Optical Nanoscopy of Cytokine-Induced Structural Alterations of the Endoplasmic Reticulum and Golgi Apparatus in Insulin-Secreting Cells
Abstract
:1. Introduction
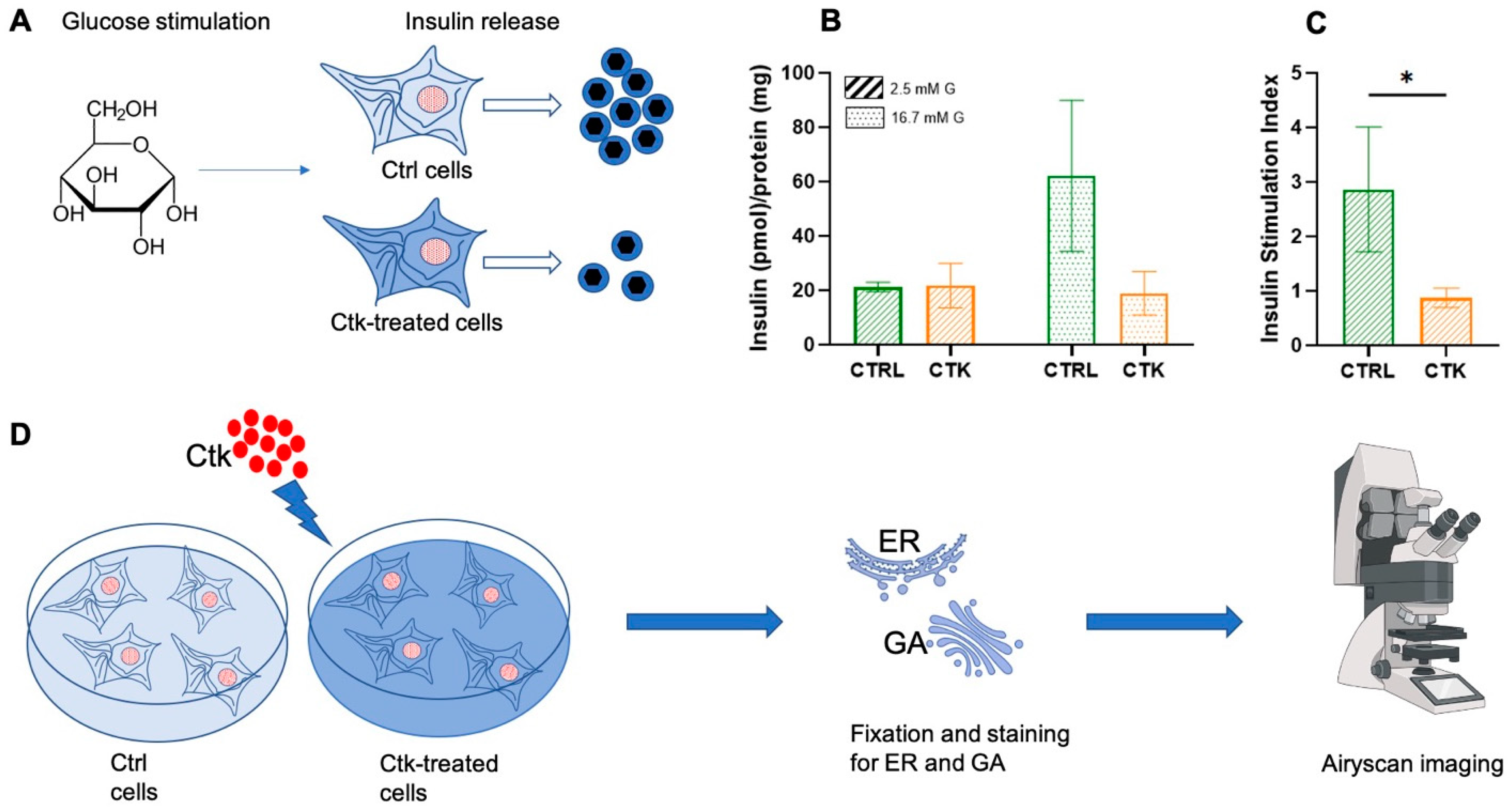
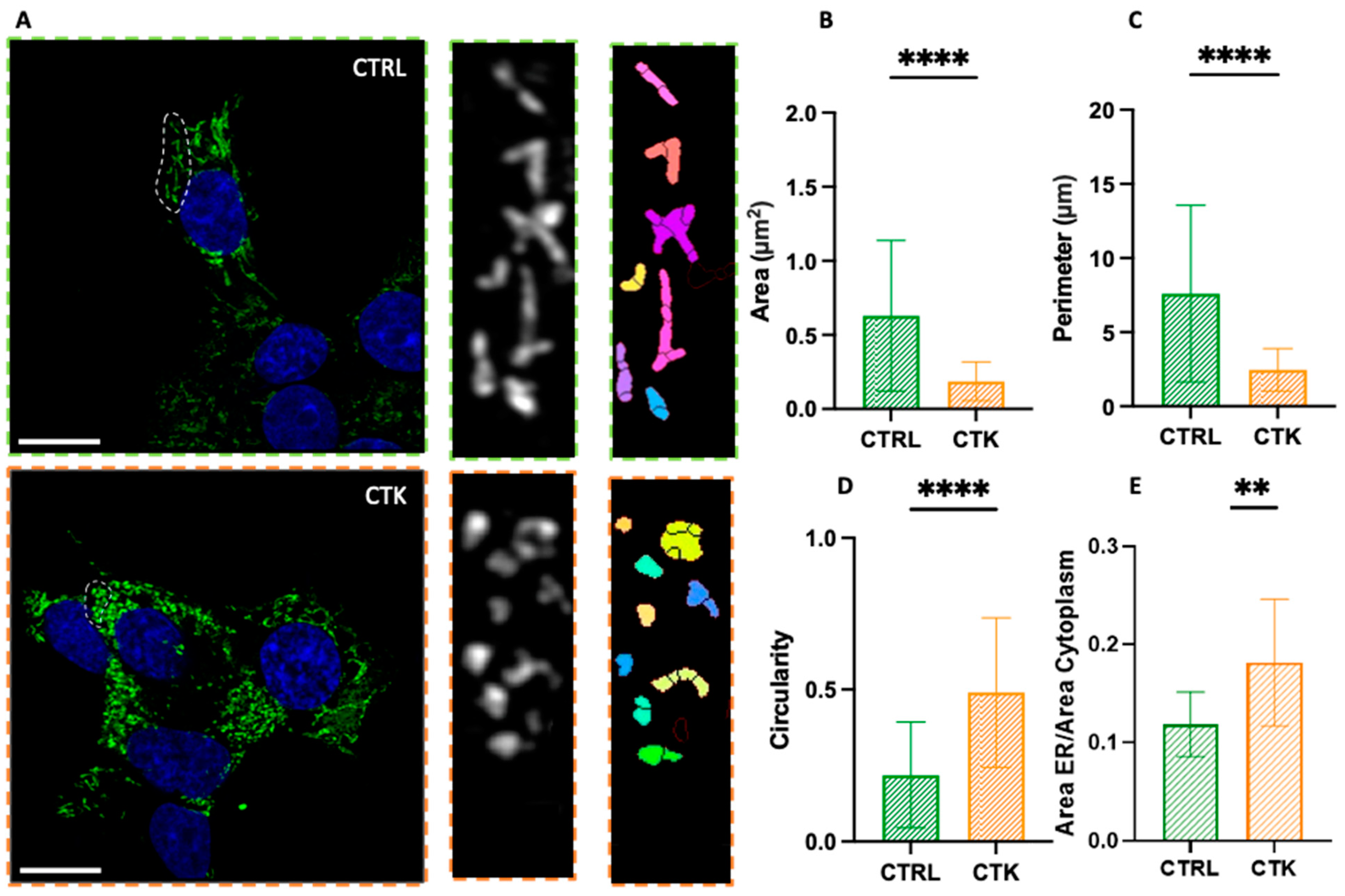
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Omar-Hmeadi, M.; Idevall-Hagren, O. Insulin granule biogenesis and exocytosis. Cell. Mol. Life Sci. 2021, 78, 1957–1970. [Google Scholar] [CrossRef] [PubMed]
- Germanos, M.; Gao, A.; Taper, M.; Yau, B.; Kebede, M.A. Inside the Insulin Secretory Granule. Metabolites 2021, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Davidson, H.W.; Rhodes, C.J.; Hutton, J.C. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic β cell via two distinct site-specific endopeptidases. Nature 1988, 333, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, S.P.; Montag, A.G.; Thomas, G.; Albiges-Rizo, C.; Carroll, R.; Benig, M.; Phillips, L.A.; Martin, S.; Ohagi, S.; Gardner, P. Proinsulin processing by the subtilisin-related proprotein convertases furin, PC2, and PC3. Proc. Natl. Acad. Sci. USA 1992, 89, 8822–8826. [Google Scholar] [CrossRef]
- Orci, L.; Ravazzola, M.; Perrelet, A. (Pro)insulin associates with Golgi membranes of pancreatic B cells. Proc. Natl. Acad. Sci. USA 1984, 81, 6743–6746. [Google Scholar] [CrossRef]
- Orci, L.; Halban, P.; Amherdt, M.; Ravazzola, M.; Vassalli, J.D.; Perrelet, A. Nonconverted, amino acid analog-modified proinsulin stays in a Golgi-derived clathrin-coated membrane compartment. J. Cell Biol. 1984, 99, 2187–2192. [Google Scholar] [CrossRef]
- Baker, E.N.; Blundell, T.L.; Cutfield, J.F.; Dodson, E.J.; Dodson, G.G.; Hodgkin, D.M.C.; Hubbard, R.E.; Isaacs, N.W.; Reynolds, C.D.; Sakabe, K.; et al. The structure of 2Zn pig insulin crystals at 1.5 Å resolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998, 319, 369–456. [Google Scholar]
- Kuliawat, R.; Klumperman, J.; Ludwig, T.; Arvan, P. Differential Sorting of Lysosomal Enzymes Out of the Regulated Secretory Pathway in Pancreatic β-Cells. J. Cell Biol. 1997, 137, 595–608. [Google Scholar] [CrossRef]
- Orci, L.; Ravazzola, M.; Storch, M.J.; Anderson, R.G.W.; Vassalli, J.D.; Perrelet, A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell 1987, 49, 865–868. [Google Scholar] [CrossRef]
- Rhodes, C.J.; Halban, P.A. Newly synthesized proinsulin/insulin and stored insulin are released from pancreatic B cells predominantly via a regulated, rather than a constitutive, pathway. J. Cell Biol. 1987, 105, 145–153. [Google Scholar] [CrossRef]
- Blair, M. Diabetes Mellitus Review. Urol. Nurs. 2016, 36, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Schuit, F.C.; In’t Veld, P.A.; Pipeleers, D.G. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc. Natl. Acad. Sci. USA 1988, 85, 3865–3869. [Google Scholar] [CrossRef]
- Boland, B.B.; Rhodes, C.J.; Grimsby, J.S. The dynamic plasticity of insulin production in β-cells. Mol. Metab. 2017, 6, 958–973. [Google Scholar] [CrossRef]
- Sun, J.; Cui, J.; He, Q.; Chen, Z.; Arvan, P.; Liu, M. Proinsulin misfolding and endoplasmic reticulum stress during the development and progression of diabetes☆. Mol. Asp. Med. 2015, 42, 105–118. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Cardozo, A.K.; Cnop, M. The Role for Endoplasmic Reticulum Stress in Diabetes Mellitus. Endocr. Rev. 2008, 29, 42–61. [Google Scholar] [CrossRef]
- Sahin, G.S.; Lee, H.; Engin, F. An accomplice more than a mere victim: The impact of β-cell ER stress on type 1 diabetes pathogenesis. Mol. Metab. 2021, 54, 101365. [Google Scholar] [CrossRef] [PubMed]
- Brozzi, F.; Nardelli, T.R.; Lopes, M.; Millard, I.; Barthson, J.; Igoillo-Esteve, M.; Grieco, F.A.; Villate, O.; Oliveira, J.M.; Casimir, M.; et al. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia 2015, 58, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.N.; Oyebamiji, O.; Talware, S.; Selvaraj, S.; Krishnan, P.; Syed, F.; Wu, H.; Evans-Molina, C. A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes. Diabetes 2020, 69, 2364–2376. [Google Scholar] [CrossRef] [PubMed]
- Tütüncü, N.B.; Verdi, H.; Yalçın, Y.; Kınık, S.; Tütüncü, T.; Ataç, F.B. Beta-Cell Golgi Stress Response to Lipotoxicity and Glucolipotoxicity: A Preliminary Study of a Potential Mechanism of Beta-Cell Failure in Posttransplant Diabetes and Intraportal Islet Transplant. Exp. Clin. Transplant. 2022, 20, 585–594. [Google Scholar] [CrossRef]
- Kim, W.K.; Choi, W.; Deshar, B.; Kang, S.; Kim, J. Golgi Stress Response: New Insights into the Pathogenesis and Therapeutic Targets of Human Diseases. Mol. Cells 2023, 46, 191–199. [Google Scholar] [CrossRef]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S. Endoplasmic reticulum stress signalling—From basic mechanisms to clinical applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Takeuchi, T.; Tanaka, S.; Kubo, S.K.; Kayo, T.; Lu, D.; Takata, K.; Koizumi, A.; Izumi, T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the Mody mouse. J. Clin. Investig. 1999, 103, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Riggs, A.C.; Bernal-Mizrachi, E.; Ohsugi, M.; Wasson, J.; Fatrai, S.; Welling, C.; Murray, J.; Schmidt, R.E.; Herrera, P.L.; Permutt, M.A. Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia 2005, 48, 2313–2321. [Google Scholar] [CrossRef]
- Akiyama, M.; Hatanaka, M.; Ohta, Y.; Ueda, K.; Yanai, A.; Uehara, Y.; Tanabe, K.; Tsuru, M.; Miyazaki, M.; Saeki, S.; et al. Increased insulin demand promotes while pioglitazone prevents pancreatic beta cell apoptosis in Wfs1 knockout mice. Diabetologia 2009, 52, 653–663. [Google Scholar] [CrossRef]
- Scheuner, D.; Mierde, D.V.; Song, B.; Flamez, D.; Creemers, J.W.; Tsukamoto, K.; Ribick, M.; Schuit, F.C.; Kaufman, R.J. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat. Med. 2005, 11, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, C.; Boland, B.B.; Uchizono, Y.; Moore, P.C.; Peterson, B.; Rajan, S.; Rhodes, O.S.; Noske, A.B.; Haataja, L.; Arvan, P.; et al. Pancreatic β-Cell Adaptive Plasticity in Obesity Increases Insulin Production but Adversely Affects Secretory Function. Diabetes 2016, 65, 438–450. [Google Scholar] [CrossRef]
- Marchetti, P.; Bugliani, M.; Lupi, R.; Marselli, L.; Masini, M.; Boggi, U.; Filipponi, F.; Weir, G.C.; Eizirik, D.L.; Cnop, M. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 2007, 50, 2486–2494. [Google Scholar] [CrossRef] [PubMed]
- Masini, M.; Marselli, L.; Bugliani, M.; Martino, L.; Masiello, P.; Marchetti, P.; De Tata, V. Ultrastructural morphometric analysis of insulin secretory granules in human type 2 diabetes. Acta Diabetol. 2012, 49, 247–252. [Google Scholar] [CrossRef]
- Pugliese, L.A.; De Lorenzi, V.; Bernardi, M.; Ghignoli, S.; Tesi, M.; Marchetti, P.; Pesce, L.; Cardarelli, F. Unveiling nanoscale optical signatures of cytokine-induced β-cell dysfunction. Sci. Rep. 2023, 13, 13342. [Google Scholar] [CrossRef]
- Snapp, E.L.; Hegde, R.S.; Francolini, M.; Lombardo, F.; Colombo, S.; Pedrazzini, E.; Borgese, N.; Lippincott-Schwartz, J. Formation of stacked ER cisternae by low affinity protein interactions. J. Cell. Biol. 2003, 163, 257–269. [Google Scholar] [CrossRef]
- Korkhov, V.M.; Zuber, B. Direct observation of molecular arrays in the organized smooth endoplasmic reticulum. BMC Cell Biol. 2009, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Dinarello, C.A.; Mandrup-Poulsen, T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat. Rev. Immunol. 2019, 19, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Merglen, A.; Theander, S.; Rubi, B.; Chaffard, G.; Wollheim, C.B.; Maechler, P. Glucose Sensitivity and Metabolism-Secretion Coupling Studied during Two-Year Continuous Culture in INS-1E Insulinoma Cells. Endocrinology 2004, 145, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Kiely, A.; McClenaghan, N.H.; Flatt, P.R.; Newsholme, P. Pro-inflammatory cytokines increase glucose, alanine and triacylglycerol utilization but inhibit insulin secretion in a clonal pancreatic β-cell line. J. Endocrinol. 2007, 195, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Pottekat, A.; Lee, K.; Raghunathan, M.; Loguercio, S.; Mir, S.A.; Paton, A.W.; Paton, J.C.; Arvan, P.; Kaufman, R.J.; et al. Inflammatory Cytokines Rewire the Proinsulin Interaction Network in Human Islets. J. Clin. Endocrinol. Metab. 2022, 107, 3100–3110. [Google Scholar] [CrossRef]
- Masini, M.; Martino, L.; Marselli, L.; Bugliani, M.; Boggi, U.; Filipponi, F.; Marchetti, P.; De Tata, V. Ultrastructural alterations of pancreatic beta cells in human diabetes mellitus. Diabetes Metab. Res. Rev. 2017, 33, e2894. [Google Scholar] [CrossRef]
- Marselli, L.; Piron, A.; Suleiman, M.; Colli, M.L.; Yi, X.; Khamis, A.; Carrat, G.R.; Rutter, G.A.; Bugliani, M.; Giusti, L.; et al. Persistent or Transient Human β Cell Dysfunction Induced by Metabolic Stress: Specific Signatures and Shared Gene Expression with Type 2 Diabetes. Cell Rep. 2020, 33, 108466. [Google Scholar] [CrossRef]
- Marhfour, I.; Lopez, X.M.; Lefkaditis, D.; Salmon, I.; Allagnat, F.; Richardson, S.J.; Morgan, N.G.; Eizirik, D.L. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia 2012, 55, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rodríguez, M.; Raurell-Vila, H.; Colli, M.L.; Alvelos, M.I.; Subirana-Granés, M.; Juan-Mateu, J.; Norris, R.; Turatsinze, J.V.; Nakayasu, E.S.; Webb-Robertson, B.J.M.; et al. The impact of proinflammatory cytokines on the β-cell regulatory landscape provides insights into the genetics of type 1 diabetes. Nat. Genet. 2019, 51, 1588–1595. [Google Scholar] [CrossRef]
- Boyer, C.K.; Bauchle, C.J.; Zhang, J.; Wang, Y.; Stephens, S.B. Synchronized proinsulin trafficking reveals delayed Golgi export accompanies β-cell secretory dysfunction in rodent models of hyperglycemia. Sci. Rep. 2023, 13, 5218. [Google Scholar] [CrossRef]
- Sanchez Caballero, L.; Gorgogietas, V.; Arroyo, M.N.; Igoillo-Esteve, M. Molecular mechanisms of β-cell dysfunction and death in monogenic forms of diabetes. Int. Rev. Cell Mol. Biol. 2021, 359, 139–256. [Google Scholar] [CrossRef] [PubMed]
- Ciregia, F.; Bugliani, M.; Ronci, M.; Giusti, L.; Boldrini, C.; Mazzoni, M.R.; Mossuto, S.; Grano, F.; Cnop, M.; Marselli, L.; et al. Palmitate-induced lipotoxicity alters acetylation of multiple proteins in clonal β cells and human pancreatic islets. Sci. Rep. 2017, 7, 13445. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliese, L.A.; De Lorenzi, V.; Tesi, M.; Marchetti, P.; Cardarelli, F. Optical Nanoscopy of Cytokine-Induced Structural Alterations of the Endoplasmic Reticulum and Golgi Apparatus in Insulin-Secreting Cells. Int. J. Mol. Sci. 2024, 25, 10391. https://doi.org/10.3390/ijms251910391
Pugliese LA, De Lorenzi V, Tesi M, Marchetti P, Cardarelli F. Optical Nanoscopy of Cytokine-Induced Structural Alterations of the Endoplasmic Reticulum and Golgi Apparatus in Insulin-Secreting Cells. International Journal of Molecular Sciences. 2024; 25(19):10391. https://doi.org/10.3390/ijms251910391
Chicago/Turabian StylePugliese, Licia Anna, Valentina De Lorenzi, Marta Tesi, Piero Marchetti, and Francesco Cardarelli. 2024. "Optical Nanoscopy of Cytokine-Induced Structural Alterations of the Endoplasmic Reticulum and Golgi Apparatus in Insulin-Secreting Cells" International Journal of Molecular Sciences 25, no. 19: 10391. https://doi.org/10.3390/ijms251910391







