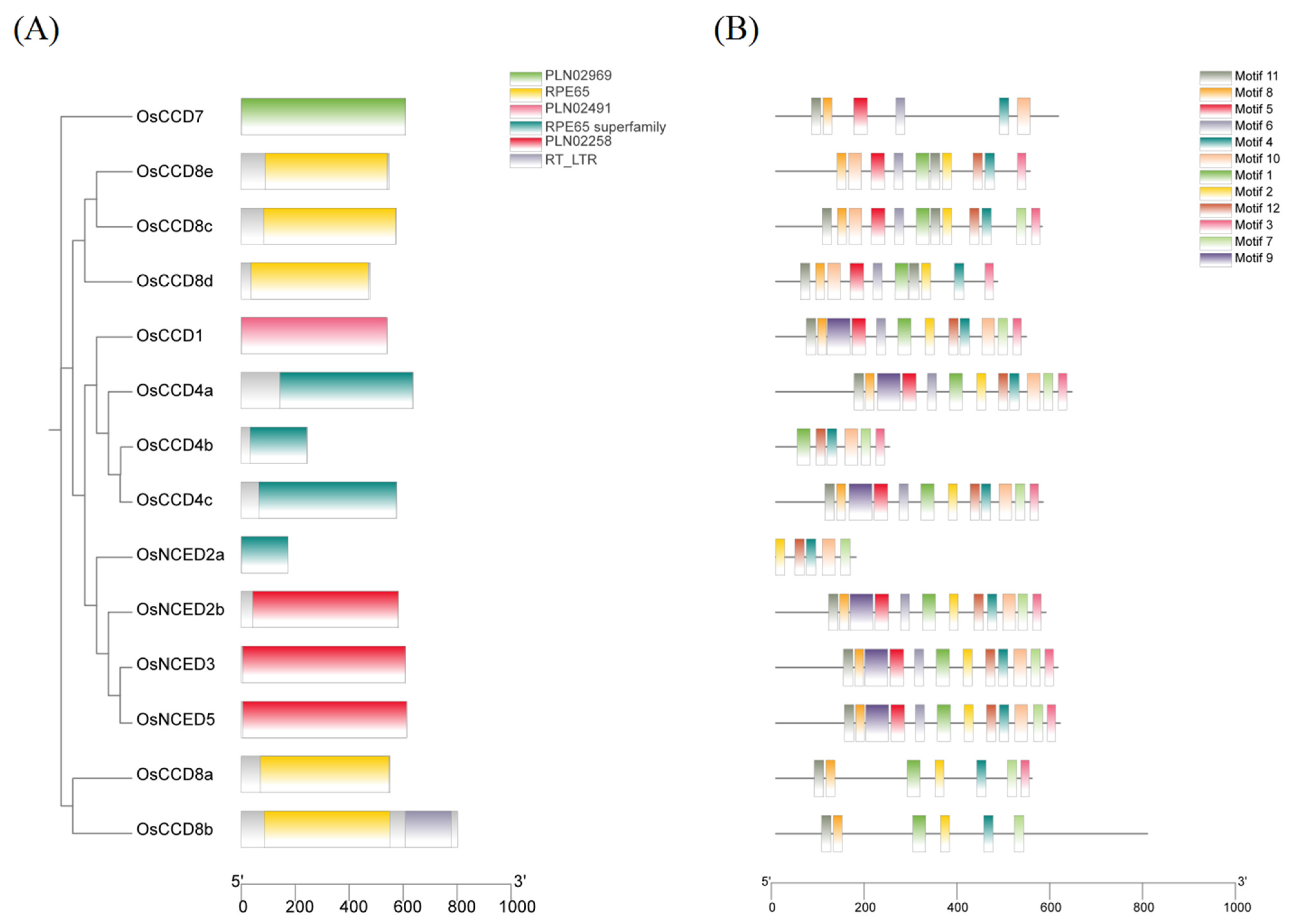
2.4. Gene Structures, Functional Domains, and Conserved Motifs of the OsCCO Family
2.5. Cis-Acting Element Analysis of the OsCCO Family Gene Promoters
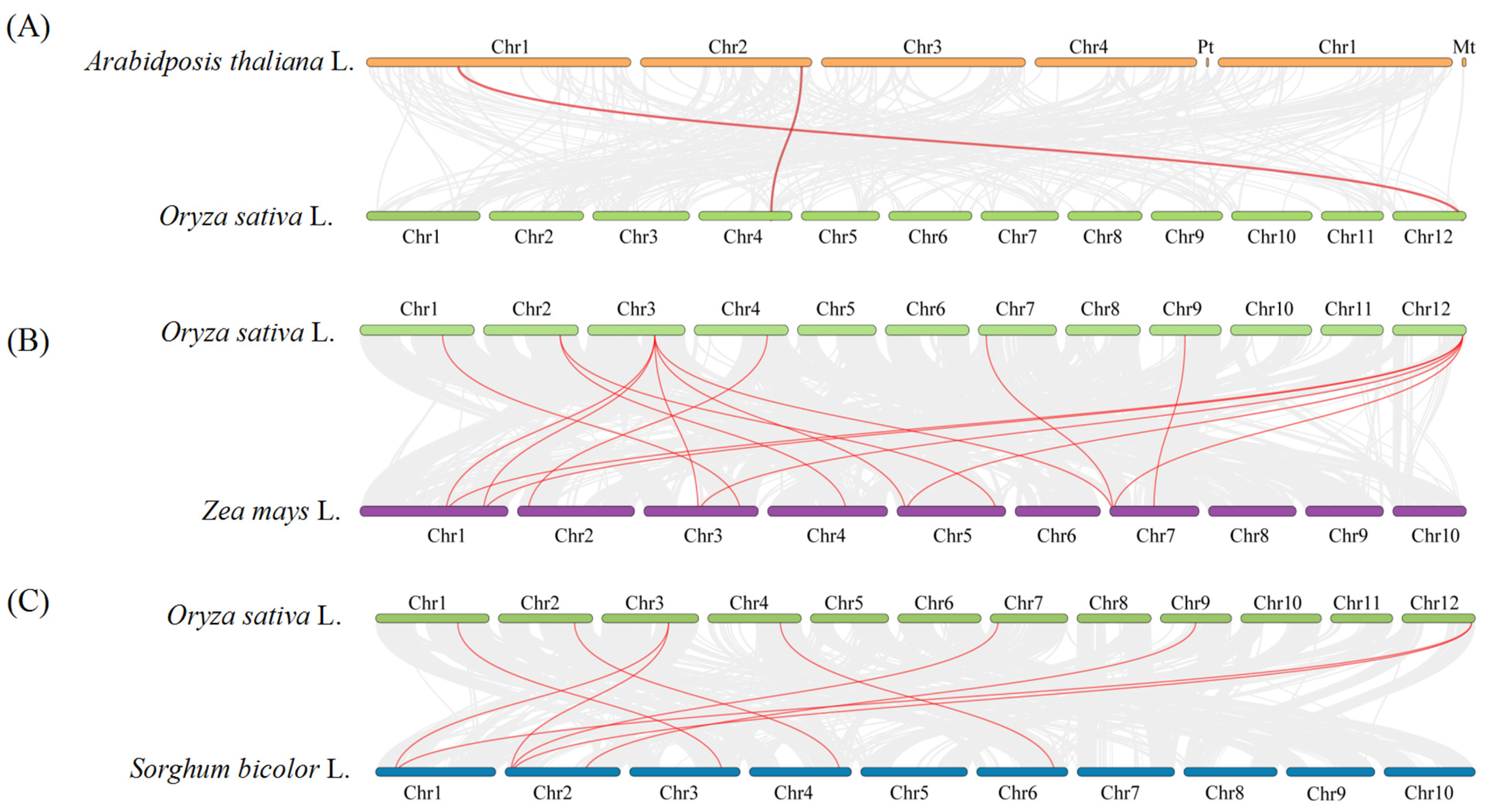
2.6. Collinearity Analysis within the OsCCO Family and Homologous Evolutionary Associations among Different Species
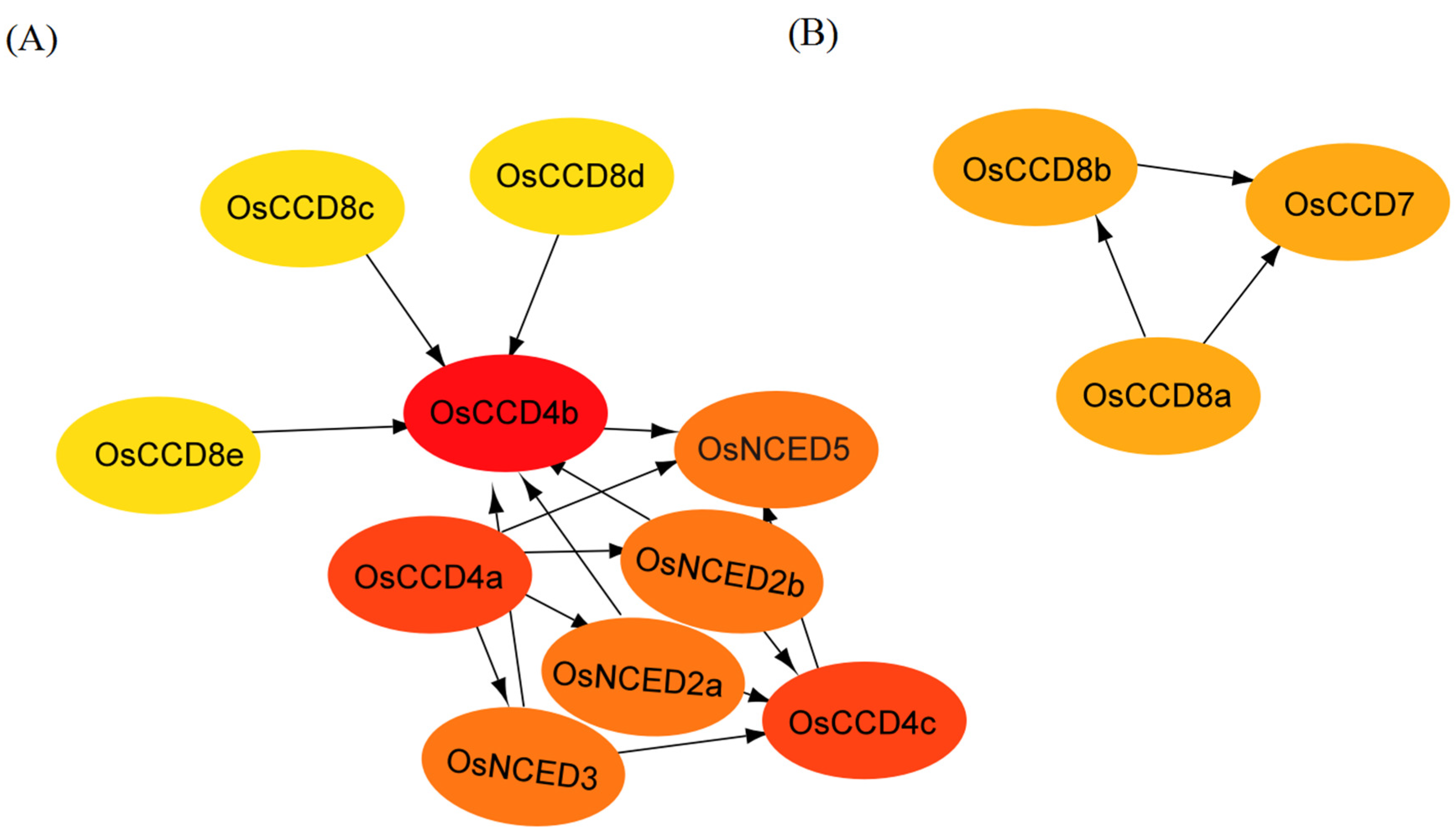
2.7. Protein Interaction Analysis of the OsCCO Family
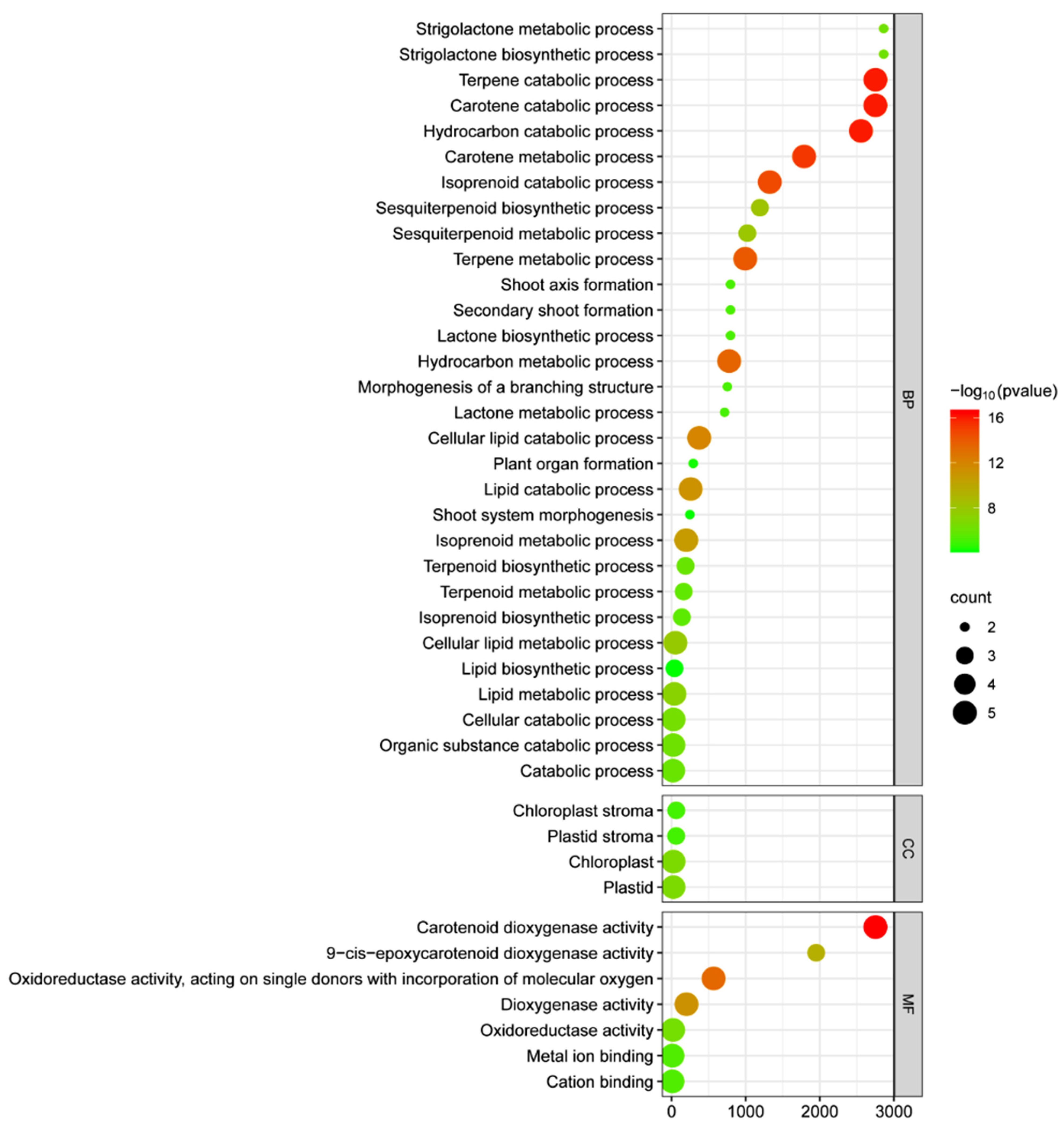
2.8. GO Annotation Analysis of the OsCCO Family
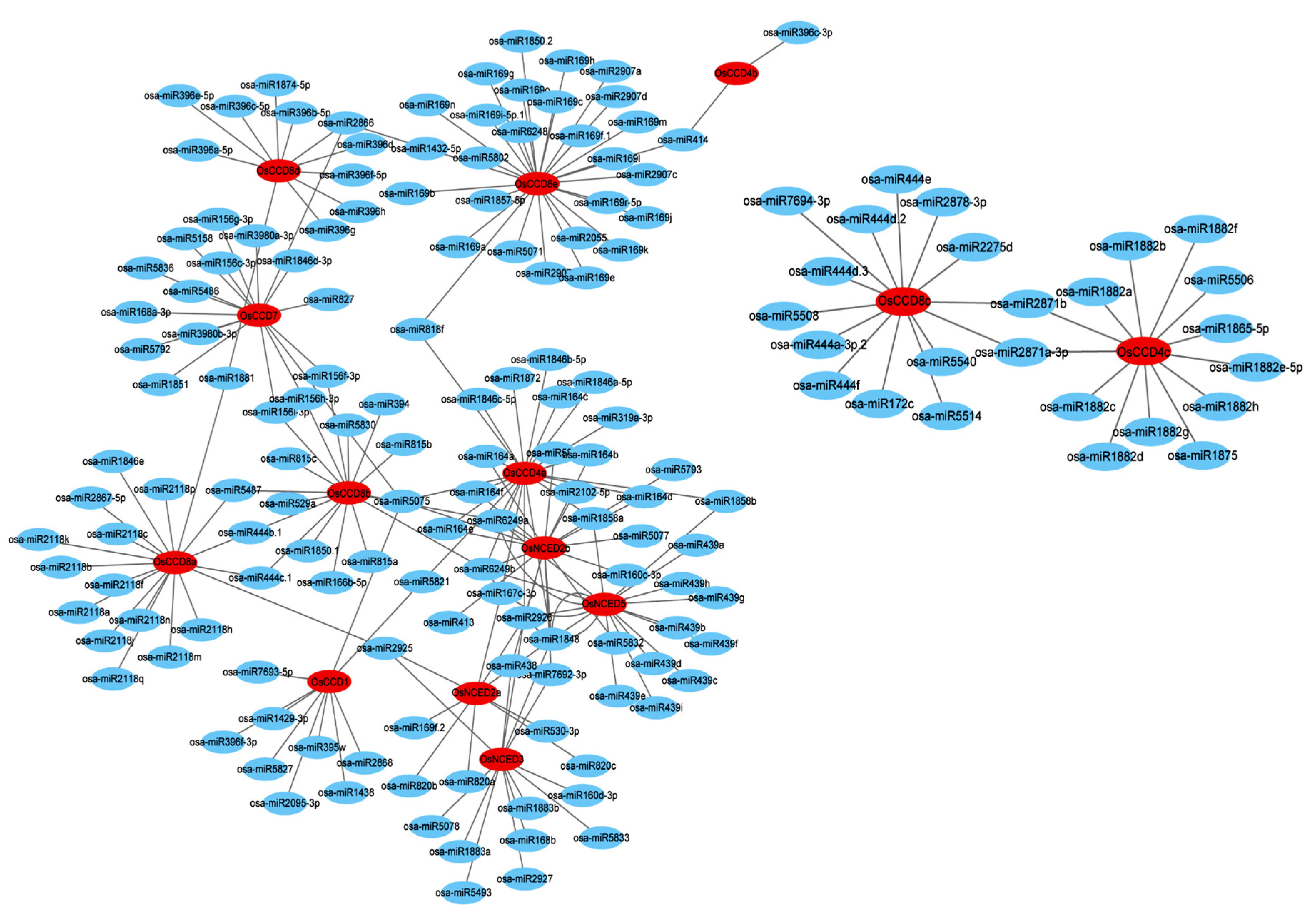
2.9. Identification of miRNA Targeting OsCCO Family Genes in Genome-Wide of Rice
2.10. Expression Patterns in the OsCCO Family Genes in Different Rice Tissues
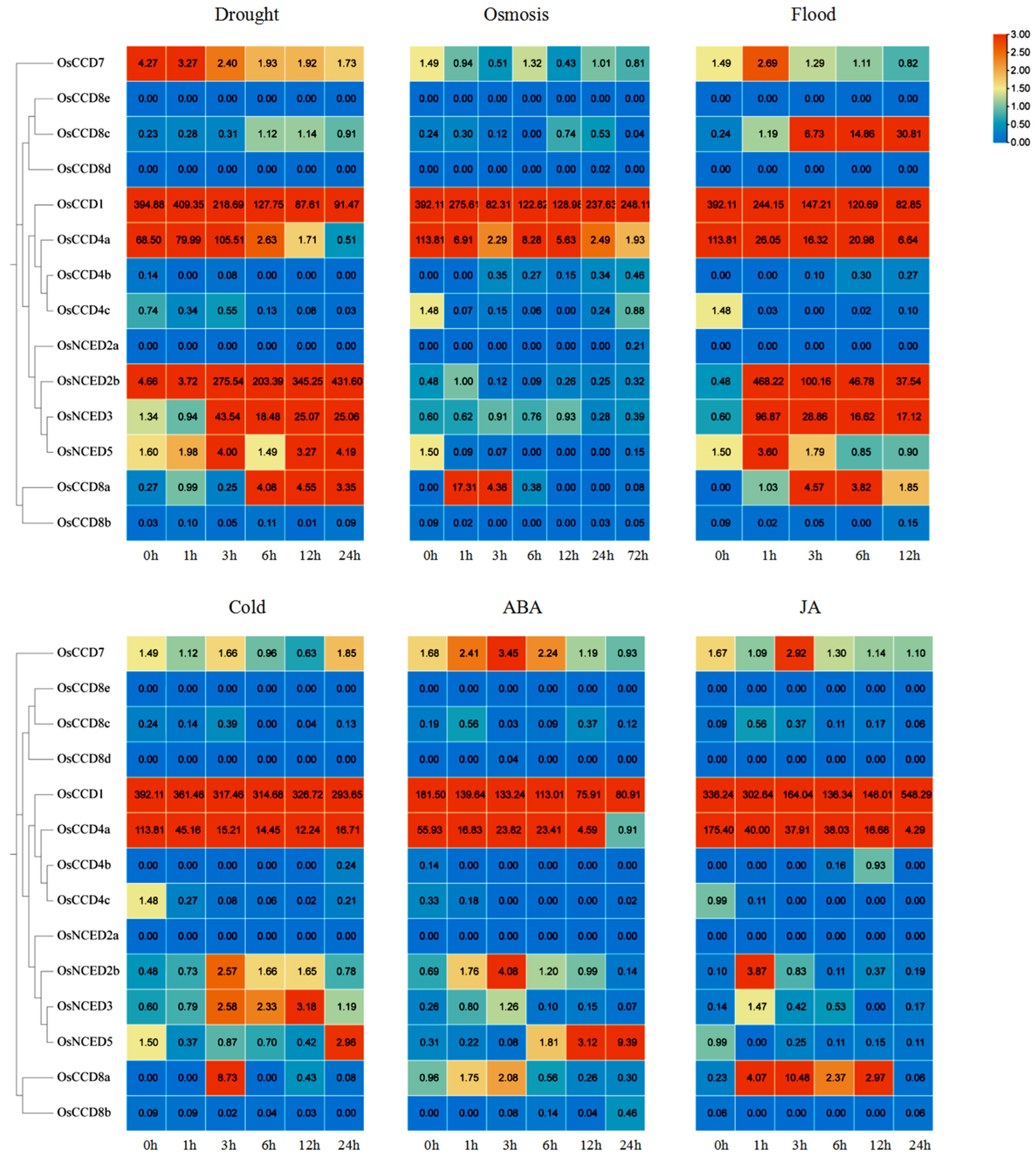
2.11. Expression Patterns of OsCCO Family Genes under Abiotic Stresses and Hormone Treatments
2.12. Expression Patterns of OsCCO Family Genes at Different Growth Stages
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Characteristics of the OsCCO Family of Rice
4.2.1. Identification of the OsCCO Family
4.2.2. Physicochemical Properties and Subcellular Localization of the OsCCO Family
4.2.3. Chromosomal Localization and Phylogenetic Tree Construction of the OsCCO Family
4.2.4. Conserved Motifs, Functional Domains, Gene Structures, and Promoters of the OsCCO Family
4.2.5. Collinearity Analysis of the OsCCO Family Genes
4.2.6. Protein Interaction and GO Enrichment Analysis of the OsCCO Family
4.2.7. Genome-Wide Analysis of miRNA Targeting OsCCO Family Genes
4.3. Tissue-Specific Expression of the OsCCO Family Members
4.4. Expression Patterns of OsCCO Family Genes under Abiotic Stress and Hormone Treatments
4.5. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, A. Carotenoid cleavage dioxygenases and their apocarotenoid products in plants. Plant Biotechnol. 2009, 26, 351–358. [Google Scholar] [CrossRef]
- Ramara, J.S.; Ramalingam, R.; Jae, K.K.; Sang, U.P. An update on biosynthesis and regulation of carotenoids in plants. S. Afr. J. Bot. 2021, 140, 290–302. [Google Scholar]
- Zhou, Q.; Li, Q.; Li, P.; Zhang, S.; Liu, C.; Jin, J.; Cao, P.; Yang, Y. Carotenoid cleavage dioxygenases: Identification, expression, and evolutionary analysis of this gene family in tobacco. Int. J. Mol. Sci. 2019, 20, 5796. [Google Scholar] [CrossRef]
- Tan, B.C.; Joseph, L.M.; Deng, W.T.; Liu, L.; Li, Q.B.; Cline, K.; McCarty, D.R. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003, 35, 44–56. [Google Scholar] [CrossRef]
- Widjaja-Adhi, M.A.; Lobo, G.P.; Golczak, M.; Von Lintig, J. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum. Mol. Genet. 2015, 24, 3206–3219. [Google Scholar] [CrossRef]
- Akram, J.; Siddique, R.; Shafiq, M.; Tabassum, B.; Manzoor, M.T.; Javed, M.A.; Anwar, S.; Nisa, B.U.; Saleem, M.H.; Javed, B.; et al. Genome-wide identification of CCO gene family in cucumber (Cucumis sativus) and its comparative analysis with A. thaliana. BMC Plant Biol. 2023, 23, 640. [Google Scholar] [CrossRef]
- Priya, R.; Sneha, P.; Dass, J.F.P.; Doss, C.G.P.; Manickavasagam, M.; Siva, R. Exploring the codon patterns between CCD and NCED genes among different plant species. Comput. Biol. Med. 2019, 114, 103449. [Google Scholar] [CrossRef]
- Simkin, A.J.; Schwartz, S.H.; Auldridge, M.; Taylor, M.G.; Klee, H.J. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles beta-ionone, pseudoionone, and geranylacetone. Plant J. 2004, 40, 882–892. [Google Scholar] [CrossRef]
- Pu, X.; Li, Z.; Tian, Y.; Gao, R.; Hao, L.; Hu, Y.; He, C.; Sun, W.; Xu, M.; Peters, R.J.; et al. The honeysuckle genome provides insight into the molecular mechanism of carotenoid metabolism underlying dynamic flower coloration. New Phytol. 2020, 227, 930–943. [Google Scholar] [CrossRef]
- Schmidt, H.; Kurtzer, R.; Eisenreich, W.; Schwab, W. The carotenase AtCCD1 from Arabidopsis thaliana is a dioxygenase. J. Biol. Chem. 2006, 281, 9845–9851. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Gutjahr, C.; Bennett, T.; Nelson, D.C. Strigolactone signaling and evolution. Annu. Rev. Plant Biol. 2017, 68, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Kitahata, N.; Saito, T.; Kobayashi, M.; Shinozaki, K.; Yoshida, S.; Asami, T. A new lead compound for abscisic acid biosynthesis inhibitors targeting 9-cis-epoxycarotenoid dioxygenase. Bioorg. Med. Chem. Lett. 2004, 14, 3033–3036. [Google Scholar] [CrossRef] [PubMed]
- Muppala, S.; Gudlavalleti, P.K.; Malireddy, K.R.; Puligundla, S.K.; Dasari, P. Development of stable transgenic maize plants tolerant for drought by manipulating ABA signaling through Agrobacterium-mediated transformation. J. Genet. Eng. Biotechnol. 2021, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Changan, S.S.; Kumar, V.; Tyagi, A. Expression pattern of candidate genes and their correlation with various metabolites of abscisic acid biosynthetic pathway under drought stress in rice. Physiol. Plant 2023, 175, e141022023. [Google Scholar] [CrossRef]
- Horváth, E.; Csiszár, J.; Gallé, Á.; Poór, P.; Szepesi, Á.; Tari, I. Hardening with salicylic acid induces concentration-dependent changes in abscisic acid biosynthesis of tomato under salt stress. J. Plant Physiol. 2015, 183, 54–63. [Google Scholar] [CrossRef]
- Xu, P.; Cai, W. Functional characterization of the BnNCED3 gene in Brassica napus. Plant Sci. 2017, 256, 16–24. [Google Scholar] [CrossRef]
- Wang, M.; Huang, L.; Kou, Y.; Li, D.; Hu, W.; Fan, D.; Cheng, S.; Yang, Y.; Zhang, Z. Differentiation of morphological traits and genome-wide expression patterns between rice subspecies indica and japonica. Genes 2023, 14, 1971. [Google Scholar] [CrossRef]
- Shang, L.; He, W.; Wang, T.; Yang, Y.; Xu, Q.; Zhao, X.; Yang, L.; Zhang, H.; Li, X.; Lv, Y.; et al. A complete assembly of the rice Nipponbare reference genome. Mol. Plant 2023, 16, 1232–1236. [Google Scholar] [CrossRef]
- Gao, J.; Liang, H.; Huang, J.; Qing, D.J.; Wu, H.; Zhou, W.Y.; Chen, W.W.; Pan, Y.H.; Dai, G.X.; Gao, L.J.; et al. Development of the PARMS marker of the TAC1 gene and its utilization in rice plant architecture breeding. Euphytica 2021, 217, 49. [Google Scholar] [CrossRef]
- Xue, G.; Hu, L.; Zhu, L.; Chen, Y.; Qiu, C.; Fan, R.; Ma, X.; Cao, Z.; Chen, J.; Shi, J.; et al. Genome-Wide identification and expression analysis of CCO Gene Family in Liriodendron chinense. Plants 2023, 12, 1975. [Google Scholar] [CrossRef] [PubMed]
- Gamage, D.G.; Gunaratne, A.; Periyannan, G.R.; Russell, T.G. Applicability of instability index for in vitro protein stability prediction. Protein Pept. Lett. 2019, 26, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Voon, C.P.; Law, Y.S.; Guan, X.; Lim, S.L.; Xu, Z.; Chu, W.T.; Zhang, R.; Sun, F.; Labs, M.; Leister, D.; et al. Modulating the activities of chloroplasts and mitochondria promotes adenosine triphosphate production and plant growth. Quant. Plant Biol. 2021, 2, e72021. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shang, L.; Yu, H.; Zeng, L.; Hu, J.; Ni, S.; Rao, Y.; Li, S.; Chu, J.; Meng, X.; et al. A strigolactone biosynthesis gene contributed to the green revolution in rice. Mol. Plant 2020, 13, 923–932. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Tan, B.C.; Gage, D.A.; Zeevaart, J.A.; McCarty, D.R. Specific oxidative cleavage of carotenoids by VP14 of maize. Science 1997, 276, 1872–1874. [Google Scholar] [CrossRef]
- Mokhtar, M.M.; Alsamman, A.M.; El, A.A. PlantLTRdb: An interactive database for 195 plant species. Front. Plant Sci. 2023, 14, 1134627. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.X.; Liu, X.J.; Xu, Y.; Lyu, Z.X.; Liu, R.; Jiu, S.T.; Zhang, C.X.; Wang, J.Y. Genome-wide identification of the NCED gene family and functional characterization of PavNCED5 related to bud dormancy in sweet cherry. Sci. Hortic. 2023, 319, 112186. [Google Scholar] [CrossRef]
- Sreekumar, J.; Muhammed Sadiq, P.A.; Raju, S.; Mukherjee, A. In silico analysis of carotenoid biosynthesis pathway in cassava (Manihot esculenta Crantz). J. Genet. 2022, 101, 102. [Google Scholar] [CrossRef]
- Cheng, G.T.; Li, Y.S.; Qi, S.M.; Wang, J.; Zhao, P.; Lou, Q.Q.; Wang, Y.F.; Zhang, X.Q.; Liang, Y. SlCCD1A Enhances the aroma quality of tomato fruits by promoting the synthesis of carotenoid-derived volatiles. Foods 2021, 10, 2678. [Google Scholar] [CrossRef]
- Frey, A.; Effroy, D.; Lefebvre, V.; Seo, M.; Perreau, F.; Berger, A.; Marion-Poll, A. Epoxycarotenoid cleavage by NCED5 fine-tunes aba accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J. 2012, 70, 501–512. [Google Scholar] [CrossRef]
- Kulkarni, K.P.; Vishwakarma, C.; Sahoo, S.P.; Lima, J.M.; Nath, M.; Dokku, P.; Gacche, R.N.; Mohapatra, T.; Robin, S.; Sarla, N.; et al. A substitution mutation in OsCCD7 cosegregates with dwarf and increased tillering phenotype in rice. J. Genet. 2014, 93, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Auldridge, M.E.; McCarty, D.R.; Klee, H.J. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr. Opin. Plant Biol. 2006, 9, 315–321. [Google Scholar] [CrossRef] [PubMed]
- González-Verdejo, C.I.; Obrero, Á.; Román, B.; Gómez, P. Expression profile of carotenoid cleavage dioxygenase genes in summer squash (Cucurbita pepo L.). Plant Foods Hum. Nutr. 2015, 70, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, Q.; Yan, J.; Sun, K.; Liang, Y.; Jia, M.; Meng, X.; Fang, S.; Wang, Y.; Jing, Y.; et al. ζ-Carotene isomerase suppresses tillering in rice through the coordinated biosynthesis of strigolactone and abscisic acid. Mol. Plant 2020, 13, 1784–1801. [Google Scholar] [CrossRef] [PubMed]
- Koop, J.; Merz, J.; Schembecker, G. Hydrophobicity, amphilicity, and flexibility: Relation between molecular protein properties and the macroscopic effects of surface activity. J. Biotechnol. 2021, 334, 11–25. [Google Scholar] [CrossRef]
- Gao, L.L.; Hong, Z.H.; Wang, Y.; Wu, G.Z. Chloroplast proteostasis: A story of birth, life, and death. Plant Commun. 2023, 4, 100424. [Google Scholar] [CrossRef]
- Ngounou Wetie, A.G.; Sokolowska, I.; Channaveerappa, D.; Dupree, E.J.; Jayathirtha, M.; Woods, A.G.; Darie, C.C. Proteomics and non-proteomics approaches to study stable and transient protein-protein interactions. Adv. Exp. Med. Biol. 2019, 1140, 121–142. [Google Scholar]
- Pan, X.; Zheng, H.; Zhao, J.; Xu, Y.; Li, X. ZmCCD7/ZpCCD7 encodes a carotenoid cleavage dioxygenase mediating shoot branching. Planta 2016, 243, 1407–1418. [Google Scholar] [CrossRef]
- Zheng, J.C.; Yu, J.; Liu, T.; Wang, X.; Zhan, Q.W.; Li, J.Q.; Xu, Z.S.; Ma, Y.Z. Identification and expression characterisation of SbERECTA family genes in Sorghum bicolor. Crop Pasture Sci. 2021, 72, 125–135. [Google Scholar] [CrossRef]
- Sami, A.; Haider, M.Z.; Shafiq, M.; Sadiq, S.; Ahmad, F. Genome-wide identification and in-silico expression analysis of CCO gene family in sunflower (Helianthus annnus) against abiotic stress. Plant Mol. Biol. 2024, 114, 34. [Google Scholar] [CrossRef]
- Norén Lindbäck, L.; Ji, Y.; Cervela-Cardona, L.; Jin, X.; Pedmale, U.V.; Strand, A. An interplay between bZIP16, bZIP68, and GBF1 regulates nuclear photosynthetic genes during photomorphogenesis in Arabidopsis. New Phytol. 2023, 240, 1082–1096. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Felemban, A.; Braguy, J.; Zurbriggen, M.D.; Al-Babili, S. Apocarotenoids involved in plant development and stress response. Front. Plant Sci. 2019, 10, 1168. [Google Scholar] [CrossRef]
- Li, C.; Zha, W.; Li, W.; Wang, J.; You, A. Advances in the biosynthesis of terpenoids and their ecological functions in plant resistance. Int. J. Mol. Sci. 2023, 24, 11561. [Google Scholar] [CrossRef]
- Fang, Y.J.; Xie, K.B.; Xiong, L.Z. Conserved miR164-targeted NAC genes negatively regulate drought resistance in rice. J. Exp. Bot. 2014, 65, 2119–2135. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhang, H.; Srivastava, A.K.; Pan, Y.J.; Bai, J.J.; Fang, J.J.; Shi, H.Z.; Zhu, J.K. Knockdown of rice microRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol. 2018, 176, 2082–2094. [Google Scholar] [CrossRef]
- Zhao, B.; Ge, L.; Liang, R.; Li, W.; Ruan, K.; Lin, H.; Jin, Y. Members of miR-169 family are induced by high salinity and transiently inhibit the NF-YA transcription factor. BMC Mol. Biol. 2009, 10, 29. [Google Scholar] [CrossRef]
- Kansal, S.; Devi, R.M.; Balyan, S.C.; Arora, M.K.; Singh, A.K.; Mathur, S.; Raghuvanshi, S. Unique miRNome during anthesis in drought-tolerant indica rice var. Nagina 22. Planta 2015, 241, 1543–1559. [Google Scholar] [CrossRef]
- Cui, N.; Sun, X.L.; Sun, M.Z.; Jia, B.W.; Duanmu, H.Z.; Lv, D.K.; Duan, X.; Zhu, Y.M. Overexpression of OsmiR156k leads to reduced tolerance to cold stress in rice (Oryza sativa). Mol. Breed. 2015, 35, 214. [Google Scholar] [CrossRef]
- Macovei, A.; Tuteja, N. microRNAs targeting DEAD-box helicases are involved in salinity stress response in rice (Oryza sativa L.). BMC Plant Biol. 2012, 12, 183. [Google Scholar] [CrossRef]
- Gao, P.; Bai, X.; Yang, L.; Lv, D.; Li, Y.; Cai, H.; Ji, W.; Guo, D.; Zhu, Y. Over-expression of osa-MIR396c decreases salt and alkali stress tolerance. Planta 2010, 231, 991–1001. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, G.; Liu, Y.; Li, Y.; Zhang, J. ABA controls H₂O₂ accumulation through the induction of OsCATB in rice leaves under water stress. Plant Cell Physiol. 2011, 52, 689–698. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Lin, J.; Zhu, T.; Liu, N.; Yang, X.; Ma, J.; Sui, S. Carotenoid Cleavage Dioxygenase Genes of Chimonanthus praecox, CpCCD7 and CpCCD8, Regulate Shoot Branching in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 8750. [Google Scholar] [CrossRef]
- Lashbrooke, J.G.; Young, P.R.; Dockrall, S.J.; Vasanth, K.; Vivier, M.A. Functional characterisation of three members of the Vitis vinifera L. carotenoid cleavage dioxygenase gene family. BMC Plant Biol. 2013, 13, 156. [Google Scholar] [CrossRef]
- Basso, M.F.; Contaldi, F.; Celso, F.L.; Karalija, E.; Paz-Carrasco, L.C.; Barone, G.; Ferrante, A.; Martinelli, F. Expression profile of the NCED/CCD genes in chickpea and lentil during abiotic stress reveals a positive correlation with increased plant tolerance. Plant Sci. 2023, 336, 111817. [Google Scholar] [CrossRef]
- Wang, R.K.; Wang, C.E.; Fei, Y.Y.; Gai, J.Y.; Zhao, T.J. Genome-wide identification and transcription analysis of soybean carotenoid oxygenase genes during abiotic stress treatments. Mol. Biol. Rep. 2013, 40, 4737–4745. [Google Scholar] [CrossRef]
- Sato, H.; Takasaki, H.; Takahashi, F.; Suzuki, T.; Iuchi, S.; Mitsuda, N.; Ohme-Takagi, M.; Ikeda, M.; Seo, M.; Yamaguchi-Shinozaki, K.; et al. Arabidopsis thaliana NGATHA1 transcription factor induces ABA biosynthesis by activating NCED3 gene during dehydration stress. Proc. Natl. Acad. Sci. USA 2018, 115, E11178. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network analysis and visualization of proteomics data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Langenbacher, A.D.; Shimizu, H.; Hsu, W.; Zhao, Y.; Borges, A.; Koehler, C.; Chen, J.N. Mitochondrial calcium uniporter deficiency in zebrafish causes cardiomyopathy with Arrhythmia. Front. Physiol. 2020, 11, 617492. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, Z.; Madgwick, P.J.; Carmo-Silva, E.; Parry, M.A.; Hu, Y.G. TaER expression is associated with transpiration efficiency traits and yield in bread wheat. PLoS ONE 2015, 10, e01284152015. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression datausing real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]





| Gene Name | Gene ID | CDS Length (bp) | Amino Acids (aa) | MW (kDa) | pI | INI | AI | GRAVY |
|---|---|---|---|---|---|---|---|---|
| OsCCD1 | LOC_Os12g44310 | 1623 | 540 | 60.95 | 5.9 | 32.57 | 76.44 | −0.322 |
| OsCCD4a | LOC_Os02g47510 | 1917 | 638 | 68.62 | 6.07 | 41.95 | 77.04 | −0.148 |
| OsCCD4b | LOC_Os10g08980 | 738 | 245 | 27.18 | 4.77 | 49.26 | 76.00 | −0.171 |
| OsCCD4c | LOC_Os12g24800 | 1731 | 576 | 63.87 | 6.26 | 42.17 | 85.62 | −0.067 |
| OsCCD7 | LOC_Os04g46470 | 1830 | 609 | 68.19 | 9.19 | 51.65 | 77.55 | −0.295 |
| OsCCD8a | LOC_Os01g38580 | 1659 | 552 | 59.92 | 5.88 | 50.25 | 78.13 | −0.188 |
| OsCCD8b | LOC_Os01g54270 | 2406 | 801 | 87.93 | 5.89 | 46.69 | 80.24 | −0.259 |
| OsCCD8c | LOC_Os08g28240 | 1725 | 574 | 64.63 | 6.33 | 46.29 | 82.33 | −0.268 |
| OsCCD8d | LOC_Os08g28410 | 1437 | 478 | 53.64 | 6.04 | 35.09 | 86.63 | −0.213 |
| OsCCD8e | LOC_Os09g15240 | 1647 | 548 | 61.07 | 5.84 | 40.88 | 85.24 | −0.181 |
| OsNCED2a | LOC_Os04g04230 | 522 | 173 | 18.65 | 5.37 | 36.21 | 86.18 | 0.029 |
| OsNCED2b | LOC_Os07g05940 | 1749 | 582 | 62.95 | 6.21 | 46.06 | 82.30 | −0.233 |
| OsNCED3 | LOC_Os03g44380 | 1827 | 608 | 65.65 | 5.77 | 36.74 | 77.57 | −0.172 |
| OsNCED5 | LOC_Os12g42280 | 1842 | 613 | 65.93 | 5.83 | 38.62 | 76.61 | −0.140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, H.; Ai, H.; Wang, Y.; Shi, J.; Ren, L.; Li, J.; Tao, Y.; Xu, Z.; Zheng, J. Molecular Characteristics and Expression Patterns of Carotenoid Cleavage Oxygenase Family Genes in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2024, 25, 10264. https://doi.org/10.3390/ijms251910264
Dai H, Ai H, Wang Y, Shi J, Ren L, Li J, Tao Y, Xu Z, Zheng J. Molecular Characteristics and Expression Patterns of Carotenoid Cleavage Oxygenase Family Genes in Rice (Oryza sativa L.). International Journal of Molecular Sciences. 2024; 25(19):10264. https://doi.org/10.3390/ijms251910264
Chicago/Turabian StyleDai, Hanjing, Hao Ai, Yingrun Wang, Jia Shi, Lantian Ren, Jieqin Li, Yulu Tao, Zhaoshi Xu, and Jiacheng Zheng. 2024. "Molecular Characteristics and Expression Patterns of Carotenoid Cleavage Oxygenase Family Genes in Rice (Oryza sativa L.)" International Journal of Molecular Sciences 25, no. 19: 10264. https://doi.org/10.3390/ijms251910264







