Polymyxin B Peptide Hydrogel Coating: A Novel Approach to Prevent Ventilator-Associated Pneumonia
Abstract
:1. Introduction
2. Results
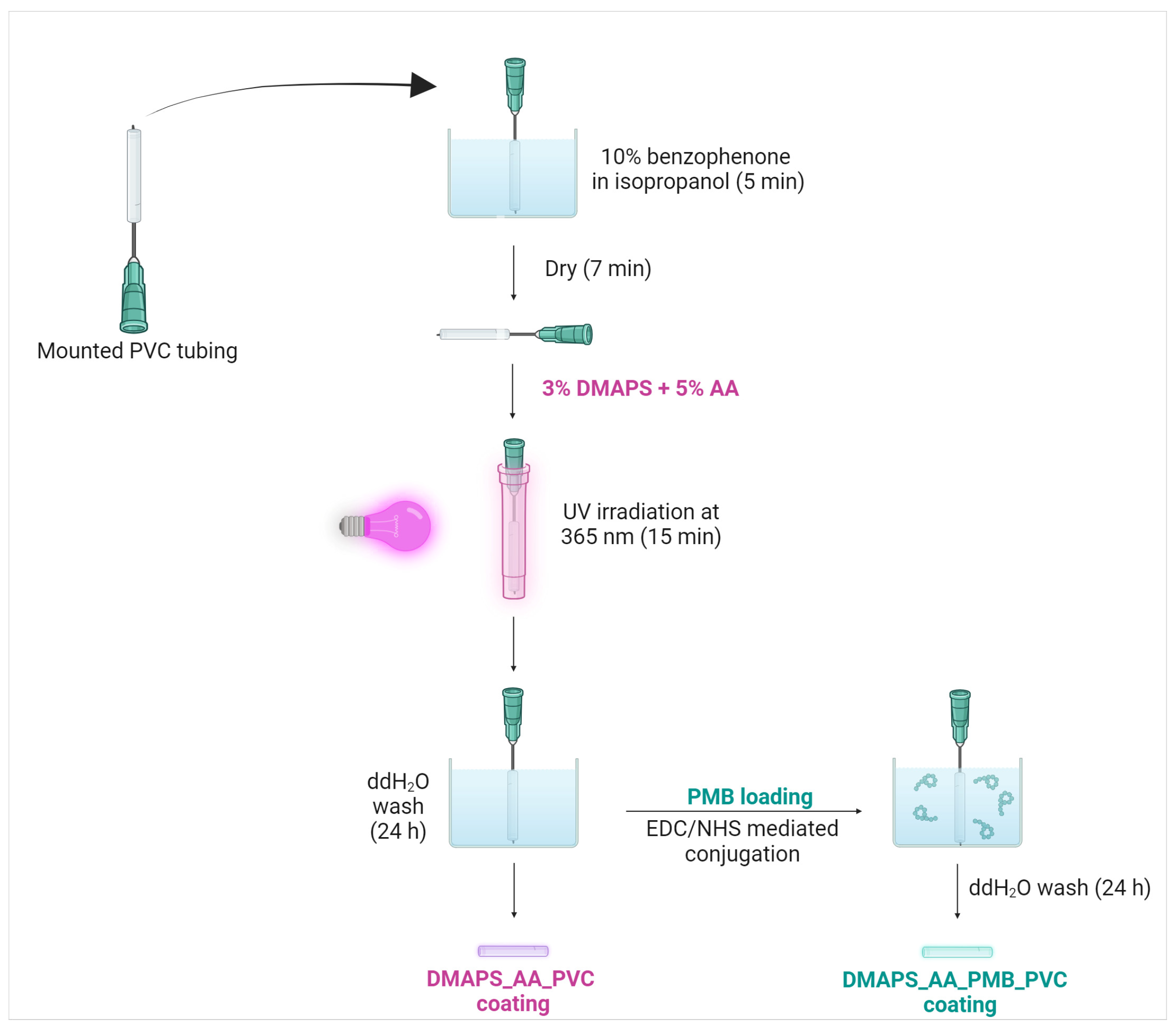
2.1. Antibacterial Hydrogel Coating Preparation
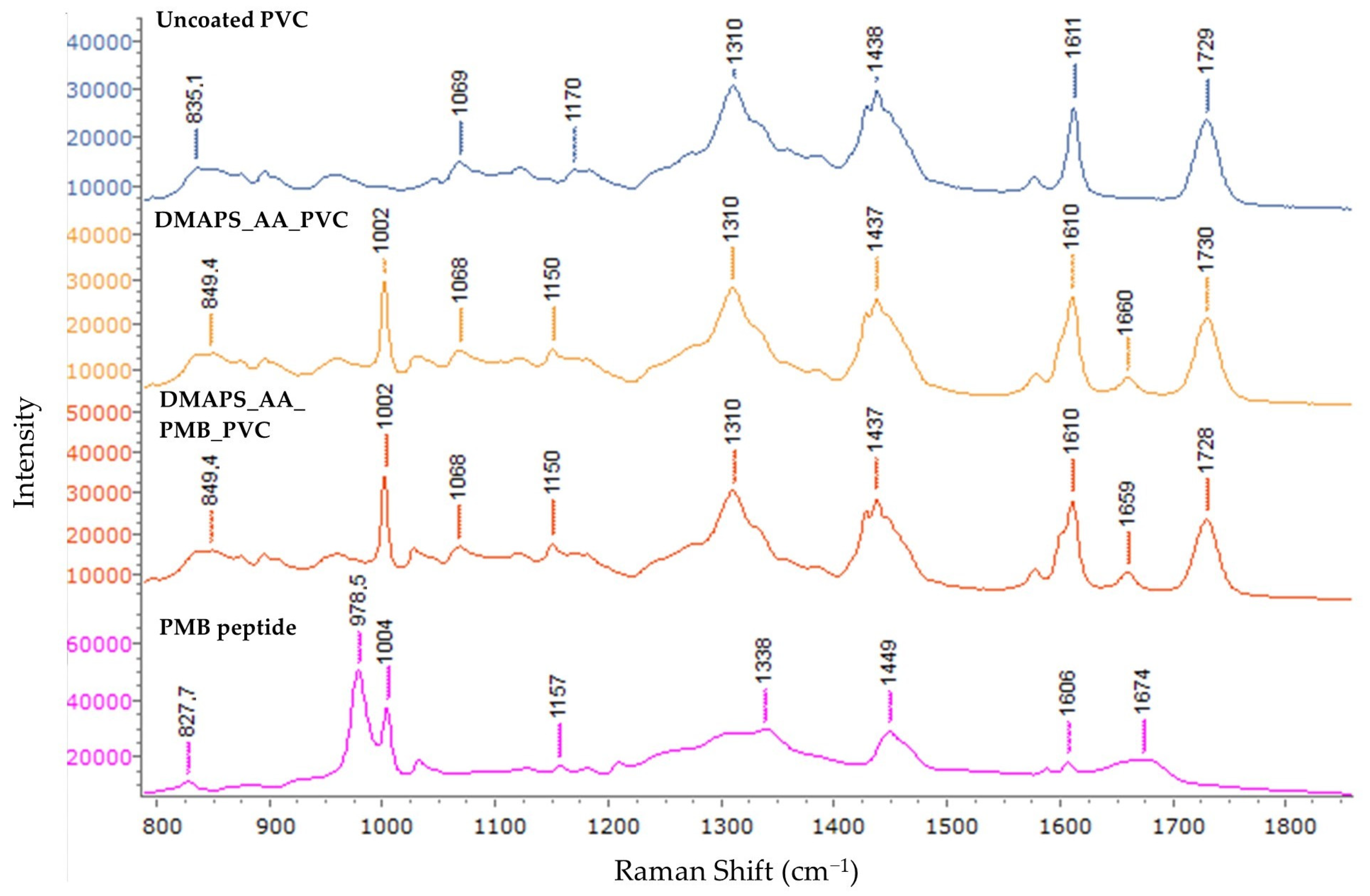
2.2. In-Depth Spectral Analysis of Coated Endotracheal Tubing
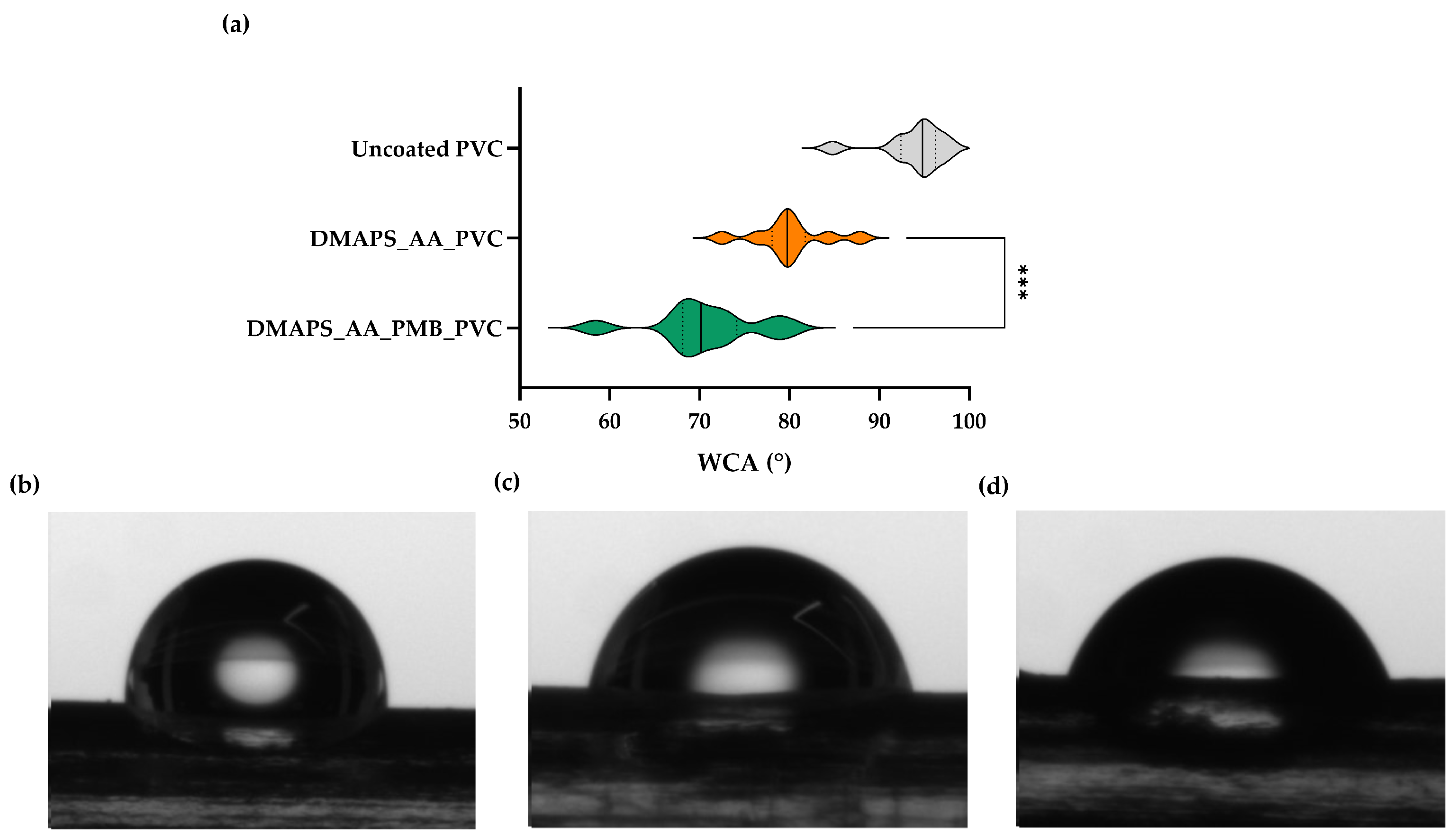
2.3. Antibacterial Hydrogel Coating Enhances Surface Hydrophilicity
2.4. The Applied Coating Can Withstand Physiological Pressures
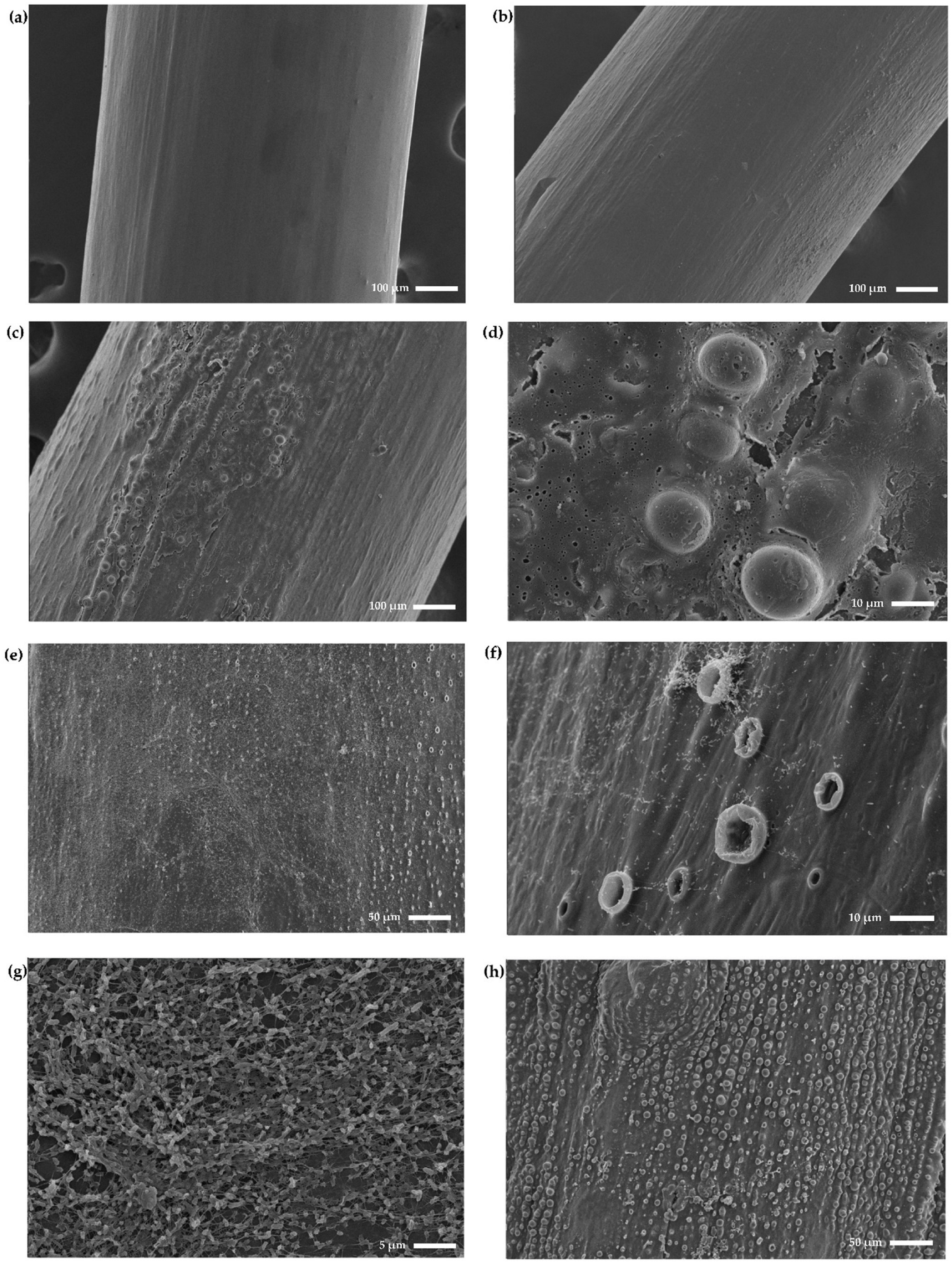
2.5. Coated Endotracheal Tubes Exhibit a Rougher and More Textured Surface
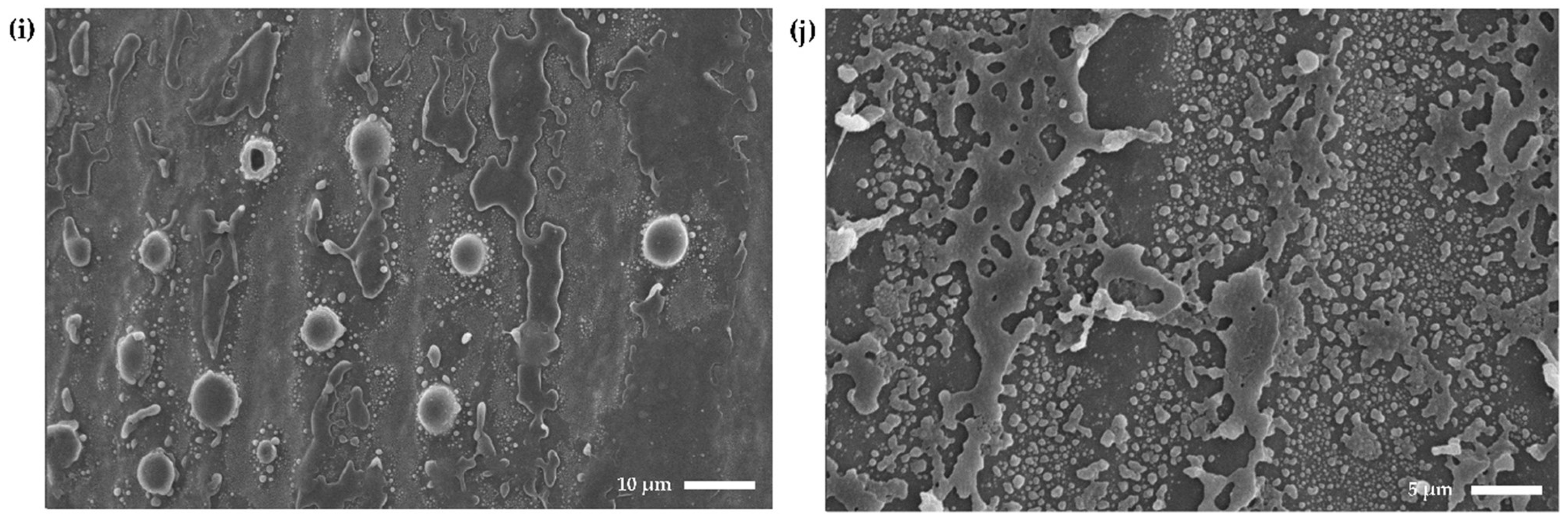
2.6. Scanning Electron Microscopy Confirms Anti-Biofilm Activity and Shows the Formation of Blister-Like Structures
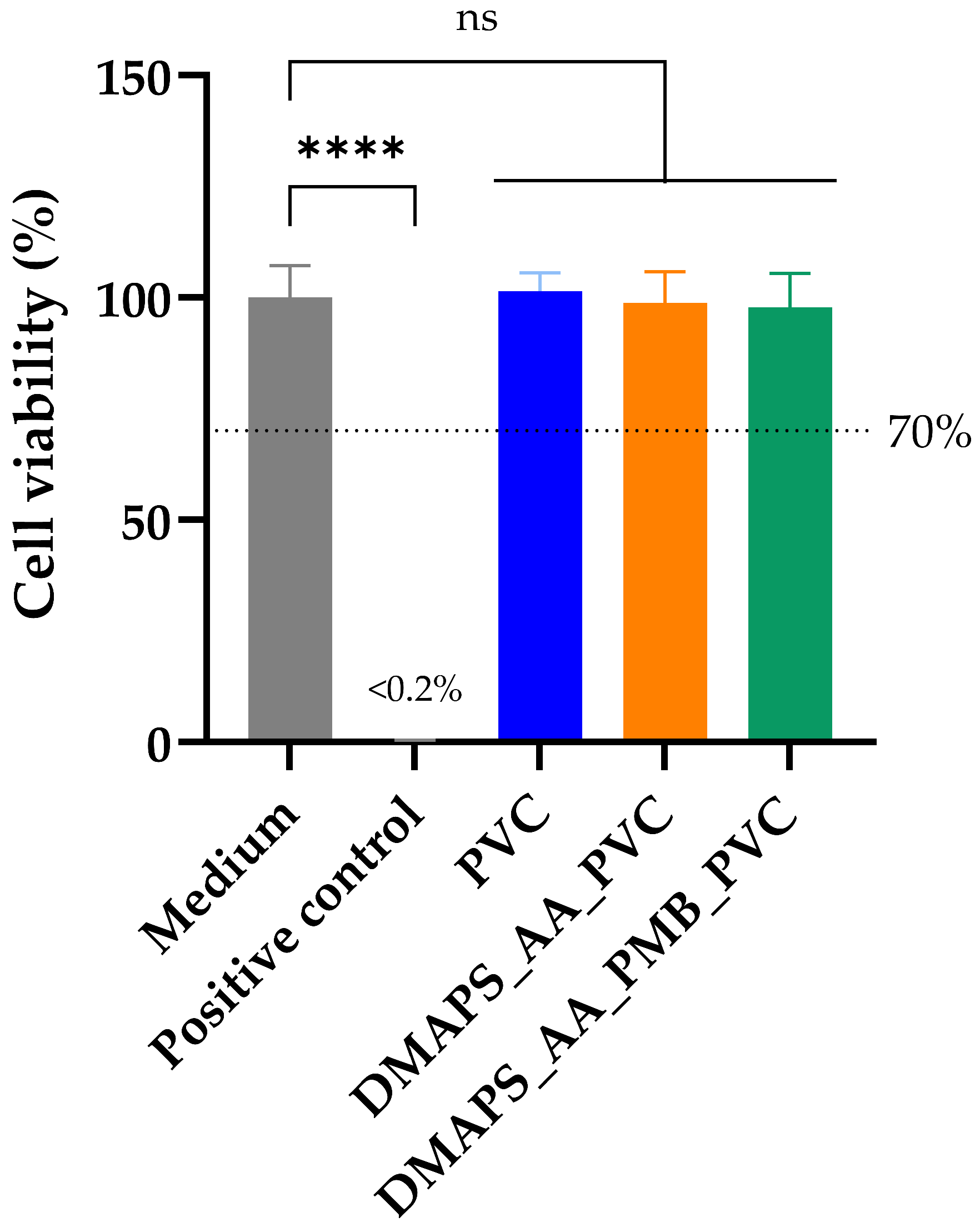
2.7. Polymyxin B-Coated Tubing Meets Biocompatibility Standards
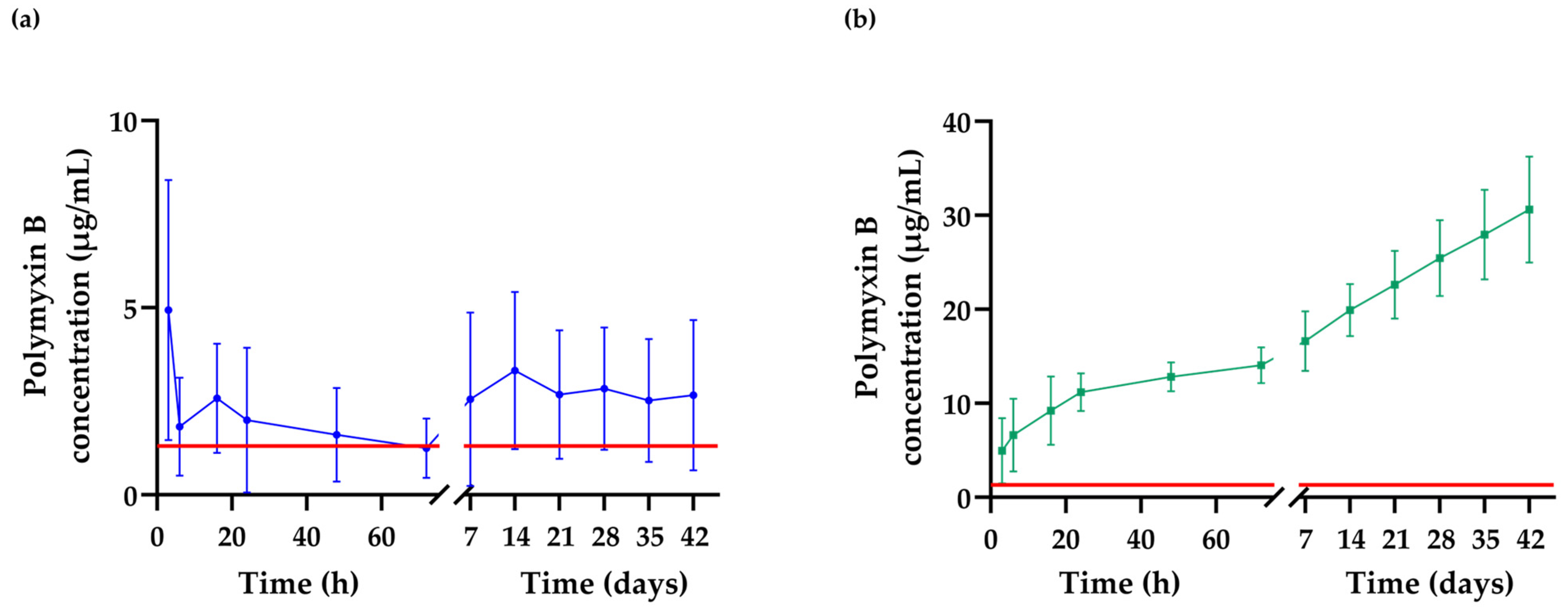
2.8. Detection of the Extended Release of Polymyxin B from the Hydrogel Matrix
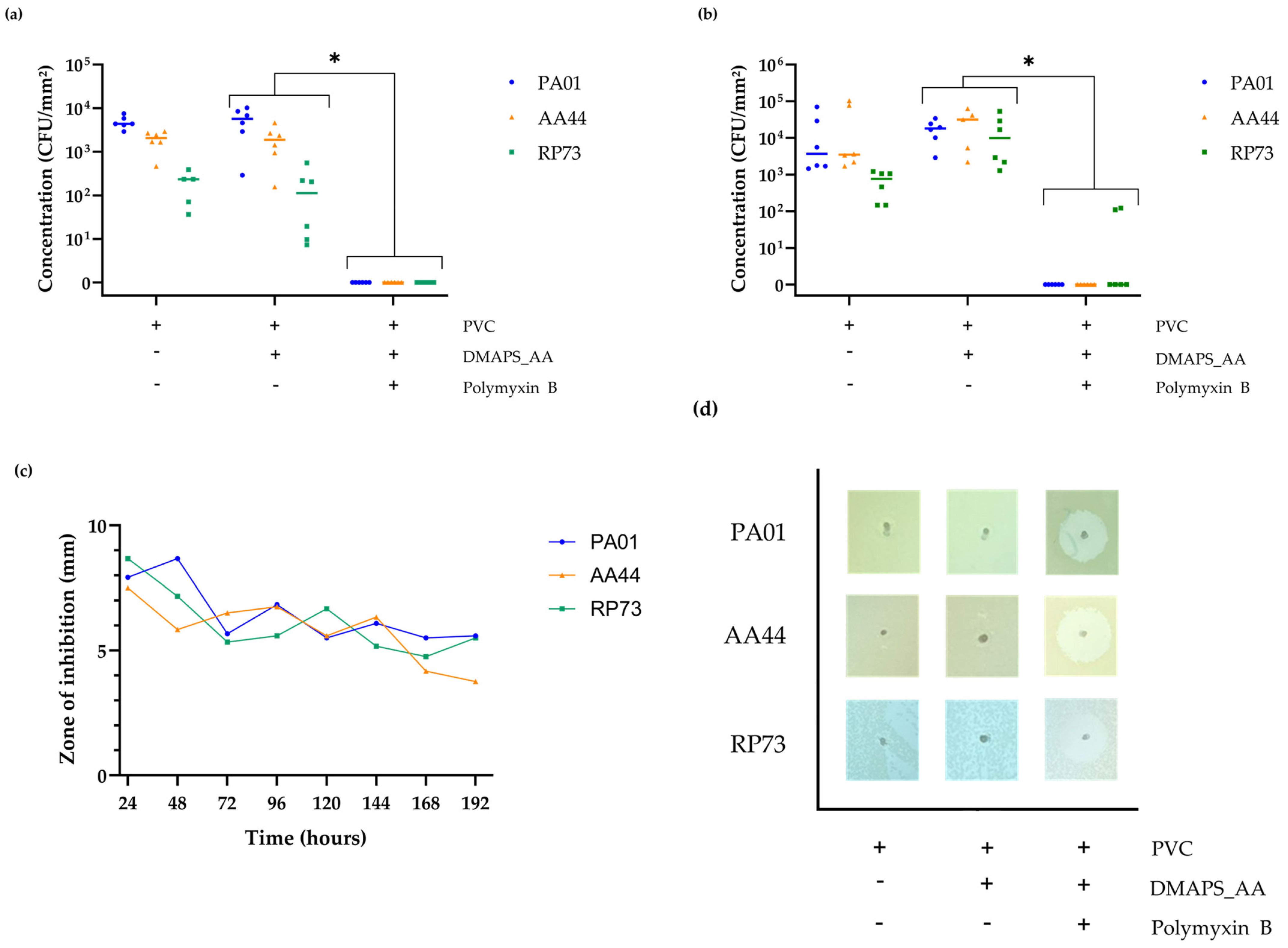
2.9. Confirmation of the In Vitro Antibacterial Activity against Various P. aeruginosa Strains
3. Discussion
4. Materials and Methods
4.1. Materials and Biologicals
4.2. Preparation of PVC Tubing
4.3. Hydrogel Coating Application
4.4. Fourier-Transform Infrared Spectroscopy and Raman Spectroscopy
4.5. Wettability (Water Contact Angle)
4.6. Compression Mechanical Analysis
4.7. Atomic Force Microscopy
4.8. Scanning Electron Microscopy
4.9. Cytotoxicity Evaluation
4.10. Polymyxin B Release Quantification
4.11. Antibacterial Evaluation
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raoofi, S.; Kan, F.P.; Rafiei, S.; Hosseinipalangi, Z.; Mejareh, Z.N.; Khani, S.; Abdollahi, B.; Talab, F.S.; Sanaei, M.; Zarabi, F.; et al. Global Prevalence of Nosocomial Infection: A Systematic Review and Meta-Analysis. PLoS ONE 2023, 18, e0274248. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control Healthcare-Associated Infections Acquired in Intensive Care Units—Annual Epidemiological Report for 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-intensive-care-units-2019 (accessed on 28 March 2024).
- Papazian, L.; Klompas, M.; Luyt, C.E. Ventilator-Associated Pneumonia in Adults: A Narrative Review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Gunalan, A.; Sastry, A.S.; Ramanathan, V.; Sistla, S. Early-vs Late-Onset Ventilator-Associated Pneumonia in Critically Ill Adults: Comparison of Risk Factors, Outcome, and Microbial Profile. Indian J. Crit. Care Med. 2023, 27, 411. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, V.D.; Jin, Z.; Memish, Z.A.; Rodrigues, C.; Myatra, S.N.; Kharbanda, M.; Valderrama-Beltran, S.L.; Mehta, Y.; Daboor, M.A.; Todi, S.K.; et al. Multinational Prospective Cohort Study of Rates and Risk Factors for Ventilator-Associated Pneumonia over 24 Years in 42 Countries of Asia, Africa, Eastern Europe, Latin America, and the Middle East: Findings of the International Nosocomial Infection Control Consortium (INICC). Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e6. [Google Scholar] [CrossRef] [PubMed]
- Mandell, L.A.; Niederman, M.S. Pneumonia. In Harrison’s Principles of Internal Medicine, 21e; Loscalzo, J., Fauci, A., Kasper, D., Hauser, S., Longo, D., Jameson, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2022; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=3095§ionid=263547796 (accessed on 28 March 2024).
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. Summary of the International Clinical Guidelines for the Management of Hospital-Acquired and Ventilator-Acquired Pneumonia. ERJ Open Res. 2018, 4, 00028-2018. [Google Scholar] [CrossRef]
- Rodrigues, J.; Sousa, P. Economic and Clinical Impact of Ventilator-Associated Pneumonia in Intensive Care Units of a University Hospital Center. In Health and Social Care Systems of the Future: Demographic Changes, Digital Age and Human Factors: Proceedings of the Healthcare Ergonomics and Patient Safety, HEPS, Lisbon, Portugal, 3–5 July 2019; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1012, pp. 135–141. [Google Scholar]
- Marcut, L.; Manescu, V.; Antoniac, A.; Paltanea, G.; Robu, A.; Mohan, A.G.; Grosu, E.; Corneschi, I.; Bodog, A.D. Antimicrobial Solutions for Endotracheal Tubes in Prevention of Ventilator-Associated Pneumonia. Materials 2023, 16, 5034. [Google Scholar] [CrossRef]
- Alves, D.; Grainha, T.; Pereira, M.O.; Lopes, S.P. Antimicrobial Materials for Endotracheal Tubes: A Review on the Last Two Decades of Technological Progress. Acta Biomater. 2023, 158, 32–55. [Google Scholar] [CrossRef]
- Drexelius, M.G.; Neundorf, I. Application of Antimicrobial Peptides on Biomedical Implants: Three Ways to Pursue Peptide Coatings. Int. J. Mol. Sci. 2021, 22, 13212. [Google Scholar] [CrossRef]
- Nicolas, M.; Beito, B.; Oliveira, M.; Martins, M.T.; Gallas, B.; Salmain, M.; Boujday, S.; Humblot, V. Strategies for Antimicrobial Peptides Immobilization on Surfaces to Prevent Biofilm Growth on Biomedical Devices. Antibiotics 2022, 11, 13. [Google Scholar] [CrossRef]
- Cao, P.; Liu, D.; Zhang, Y.; Xiao, F.; Yuan, C.; Liang, F.; Liu, X.; Zhang, C. Dopamine-Assisted Sustainable Antimicrobial Peptide Coating with Antifouling and Anticorrosion Properties. Appl. Surf. Sci. 2022, 589, 153019. [Google Scholar] [CrossRef]
- Copling, A.; Akantibila, M.; Kumaresan, R.; Fleischer, G.; Cortes, D.; Tripathi, R.S.; Carabetta, V.J.; Vega, S.L. Recent Advances in Antimicrobial Peptide Hydrogels. Int. J. Mol. Sci. 2023, 24, 7563. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Begines, B.; Pajuelo, E.; Vázquez, J.; Rodriguez-Albelo, L.M.; Cofini, D.; Torres, Y.; Alcudia, A. Versatile Biodegradable Poly(Acrylic Acid)-Based Hydrogels Infiltrated in Porous Titanium Implants to Improve the Biofunctional Performance. Biomacromolecules 2023, 24, 4743–4758. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh Pakdel, P.; Peighambardoust, S.J. A Review on Acrylic Based Hydrogels and Their Applications in Wastewater Treatment. J. Environ. Manag. 2018, 217, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Avedissian, S.N.; Liu, J.; Rhodes, N.J.; Lee, A.; Pais, G.M.; Hauser, A.R.; Scheetz, M.H. A Review of the Clinical Pharmacokinetics of Polymyxin B. Antibiotics 2019, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Shatri, G.; Tadi, P. Polymyxin; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Dubashynskaya, N.V.; Skorik, Y.A. Polymyxin Delivery Systems: Recent Advances and Challenges. Pharmaceuticals 2020, 13, 83. [Google Scholar] [CrossRef]
- Browne, K.; Kuppusamy, R.; Chen, R.; Willcox, M.D.P.; Walsh, W.R.; Black, D.S.; Kumar, N. Bioinspired Polydopamine Coatings Facilitate Attachment of Antimicrobial Peptidomimetics with Broad-Spectrum Antibacterial Activity. Int. J. Mol. Sci. 2022, 23, 2952. [Google Scholar] [CrossRef]
- Peng, T.; Shi, Q.; Chen, M.; Yu, W.; Yang, T. Antibacterial-Based Hydrogel Coatings and Their Application in the Biomedical Field—A Review. J. Funct. Biomater. 2023, 14, 243. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, F.; Wei, Y.; Hu, Q.; Luo, Q.; Chen, C.; Wang, J.; Yang, L.; Luo, R.; Wang, Y. Dressing Blood-Contacting Materials by a Stable Hydrogel Coating with Embedded Antimicrobial Peptides for Robust Antibacterial and Antithrombus Properties. ACS Appl. Mater. Interfaces 2021, 13, 38947–38958. [Google Scholar] [CrossRef]
- Yu, Y.; Yuk, H.; Parada, G.A.; Wu, Y.; Liu, X.; Nabzdyk, C.S.; Youcef-Toumi, K.; Zang, J.; Zhao, X.; Yu, Y.; et al. Multifunctional “Hydrogel Skins” on Diverse Polymers with Arbitrary Shapes. Adv. Mater. 2019, 31, 1807101. [Google Scholar] [CrossRef]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional Hydrogel Coatings. Natl. Sci. Rev. 2021, 8, 2021. [Google Scholar] [CrossRef]
- Zhu, Z.; Gao, Q.; Long, Z.; Huo, Q.; Ge, Y.; Vianney, N.; Daliko, N.A.; Meng, Y.; Qu, J.; Chen, H.; et al. Polydopamine/Poly(Sulfobetaine Methacrylate) Co-Deposition Coatings Triggered by CuSO4/H2O2 on Implants for Improved Surface Hemocompatibility and Antibacterial Activity. Bioact. Mater. 2021, 6, 2546–2556. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sha, D.; Jiang, H.; Shi, K.; Xu, J.D.; Yu, C.; Wei, H.; Wang, B.L.; Ji, X.L. Preparation of Antibacterial Poly(Sulfobetaine Methacrylate) Grafted on Poly(Vinyl Alcohol)-Formaldehyde Sponges and Their Properties. J. Appl. Polym. Sci. 2019, 136, 47047. [Google Scholar] [CrossRef]
- Stillger, L.; Müller, D. Peptide-Coating Combating Antimicrobial Contaminations: A Review of Covalent Immobilization Strategies for Industrial Applications. J. Mater. Sci. 2022, 57, 10863–10885. [Google Scholar] [CrossRef]
- Thébault, P.; Ammoun, M.; Boudjemaa, R.; Ouvrard, A.; Steenkeste, K.; Bourguignon, B.; Fontaine-Aupart, M.P. Surface Functionalization Strategy to Enhance the Antibacterial Effect of Nisin Z Peptide. Surf. Interfaces 2022, 30, 101822. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Hu, L.; Zhang, Q.; Chen, E.; Wang, Z.; Shi, Y.; Tan, L.; Xiao, S. A Universal Coating Strategy for Inhibiting the Growth of Bacteria on Materials Surfaces. Front. Chem. 2022, 10, 1043353. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, S.; Rajendran, A. Synthesis, Characterization and Electrical Properties of Nano Metal and Metal-Oxide Doped with Conducting Polymer Composites by in-Situ Chemical Polymerization. MOJ Polym. Sci. 2017, 1, 1–4. [Google Scholar] [CrossRef]
- Klempová, S.; Oravec, M.; Vizárová, K. Analysis of Thermally and UV-Vis Aged Plasticized PVC Using UV-Vis, ATR-FTIR and Raman Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 294, 122541. [Google Scholar] [CrossRef]
- Royaux, A.; Fabre-Francke, I.; Balcar, N.; Barabant, G.; Bollard, C.; Lavédrine, B.; Cantin, S. Aging of Plasticized Polyvinyl Chloride in Heritage Collections: The Impact of Conditioning and Cleaning Treatments. Polym. Degrad. Stab. 2017, 137, 109–121. [Google Scholar] [CrossRef]
- Ul-Hamid, A.; Soufi, K.Y.; Al-Hadhrami, L.M.; Shemsi, A.M. Failure Investigation of an Underground Low Voltage XLPE Insulated Cable. Anti-Corros. Methods Mater. 2015, 62, 281–287. [Google Scholar] [CrossRef]
- Jalil, A.; Asim, M.H.; Nazir, I.; Matuszczak, B.; Bernkop-Schnürch, A. Self-Emulsifying Drug Delivery Systems Containing Hydrophobic Ion Pairs of Polymyxin B and Agaric Acid: A Decisive Strategy for Enhanced Antimicrobial Activity. J. Mol. Liq. 2020, 311, 113298. [Google Scholar] [CrossRef]
- Naim, A.F.A.; Alfannakh, H.; Arafat, S.; Ibrahim, S.S. Characterization of PVC/MWCNTs Nanocomposite: Solvent Blend. Sci. Eng. Compos. Mater. 2020, 27, 55–64. [Google Scholar] [CrossRef]
- Nørbygaard, T.; Berg, R.W. Analysis of Phthalate Ester Content in Poly(Vinyl Chloride) Plastics by Means of Fourier Transform Raman Spectroscopy. Appl. Spectrosc. 2004, 58, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.G.M.; Brown, D.R.; Dale, J.A.; Plant, S. Raman Spectroscopy of Sulfonated Polystyrene Resins. Vib. Spectrosc. 2000, 24, 213–224. [Google Scholar] [CrossRef]
- Solodovnichenko, V.S.; Polyboyarov, V.A.; Zhdanok, A.A.; Arbuzov, A.B.; Zapevalova, E.S.; Kryazhev, Y.G.; Likholobov, V.A. Synthesis of Carbon Materials by the Short-Term Mechanochemical Activation of Polyvinyl Chloride. Procedia Eng. 2016, 152, 747–752. [Google Scholar] [CrossRef]
- Li, X.; Silge, S.; Saal, A.; Kircher, G.; Koynov, K.; Berger, R.; Butt, H.J. Adaptation of a Styrene-Acrylic Acid Copolymer Surface to Water. Langmuir 2021, 37, 1571–1577. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Shen, H.X.; Wang, C.F.; Chen, S. Rapid Preparation of Superabsorbent Self-Healing Hydrogels by Frontal Polymerization. Gels 2023, 9, 380. [Google Scholar] [CrossRef]
- Ivanova, A.; Ivanova, K.; Tzanov, T. Simultaneous Ultrasound-Assisted Hybrid Polyzwitterion/Antimicrobial Peptide Nanoparticles Synthesis and Deposition on Silicone Urinary Catheters for Prevention of Biofilm-Associated Infections. Nanomaterials 2021, 11, 3143. [Google Scholar] [CrossRef]
- Pan, C.; Chen, L.; Zhang, Y.H.; Liu, W.; Urbino, R.; Marco Ranieri, V.; Qiu, H.B.; Yang, Y. Physiological Correlation of Airway Pressure and Transpulmonary Pressure Stress Index on Respiratory Mechanics in Acute Respiratory Failure. Chin. Med. J. 2016, 129, 1652–1657. [Google Scholar] [CrossRef]
- Wilder, N.A.; Orr, J.; Westenskow, D. Clinical Evaluation of Tracheal Pressure Estimation from the Endotracheal Tube Cuff Pressure. J. Clin. Monit. Comput. 1998, 14, 29–34. [Google Scholar] [CrossRef]
- Lucangelo, U.; Ajčević, M.; Lena, E.; Ferluga, M.; Comuzzi, L.; Accardo, A.; Zin, W.A. On Some Factors Determining the Pressure Drop across Tracheal Tubes during High-Frequency Percussive Ventilation: A Flow-Independent Model. J. Clin. Monit. Comput. 2021, 35, 885–890. [Google Scholar] [CrossRef]
- Moon, K.M.; Donaworth, S.; Hagele, M.S.; Kim, S.S.; Willer, B.L.; Tobias, J.D. Endotracheal Tube Cuff Pressures in the Operating Room of a Pediatric Hospital: A Quality Improvement Initiative. Pediatr. Qual. Saf. 2022, 7, e619. [Google Scholar] [CrossRef] [PubMed]
- Kanďárová, H.; Pôbiš, P. The “Big Three” in Biocompatibility Testing of Medical Devices: Implementation of Alternatives to Animal Experimentation—Are We There Yet? Front. Toxicol. 2023, 5, 1337468. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- LI, W.; Zhou, J.; Xu, Y. Study of the in Vitro Cytotoxicity Testing of Medical Devices. Biomed. Rep. 2015, 3, 617. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.; Nickel, A. Toxic or Not Toxic? The Specifications of the Standard ISO 10993-5 Are Not Explicit Enough to Yield Comparable Results in the Cytotoxicity Assessment of an Identical Medical Device. Front. Med. Technol. 2023, 5, 1195529. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Mohid, S.A.; Bhunia, A. Atomic-Resolution Structures and Mode of Action of Clinically Relevant Antimicrobial Peptides. Int. J. Mol. Sci. 2022, 23, 4558. [Google Scholar] [CrossRef]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Thompson, P.E.; Li, J. Pharmacology of Polymyxins: New Insights into an “old” Class of Antibiotics. Future Microbiol. 2013, 8, 711–724. [Google Scholar] [CrossRef]
- Yun, B.; Zhang, T.; Azad, M.A.K.; Wang, J.; Nowell, C.J.; Kalitsis, P.; Velkov, T.; Hudson, D.F.; Li, J. Polymyxin B Causes DNA Damage in HK-2 Cells and Mice. Arch. Toxicol. 2018, 92, 2259. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Velkov, T.; Lin, Y.W.; Yun, B.; Nowell, C.J.; Zhou, F.; Zhou, Q.T.; Chan, K.; Azad, M.A.K.; Li, J. Potential Toxicity of Polymyxins in Human Lung Epithelial Cells. Antimicrob. Agents Chemother. 2017, 61, 10-1128. [Google Scholar] [CrossRef]
- Auletta, S.; Galli, F.; Varani, M.; Campagna, G.; Conserva, M.; Martinelli, D.; Santino, I.; Signore, A. In Vitro and In Vivo Evaluation of 99mTc-Polymyxin B for Specific Targeting of Gram-Bacteria. Biomolecules 2021, 11, 232. [Google Scholar] [CrossRef]
- Stokniene, J.; Powell, L.C.; Aarstad, O.A.; Aachmann, F.L.; Rye, P.D.; Hill, K.E.; Thomas, D.W.; Ferguson, E.L. Bi-Functional Alginate Oligosaccharide–Polymyxin Conjugates for Improved Treatment of Multidrug-Resistant Gram-Negative Bacterial Infections. Pharmaceutics 2020, 12, 1080. [Google Scholar] [CrossRef]
- Grace, A.; Sahu, R.; Owen, D.R.; Dennis, V.A. Pseudomonas aeruginosa Reference Strains PAO1 and PA14: A Genomic, Phenotypic, and Therapeutic Review. Front. Microbiol. 2022, 13, 1023523. [Google Scholar] [CrossRef] [PubMed]
- Lorè, N.I.; Cigana, C.; De Fino, I.; Riva, C.; Juhas, M.; Schwager, S.; Eberl, L.; Bragonzi, A. Cystic Fibrosis-Niche Adaptation of Pseudomonas Aeruginosa Reduces Virulence in Multiple Infection Hosts. PLoS ONE 2012, 7, 35648. [Google Scholar] [CrossRef] [PubMed]
- Cigana, C.; Curcuru, L.; Leone, M.R.; Ierano, T.; Lore, N.I.; Bianconi, I.; Silipo, A.; Cozzolino, F.; Lanzetta, R.; Molinaro, A.; et al. Pseudomonas aeruginosa Exploits Lipid A and Muropeptides Modification as a Strategy to Lower Innate Immunity during Cystic Fibrosis Lung Infection. PLoS ONE 2009, 4, e8439. [Google Scholar] [CrossRef] [PubMed]
- Jeukens, J.; Boyle, B.; Bianconi, I.; Kukavica-Ibrulj, I.; Tümmler, B.; Bragonzi, A.; Levesque, R.C. Complete Genome Sequence of Persistent Cystic Fibrosis Isolate Pseudomonas Aeruginosa Strain RP73. Genome Announc. 2013, 1, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Impact Initiatives and Research Coordination (IRC) WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 20 May 2024).
- Velásquez-Garcia, L.; Mejia-Sanjuanelo, A.; Viasus, D.; Carratalà, J. Causative Agents of Ventilator-Associated Pneumonia and Resistance to Antibiotics in COVID-19 Patients: A Systematic Review. Biomedicines 2022, 10, 1226. [Google Scholar] [CrossRef]
- Li, Y.; Roberts, J.A.; Walker, M.M.; Aslan, A.T.; Harris, P.N.A.; Sime, F.B. The Global Epidemiology of Ventilator-Associated Pneumonia Caused by Multi-Drug Resistant Pseudomonas Aeruginosa: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2024, 139, 78–85. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B.; Groza, A. Polymeric Coatings and Antimicrobial Peptides as Efficient Systems for Treating Implantable Medical Devices Associated-Infections. Polymers 2022, 14, 1611. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface Characteristics Influencing Bacterial Adhesion to Polymeric Substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Thakur, S. Sustainable Nanostructural Materials for Shape Memory, Self-Healing, and Self-Cleaning Applications. In Dynamics of Advanced Sustainable Nanomaterials and Their Related Nanocomposites at the Bio-Nano Interface; Elsevier: Amsterdam, The Netherlands, 2019; pp. 235–250. [Google Scholar] [CrossRef]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The Influence of Surface Chemistry on the Kinetics and Thermodynamics of Bacterial Adhesion. Sci. Rep. 2018, 8, 17247. [Google Scholar] [CrossRef]
- Peng, W.; Liu, P.; Zhang, X.; Peng, J.; Gu, Y.; Dong, X.; Ma, Z.; Liu, P.; Shen, J. Multi-Functional Zwitterionic Coating for Silicone-Based Biomedical Devices. Chem. Eng. J. 2020, 398, 125663. [Google Scholar] [CrossRef]
- Mu, M.; Liu, S.; DeFlorio, W.; Hao, L.; Wang, X.; Salazar, K.S.; Taylor, M.; Castillo, A.; Cisneros-Zevallos, L.; Oh, J.K.; et al. Influence of Surface Roughness, Nanostructure, and Wetting on Bacterial Adhesion. Langmuir 2023, 39, 5426–5439. [Google Scholar] [CrossRef] [PubMed]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the Oral Cavity and Approaches for Biofilm Management by Surface Modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, X.; Qi, X. Raman Spectroscopic Investigation of Sulfates Using Mosaic Grating Spatial Heterodyne Raman Spectrometer. IEEE Photonics J. 2019, 11, 1–12. [Google Scholar] [CrossRef]
- Ben Mabrouk, K.; Kauffmann, T.H.; Aroui, H.; Fontana, M.D. Raman Study of Cation Effect on Sulfate Vibration Modes in Solid State and in Aqueous Solutions. J. Raman Spectrosc. 2013, 44, 1603–1608. [Google Scholar] [CrossRef]
- Babin, Y.; D’Amour, J.; Pigeon, M.; Pézolet, M. A Study of the Structure of Polymyxin B-Dipalmitoylphosphatidylglycerol Complexes by Vibrational Spectroscopy. Biochim. Et. Biophys. Acta (BBA)—Biomembr. 1987, 903, 78–88. [Google Scholar] [CrossRef]
- Griffiths, P.R.; De Haseth, J.A. Fourier Transform Infrared Spectrometry: Second Edition. Fourier Transform. InfraredSpectrom. Second. Ed. 2006, 1–529. [Google Scholar] [CrossRef]
- Wärnheim, A.; Kotov, N.; Dobryden, I.; Telaretti Leggieri, R.; Edvinsson, C.; Heydari, G.; Sundell, P.E.; Deltin, T.; Johnson, C.M.; Persson, D.; et al. Nanomechanical and Nano-FTIR Analysis of Polyester Coil Coatings before and after Artificial Weathering Experiments. Prog. Org. Coat. 2024, 190, 108355. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Dwibedy, S.K.; Padhy, I. Polymyxins, the Last-Resort Antibiotics: Mode of Action, Resistance Emergence, and Potential Solutions. J. Biosci. 2021, 46, 85. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of Polymyxins: A Systematic Review of the Evidence from Old and Recent Studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef]
- van Gent, M.E.; Ali, M.; Nibbering, P.H.; Kłodzińska, S.N. Current Advances in Lipid and Polymeric Antimicrobial Peptide Delivery Systems and Coatings for the Prevention and Treatment of Bacterial Infections. Pharmaceutics 2021, 13, 1840. [Google Scholar] [CrossRef]
- Song, Q.; Chan, S.Y.; Xiao, Z.; Zhao, R.; Zhang, Y.; Chen, X.; Liu, T.; Yan, Y.; Zhang, B.; Han, F.; et al. Contact-Killing Antibacterial Mechanisms of Polycationic Coatings: A Review. Prog. Org. Coat. 2024, 188, 108214. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Akay, S.; Yaghmur, A. Recent Advances in Antibacterial Coatings to Combat Orthopedic Implant-Associated Infections. Molecules 2024, 29, 1172. [Google Scholar] [CrossRef] [PubMed]
- Sahetya, S.; Allgood, S.; Gay, P.C.; Lechtzin, N. Long-Term Mechanical Ventilation. Clin. Chest Med. 2016, 37, 753. [Google Scholar] [CrossRef]
- Lefebvre, C.W.; Babich, J.P.; Grendell, J.H.; Grendell, J.H.; Heffner, J.E.; Thibault, R.; Pichard, C.; Monnet, X.; Teboul, J.-L.; Sinderby, C.A.; et al. Ventilator-Associated Pneumonia. Encycl. Intensive Care Med. 2023, 165, 1773–1782. [Google Scholar] [CrossRef]
- Van Moll, L.; Wouters, M.; De Smet, J.; De Vooght, L.; Delputte, P.; Van Der Borght, M.; Cos, P. In-Depth Biological Characterization of Two Black Soldier Fly Anti- Pseudomonas Peptides Reveals LPS-Binding and Immunomodulating Effects. mSphere 2023, 8, e00454-23. [Google Scholar] [CrossRef]
- Crouzet, M.; Claverol, S.; Lomenech, A.M.; Le Sénéchal, C.; Costaglioli, P.; Barthe, C.; Garbay, B.; Bonneu, M.; Vilain, S. Pseudomonas aeruginosa Cells Attached to a Surface Display a Typical Proteome Early as 20 Minutes of Incubation. PLoS ONE 2017, 12, e0180341. [Google Scholar] [CrossRef]
- Mlyniec, A.; Dabrowska, S.; Heljak, M.; Weglarz, W.P.; Wojcik, K.; Ekiert-Radecka, M.; Obuchowicz, R.; Swieszkowski, W. The Dispersion of Viscoelastic Properties of Fascicle Bundles within the Tendon Results from the Presence of Interfascicular Matrix and Flow of Body Fluids. Mater. Sci. Eng. C 2021, 130, 112435. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wouters, M.; Van Moll, L.; De Vooght, L.; Choińska, E.; Idaszek, J.; Szlązak, K.; Heljak, M.K.; Święszkowski, W.; Cos, P. Polymyxin B Peptide Hydrogel Coating: A Novel Approach to Prevent Ventilator-Associated Pneumonia. Int. J. Mol. Sci. 2024, 25, 10269. https://doi.org/10.3390/ijms251910269
Wouters M, Van Moll L, De Vooght L, Choińska E, Idaszek J, Szlązak K, Heljak MK, Święszkowski W, Cos P. Polymyxin B Peptide Hydrogel Coating: A Novel Approach to Prevent Ventilator-Associated Pneumonia. International Journal of Molecular Sciences. 2024; 25(19):10269. https://doi.org/10.3390/ijms251910269
Chicago/Turabian StyleWouters, Milan, Laurence Van Moll, Linda De Vooght, Emilia Choińska, Joanna Idaszek, Karol Szlązak, Marcin K. Heljak, Wojciech Święszkowski, and Paul Cos. 2024. "Polymyxin B Peptide Hydrogel Coating: A Novel Approach to Prevent Ventilator-Associated Pneumonia" International Journal of Molecular Sciences 25, no. 19: 10269. https://doi.org/10.3390/ijms251910269










