Metabolome and Transcriptome Joint Analysis Reveals That Different Sucrose Levels Regulate the Production of Flavonoids and Stilbenes in Grape Callus Culture
Abstract
:1. Introduction
2. Results
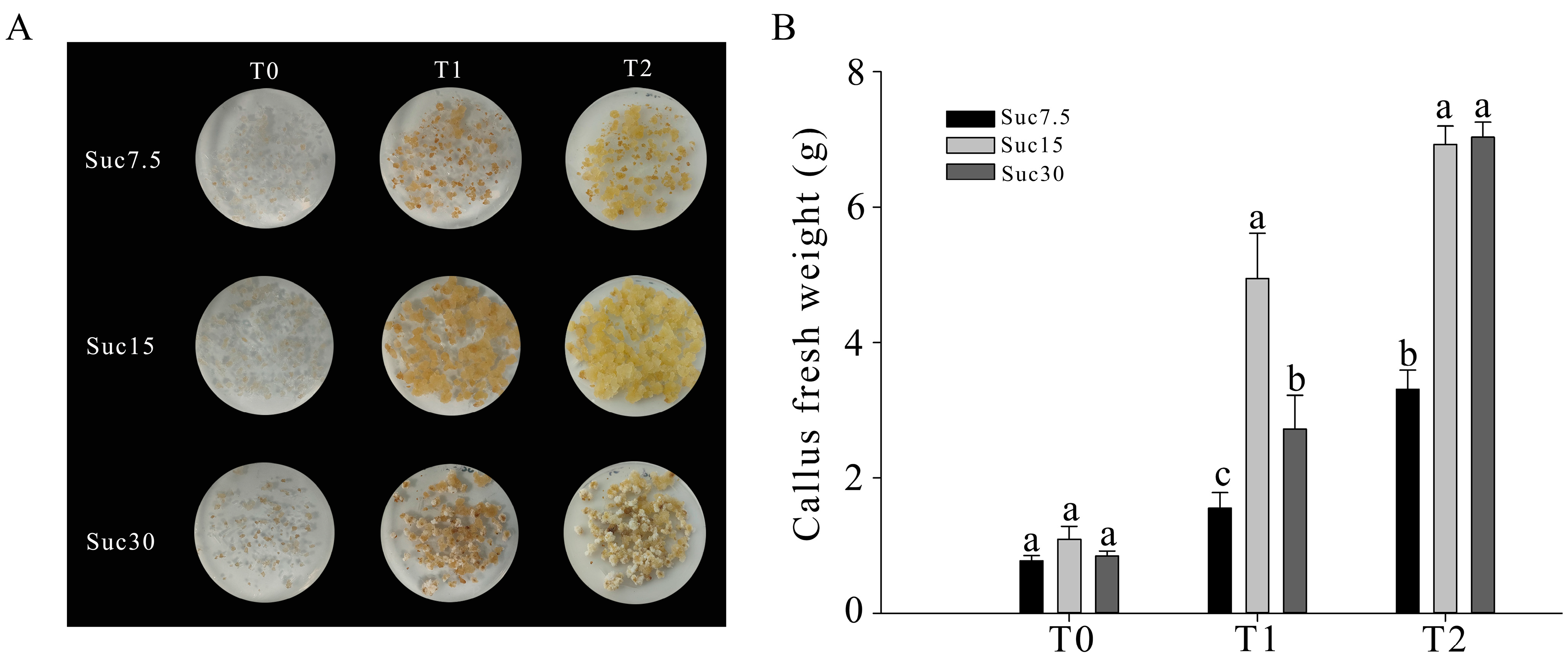
2.1. Phenotype and Proliferation Rate of Grape Callus under Different Sucrose Concentrations and Cultivation Times
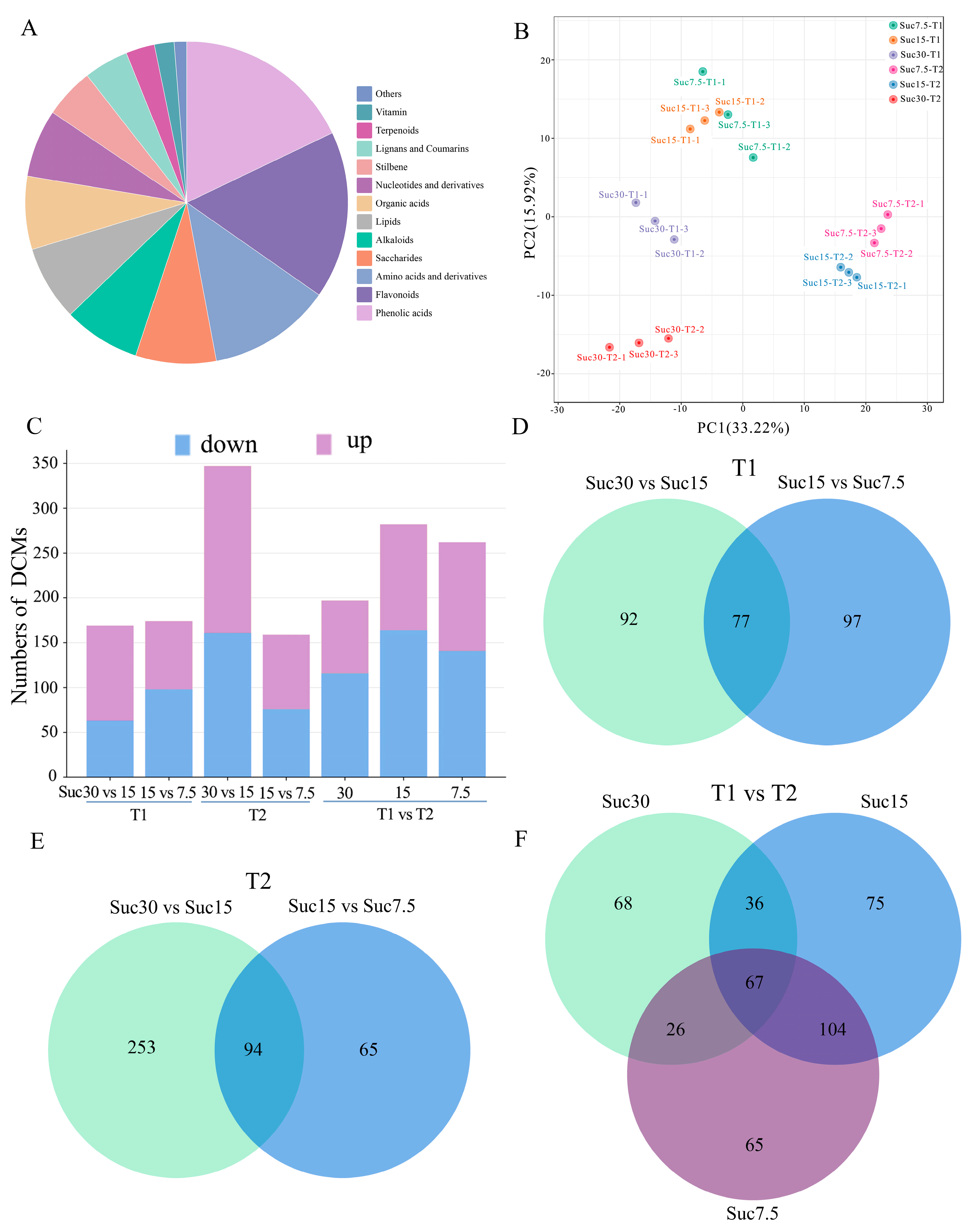
2.2. Identification of Metabolites with Differential Contents
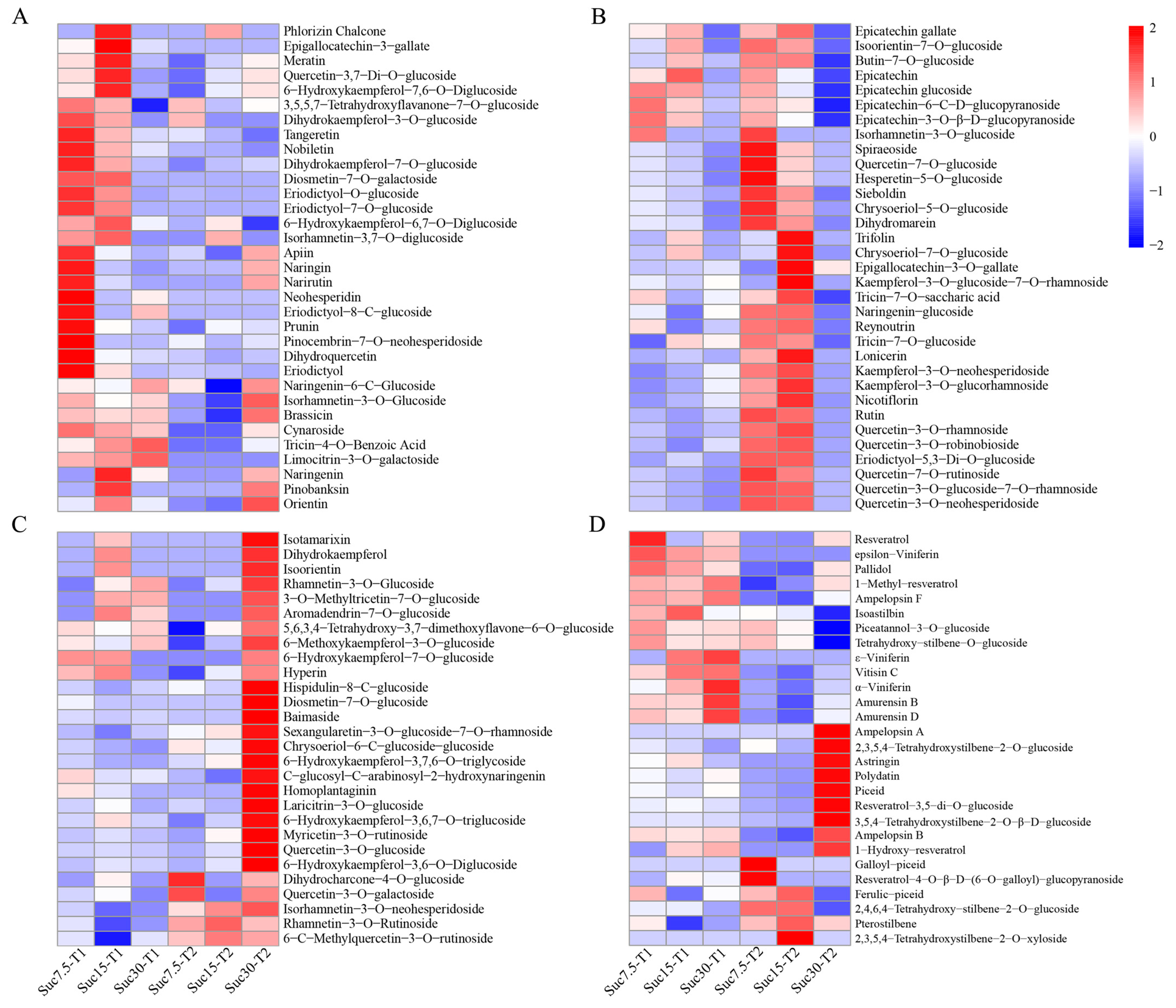
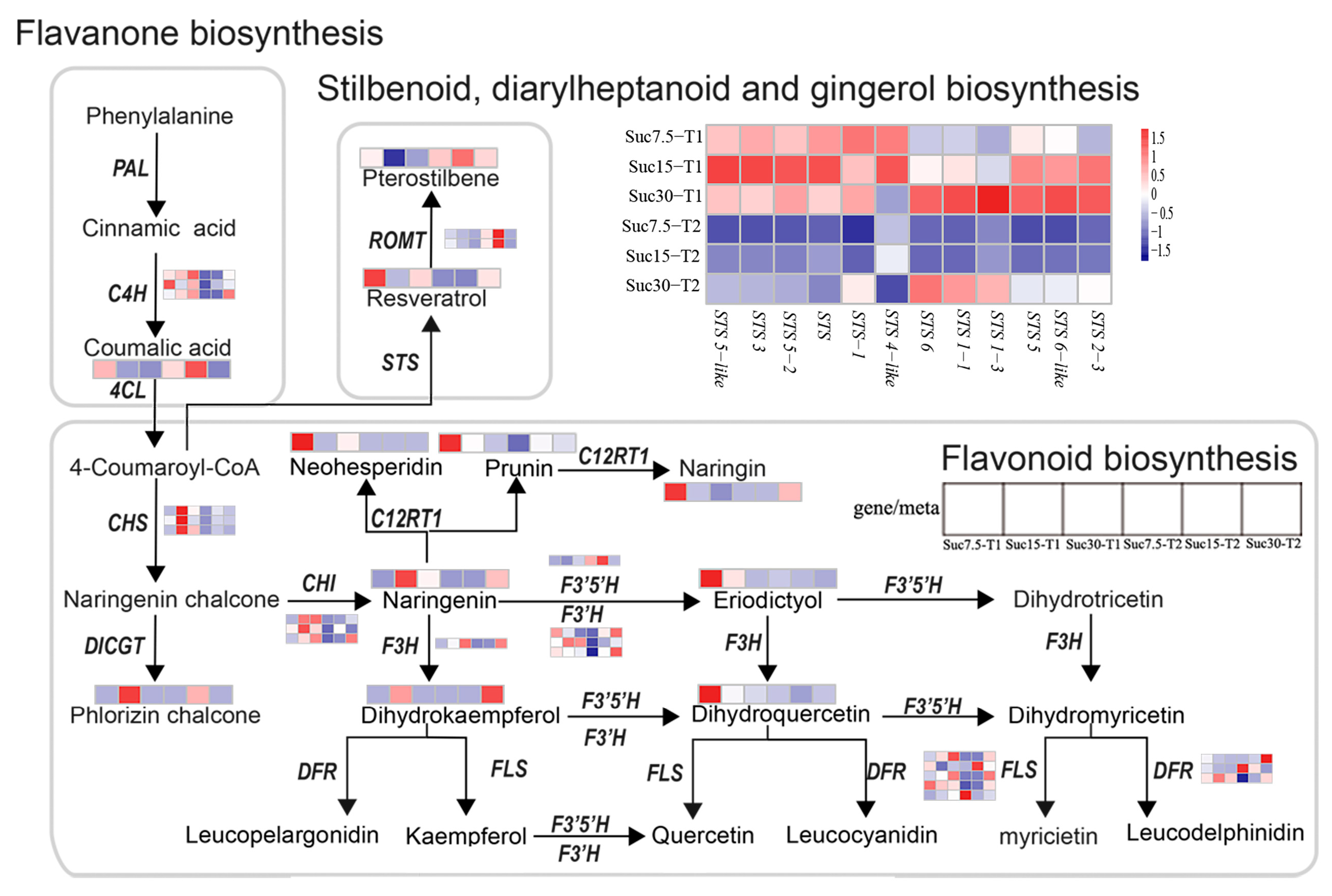
2.3. Differential Accumulation of Flavonoids and Stilbenes in Grape Callus
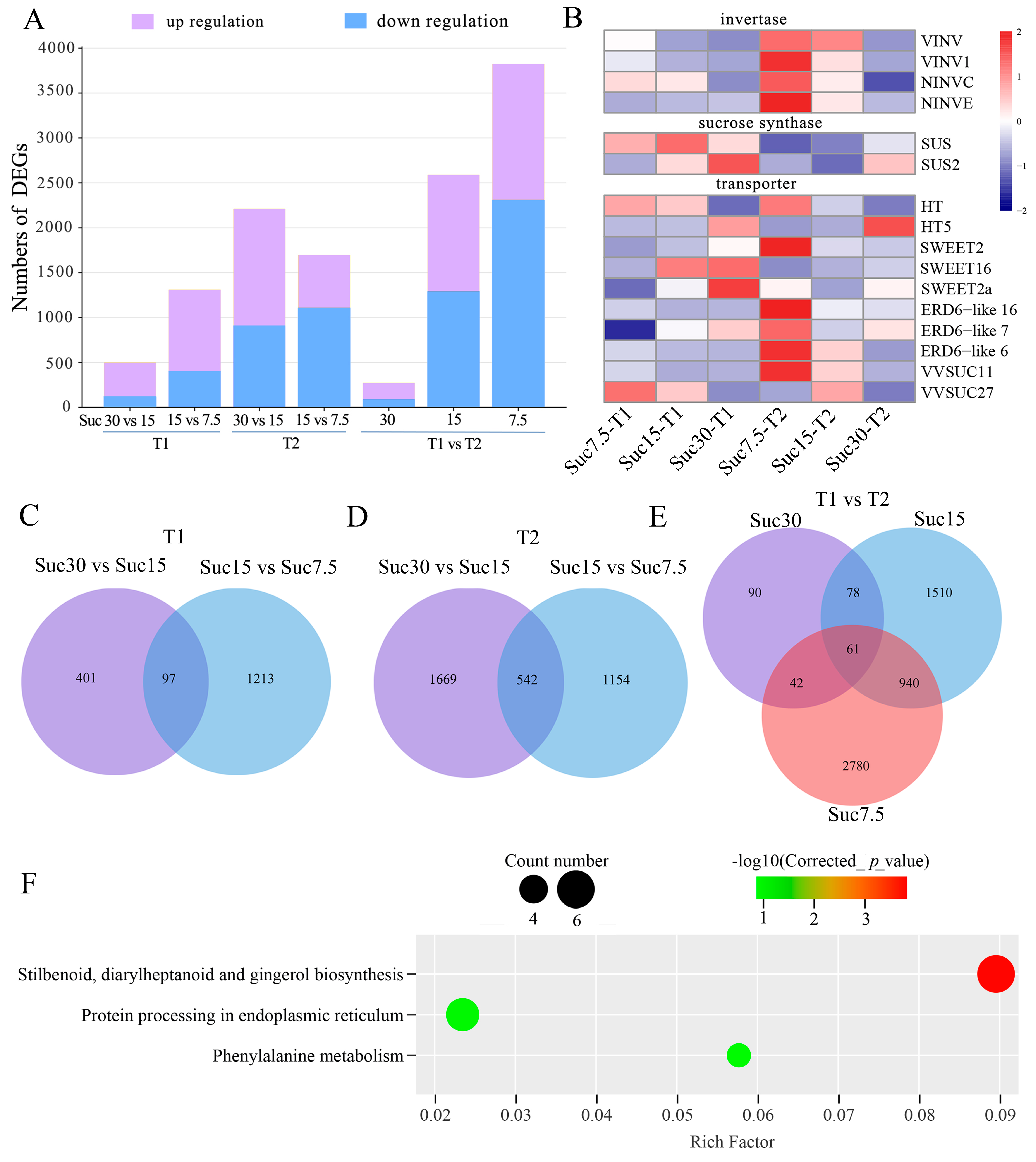
2.4. Identification of DEGs and Enrichment Analysis
2.5. DEGs in the Sucrose Metabolism Pathway
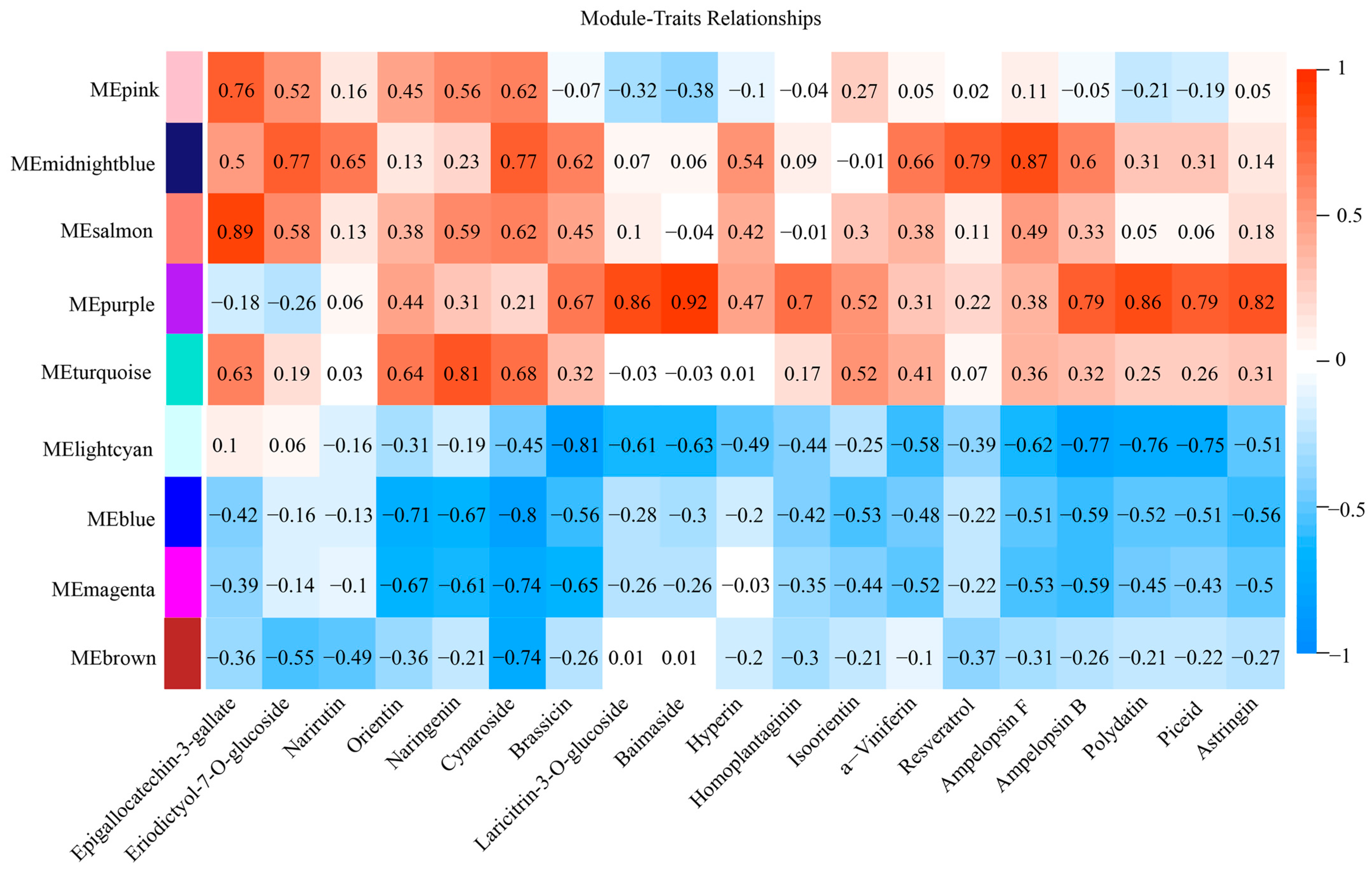
2.6. Metabolism and Gene Co-Expression Networks in Flavonoid and Stilbene Biosynthesis
2.7. Correlation Analysis between Metabolomic and Transcriptomic Results
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Metabolite Profiling
4.3. Transcriptome Analysis
4.4. Association Analysis of Metabolome and Transcriptome Data
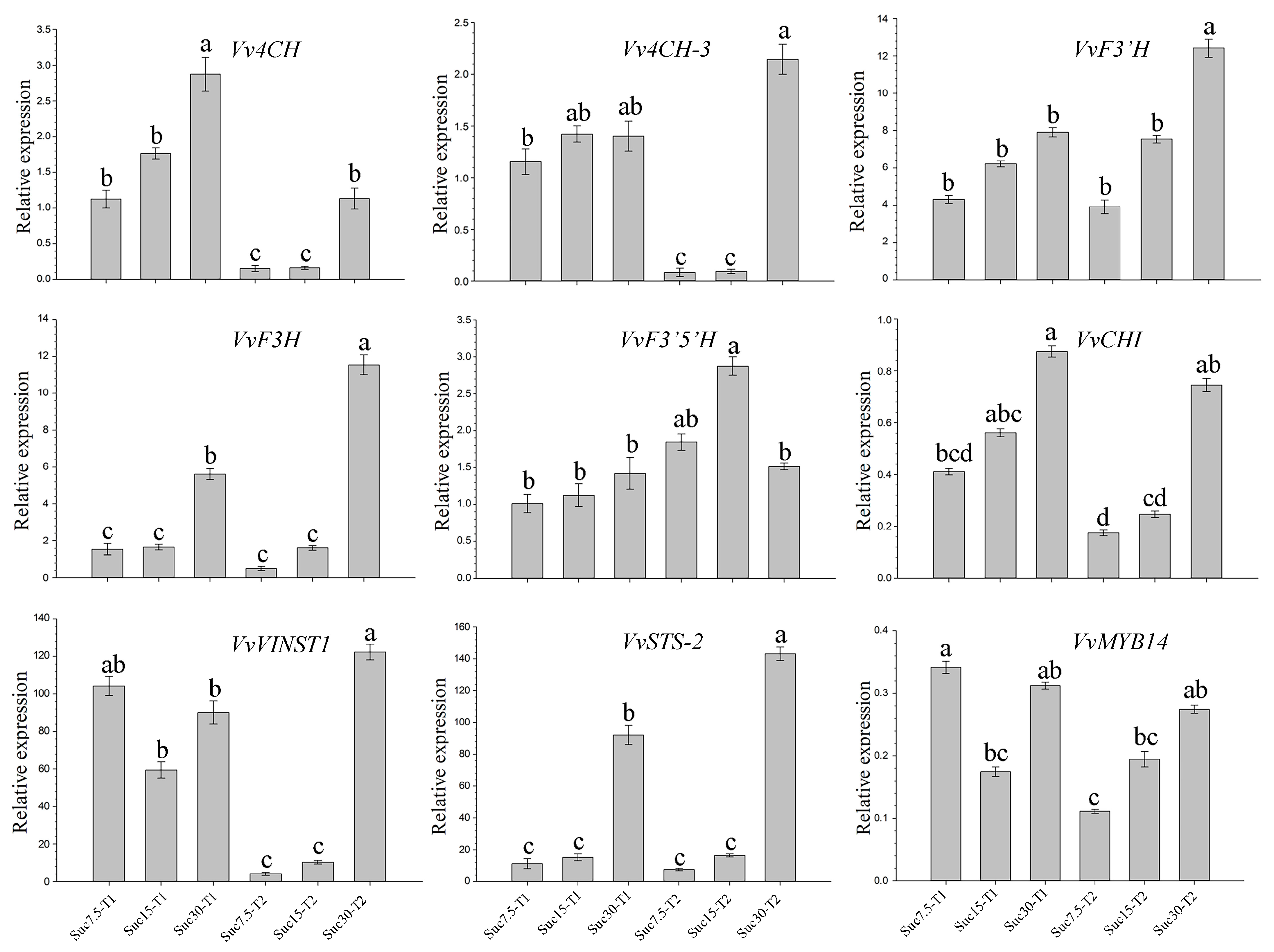
4.5. qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clifford, M.N. Anthocyanins—Nature, Occurrence and Dietary Burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; TomÁS-BarberÁN, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in Food and Their Health Benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant Properties of Resveratrol: A Structure–Activity Insight. Innov. Food Sci. Emerg. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an Anti-Cancer Agent: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Brinke, A.S.; Janssens-Böcker, C.; Kerscher, M. Skin anti-aging benefits of a 2% resveratrol emulsion. J. Cosmet. Dermatol. Sci. Appl. 2021, 11, 155–168. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Kallscheuer, N.; Vogt, M.; Marienhagen, J. A Novel Synthetic Pathway Enables Microbial Production of Polyphenols Independent from the Endogenous Aromatic Amino Acid Metabolism. ACS Synth. Biol. 2017, 6, 410–415. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Höll, J.; Vannozzi, A.; Czemmel, S.; Rausch, T.; Lucchin, M.; Boss, P.K.; Dry, I.B.; Bogs, J. The R2R3-MYB Transcription Factors MYB14 and MYB15 Regulate Stilbene Biosynthesis in Vitis vinifera. Plant Cell. 2013, 25, 4135–4149. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and Roles of Stilbenes in Plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of Stilbene Biosynthesis in Plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef] [PubMed]
- Orallo, F. Comparative Studies of the Antioxidant Effects of Cis- and Trans- Resveratrol. Curr. Med. Chem. 2006, 13, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Vannozzi, A.; Dry, I.B.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-Wide Analysis of the Grapevine Stilbene Synthase Multigenic Family: Genomic Organization and Expression Profiles upon Biotic and Abiotic Stresses. BMC Plant Biol. 2012, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Shi, N. Metabolomics of Vitis davidii Foëx. Grapes from Southern China: Flavonoids and Volatiles Reveal the Flavor Profiles of Five Spine Grape Varieties. Food Chem. 2024, 454, 139732. [Google Scholar] [CrossRef]
- Donnez, D.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of Resveratrol and Stilbene Derivatives by Plant Cells and Microorganisms. Trends Biotechnol. 2009, 27, 706–713. [Google Scholar] [CrossRef]
- Hussain, S.; Fareed, S.; Ansari, S.; Rahman, A.; Ahmad, I.Z.; Saeed, M. Current Approaches toward Production of Secondary Plant Metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef]
- Mao, J. Different Exogenous Sugars Affect the Hormone Signal Pathway and Sugar Metabolism in “Red Globe” (Vitis vinifera L.) Plantlets Grown in Vitro as Shown by Transcriptomic Analysis. Planta 2017, 246, 537–552. [Google Scholar] [CrossRef]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-Specific Induction of the Anthocyanin Biosynthetic Pathway in Arabidopsis. Plant Physiol. 2006, 140, 637–646. [Google Scholar] [CrossRef]
- Teng, S.; Keurentjes, J.; Bentsink, L.; Koornneef, M.; Smeekens, S. Sucrose-Specific Induction of Anthocyanin Biosynthesis in Arabidopsis Requires the MYB75/PAP1 Gene. Plant Physiol. 2005, 139, 1840–1852. [Google Scholar] [CrossRef]
- Ferri, M.; Righetti, L.; Tassoni, A. Increasing Sucrose Concentrations Promote Phenylpropanoid Biosynthesis in Grapevine Cell Cultures. J. Plant Physiol. 2011, 168, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Barbulova, A.; Apone, F.; Colucci, G. Plant cell cultures as source of cosmetic active ingredients. Cosmetics 2014, 1, 94–104. [Google Scholar] [CrossRef]
- Georgiev, V. Mass Propagation of Plant Cells—An Emerging Technology Platform for Sustainable Production of Biopharmaceuticals. Biochem. Pharmacol. 2015, 4, e180. [Google Scholar] [CrossRef]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant Cell Culture as Emerging Technology for Production of Active Cosmetic Ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In Vitro Plant Tissue Culture: Means for Production of Biological Active Compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Jang, M.O.; Kim, I.S.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Cambial Meristematic Cells: A Platform for the Production of Plant Natural Products. New Biotechnol. 2015, 32, 581–587. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, J.; Zhu, J.; He, S.; Zhang, W.; Yu, R.; Zi, J.; Song, L.; Huang, X. Effects of β-Cyclodextrin and Methyl Jasmonate on the Production of Vindoline, Catharanthine, and Ajmalicine in Catharanthus Roseus Cambial Meristematic Cell Cultures. Appl. Microbiol. Biot. 2015, 99, 7035–7045. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.A.; Asaf, S.; Lee, I.-J.; Kim, K.-M. Modulation of Sugar and Nitrogen in Callus Induction Media Alter PAL Pathway, SA and Biomass Accumulation in Rice Callus. Plant Cell Tiss. Org. 2020, 143, 517–530. [Google Scholar] [CrossRef]
- Lee, K.; Seo, P.J. Arabidopsis TOR Signaling Is Essential for Sugar-Regulated Callus Formation. J. Integr. Plant Biol. 2017, 59, 742–746. [Google Scholar] [CrossRef]
- Perera, P.I.P.; Pathirana, R.; Vidhanaarchchi, V.R.M. Somatic Embryogenesis in Anther-Derived Fast-Growing Callus as a Long-Term Source for Doubled-Haploid Production of Coconut (Cocos nucifera L.). J. Natn. Sci. Found. Sri Lanka. 2021, 49, 39. [Google Scholar] [CrossRef]
- Htwe, N.N.; Maziah, M.; Ling, H.C.; Zaman, F.Q.; Zain, M. Responses of Some Selected Malaysian Rice Genotypes to Callus Induction under in Vitro Salt Stress. Afr. J. Biotechnol. 2011, 10, 350–362. [Google Scholar]
- Yildiz, M.; Önde, S.; Özgen, M. Sucrose Effects on Phenolic Concentration and Plant Regeneration from Sugarbeet Leaf and Petiole Explants. J. Sugar Beet Res. 2007, 44, 1–15. [Google Scholar] [CrossRef]
- Tognetti, J.A.; Pontis, H.G.; Martínez-Noël, G.M.A. Sucrose Signaling in Plants: A World yet to Be Explored. Plant Signal. Behav. 2013, 8, e23316. [Google Scholar] [CrossRef] [PubMed]
- Suan See, K.; Bhatt, A.; Lai Keng, C. Effect of Sucrose and Methyl Jasmonate on Biomass and Anthocyanin Production in Cell Suspension Culture of Melastoma malabathricum (Melastomaceae). Rev. Biol. Trop. 2011, 59, 597–606. [Google Scholar] [PubMed]
- Winson, K.W.S.; Chew, B.L.; Sathasivam, K.; Subramaniam, S. The Establishment of Callus and Cell Suspension Cultures of Hylocereus Costaricensis for the Production of Betalain Pigments with Antioxidant Potential. Ind. Crop. Prod. 2020, 155, 112750. [Google Scholar] [CrossRef]
- Cavallini, E.; Matus, J.T.; Finezzo, L.; Zenoni, S.; Loyola, R.; Guzzo, F.; Schlechter, R.; Ageorges, A.; Arce-Johnson, P.; Tornielli, G.B. The phenylpropanoid pathway is controlled at different branches by a set of R2R3-MYB C2 repressors in grapevine. Plant Physiol. 2015, 167, 1448–1470. [Google Scholar] [CrossRef]
- Deluc, L.; Bogs, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.-M.; Robinson, S.P. The Transcription Factor VvMYB5b Contributes to the Regulation of Anthocyanin and Proanthocyanidin Biosynthesis in Developing Grape Berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar] [CrossRef]
- Deluc, L.; Barrieu, F.; Marchive, C.; Lauvergeat, V.; Decendit, A.; Richard, T.; Carde, J.-P.; Mérillon, J.-M.; Hamdi, S. Characterization of a Grapevine R2R3-MYB Transcription Factor That Regulates the Phenylpropanoid Pathway. Plant Physiol. 2006, 140, 499–511. [Google Scholar] [CrossRef]
- Mu, H.; Li, Y.; Yuan, L.; Jiang, J.; Wei, Y.; Duan, W.; Fan, P.; Li, S.; Liang, Z.; Wang, L. MYB30 and MYB14 Form a Repressor–Activator Module with WRKY8 That Controls Stilbene Biosynthesis in Grapevine. Plant Cell. 2023, 35, 552–573. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Zhuge, Y.; Zhang, M.; Liu, C.; Jia, H.; Fang, J. Integrative Analyses of Metabolomes and Transcriptomes Provide Insights into Flavonoid Variation in Grape Berries. J. Agric. Food Chem. 2021, 69, 12354–12367. [Google Scholar] [CrossRef]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent Advances in the Transcriptional Regulation of the Flavonoid Biosynthetic Pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Osbourn, A.; Ma, P. MYB Transcription Factors as Regulators of Phenylpropanoid Metabolism in Plants. Mol. Plant. 2015, 8, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Czemmel, S.; Heppel, S.C.; Bogs, J. R2R3 MYB Transcription Factors: Key Regulators of the Flavonoid Biosynthetic Pathway in Grapevine. Protoplasma 2012, 249, S109–S118. [Google Scholar] [CrossRef]
- Terrier, N.; Torregrosa, L.; Ageorges, A.; Vialet, S.; Verriès, C.; Cheynier, V.; Romieu, C. Ectopic Expression of VvMybPA2 Promotes Proanthocyanidin Biosynthesis in Grapevine and Suggests Additional Targets in the Pathway. Plant Physiol. 2009, 149, 1028–1041. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, C.; Liu, W.; Wang, Y. The WRKY53 Transcription Factor Enhances Stilbene Synthesis and Disease Resistance by Interacting with MYB14 and MYB15 in Chinese Wild Grape. J. Exp. Bot. 2020, 71, 3211–3226. [Google Scholar] [CrossRef]
- Li Yan; Wang Jianhua; Wang Ketao; Lyu Shiheng; Ren Liying; Huang Chunying; Pei Dong; Xing Yulin; Wang Yige; Xu Yifan; et al. Comparison Analysis of Widely-Targeted Metabolomics Revealed the Variation of Potential Astringent Ingredients and Their Dynamic Accumulation in the Seed Coats of Both Carya Cathayensis and Carya Illinoinensis. Food Chem. 2022, 374, 131688. [CrossRef]
- Wang, Z.; Song, M.; Wang, Z.; Chen, S.; Ma, H. Metabolome and Transcriptome Analysis of Flavor Components and Flavonoid Biosynthesis in Fig Female Flower Tissues (Ficus carica L.) after Bagging. BMC Plant Biol. 2021, 21, 396. [Google Scholar] [CrossRef]
- Gambino, G.; Perrone, I.; Gribaudo, I. A Rapid and Effective Method for RNA Extraction from Different Tissues of Grapevine and Other Woody Plants. Phytochem. Anal. 2008, 19, 520–525. [Google Scholar] [CrossRef]
- Wang, R.; Shu, P.; Zhang, C.; Zhang, J.; Chen, Y.; Zhang, Y.; Du, K.; Xie, Y.; Li, M.; Ma, T.; et al. Integrative Analyses of Metabolome and Genome-wide Transcriptome Reveal the Regulatory Network Governing Flavor Formation in Kiwifruit (Actinidia chinensis). New Phytol. 2022, 233, 373–389. [Google Scholar] [CrossRef]
| Compounds | Suc7.5-T1 | Suc15-T1 | Suc30-T1 | Suc7.5-T2 | Suc15-T2 | Suc30-T2 |
|---|---|---|---|---|---|---|
| Flavonoids | ||||||
| Epigallocatechin-3-gallate | 9.72 × 104 | 3.97 × 105 | 4.91 × 104 | ND | ND | ND |
| Eriodictyol-7-O-glucoside | 1.57× 105 | 1.14 × 105 | ND | ND | ND | ND |
| Narirutin | 1.03 × 105 | 1.75 × 104 | ND | ND | ND | 5.94 × 104 |
| Orientin | 1.92 × 104 | 4.28 × 104 | 1.94 × 104 | 4.40 × 103 | ND | 5.01 × 104 |
| Naringenin | ND | 3.85 × 104 | 1.37 × 104 | ND | ND | 2.15 × 104 |
| Cynaroside | 4.20 × 105 | 3.47 × 105 | 3.01 × 105 | ND | ND | 2.65 × 105 |
| Brassicin | 3.11 × 105 | 2.84 × 105 | 3.04 × 105 | 1.28 × 105 | ND | 4.04 × 105 |
| Laricitrin-3-O-glucoside | 9.94 × 105 | 1.15 × 106 | 8.90 × 105 | 1.02 × 106 | 9.28 × 105 | 1.94 × 106 |
| Baimaside | 2.19 × 105 | 2.00 × 105 | 2.14 × 105 | 1.91 × 105 | 1.94 × 105 | 6.43 × 105 |
| Hyperin | 8.37 × 105 | 9.22 × 105 | 5.44 × 105 | 4.31 × 105 | 6.92 × 105 | 9.18 × 105 |
| Homoplantaginin | 9.10 × 104 | 4.37 × 104 | ND | ND | ND | 2.77 × 105 |
| Isoorientin | ND | 2.60 × 104 | ND | ND | ND | 3.93 × 104 |
| Stilbenes | ||||||
| α-Viniferin | 1.24 × 106 | 1.85 × 106 | 2.87 × 106 | 6.13 × 105 | 2.27 × 105 | 9.20 × 105 |
| Resveratrol | 2.32 × 107 | 4.22 × 106 | 1.21 × 107 | 1.26 × 106 | 9.28 × 105 | 1.12 × 107 |
| Ampelopsin F | 3.55 × 105 | 3.27 × 105 | 4.02 × 105 | 6.67 × 104 | 2.73 × 104 | 2.23 × 105 |
| Ampelopsin B | 1.05 × 107 | 1.02 × 107 | 1.07 × 107 | 4.16 × 106 | 2.59 × 106 | 1.59 × 107 |
| Polydatin | 4.08 × 106 | 3.21 × 106 | 4.40 × 106 | 1.89 × 106 | 1.63 × 106 | 1.01 × 107 |
| Piceid | 2.57 × 107 | 2.15 × 107 | 2.71 × 107 | 1.54 × 107 | 1.28 × 107 | 5.67 × 107 |
| Astringin | 1.94 × 104 | 2.68 × 104 | 7.76 × 103 | ND | ND | 6.60 × 104 |
| Gene ID | Name | kTotal | kWithin |
|---|---|---|---|
| LOC100258294 | STS 1-like | 194.23 | 47.26 |
| VINST1 | STS-1 | 375.33 | 43.10 |
| LOC100246283 | STS 5-1 | 143.34 | 41.90 |
| LOC100855299 | STS 2-1 | 244.78 | 41.59 |
| LOC100242903 | STS 4-1 | 150.72 | 40.32 |
| LOC100261661 | STS 1-2 | 418.83 | 40.13 |
| LOC100245181 | STS 5 | 551.28 | 36.40 |
| LOC100853590 | STS 4-like-3 | 135.31 | 36.17 |
| LOC100853745 | STS 4-like-5 | 208.68 | 35.54 |
| STS | STS | 259.87 | 35.04 |
| LOC100241164 | STS 6-like | 495.22 | 32.88 |
| LOC100853820 | STS 4-like-6 | 126.42 | 32.82 |
| LOC100266562 | STS 2-4 | 200.09 | 31.27 |
| LOC109121404 | STS 4-like-7 | 131.97 | 30.98 |
| LOC100240862 | STS 2-2 | 155.77 | 30.54 |
| LOC100853675 | STS 4 | 194.21 | 30.42 |
| LOC100233012 | PAL G1 | 335.58 | 42.80 |
| LOC100855356 | PAL-1 | 225.87 | 42.47 |
| LOC104881994 | PAL1-like | 256.86 | 41.46 |
| LOC100266593 | PAL-2 | 318.58 | 39.04 |
| LOC100240904 | PAL-like-1 | 244.57 | 36.32 |
| LOC100256293 | PAL-like-2 | 268.50 | 32.90 |
| LOC100254698 | 4CL-2 | 249.92 | 39.96 |
| Gene ID | Name | kTotal | kWithin | Module Colors |
|---|---|---|---|---|
| LOC100256680 | bHLH30 | 210.06 | 32.46 | midnight blue |
| MYB14 | MYB14 | 569.75 | 32.46 | midnight blue |
| LOC100240970 | ERF3 | 237.17 | 28.97 | midnight blue |
| LOC100267475 | EFM | 511.43 | 26.98 | midnight blue |
| LOC100251862 | WRKY22 | 390.74 | 48.26 | salmon |
| LOC100243880 | MYB36 | 270.73 | 47.09 | salmon |
| LOC100254518 | MYB15 | 378.42 | 45.66 | salmon |
| LOC100256922 | WRKY53 | 273.87 | 45.06 | salmon |
| LOC100256868 | WRKY44 | 377.95 | 43.76 | salmon |
| LOC100266459 | NAC100 | 685.91 | 196.90 | purple |
| LOC104882333 | ORR26 | 369.73 | 184.61 | purple |
| LOC100251455 | bHLH87 | 711.39 | 178.60 | purple |
| LOC100245739 | ZFP3 | 722.76 | 221.87 | magenta |
| LOC100245013 | TCP15 | 966.92 | 221.44 | magenta |
| LOC100257488 | BEL1-like protein 3 | 1816.60 | 865.65 | blue |
| LOC100264680 | bZIP9 | 1764.46 | 857.84 | blue |
| LOC100249486 | E2FA | 1581.44 | 623.29 | turquoise |
| LOC100261443 | bHLH96 | 1223.52 | 581.65 | turquoise |
| LOC100241701 | E2FE | 809.73 | 295.98 | pink |
| LOC100261001 | MYB3R-4 | 752.70 | 277.57 | pink |
| LOC100250157 | AGL65 | 1158.16 | 525.52 | brown |
| WRKY20 | WRKY20 | 1192.95 | 508.63 | brown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Fan, Z.; Wang, Y.; He, J.; Zheng, C.; Ma, H. Metabolome and Transcriptome Joint Analysis Reveals That Different Sucrose Levels Regulate the Production of Flavonoids and Stilbenes in Grape Callus Culture. Int. J. Mol. Sci. 2024, 25, 10398. https://doi.org/10.3390/ijms251910398
Gu X, Fan Z, Wang Y, He J, Zheng C, Ma H. Metabolome and Transcriptome Joint Analysis Reveals That Different Sucrose Levels Regulate the Production of Flavonoids and Stilbenes in Grape Callus Culture. International Journal of Molecular Sciences. 2024; 25(19):10398. https://doi.org/10.3390/ijms251910398
Chicago/Turabian StyleGu, Xiaojiao, Zhiyi Fan, Yuan Wang, Jiajun He, Chuanlin Zheng, and Huiqin Ma. 2024. "Metabolome and Transcriptome Joint Analysis Reveals That Different Sucrose Levels Regulate the Production of Flavonoids and Stilbenes in Grape Callus Culture" International Journal of Molecular Sciences 25, no. 19: 10398. https://doi.org/10.3390/ijms251910398






