Variation in Ants’ Chemical Recognition Signals across Vineyard Agroecosystems
Abstract
:1. Introduction
2. Results
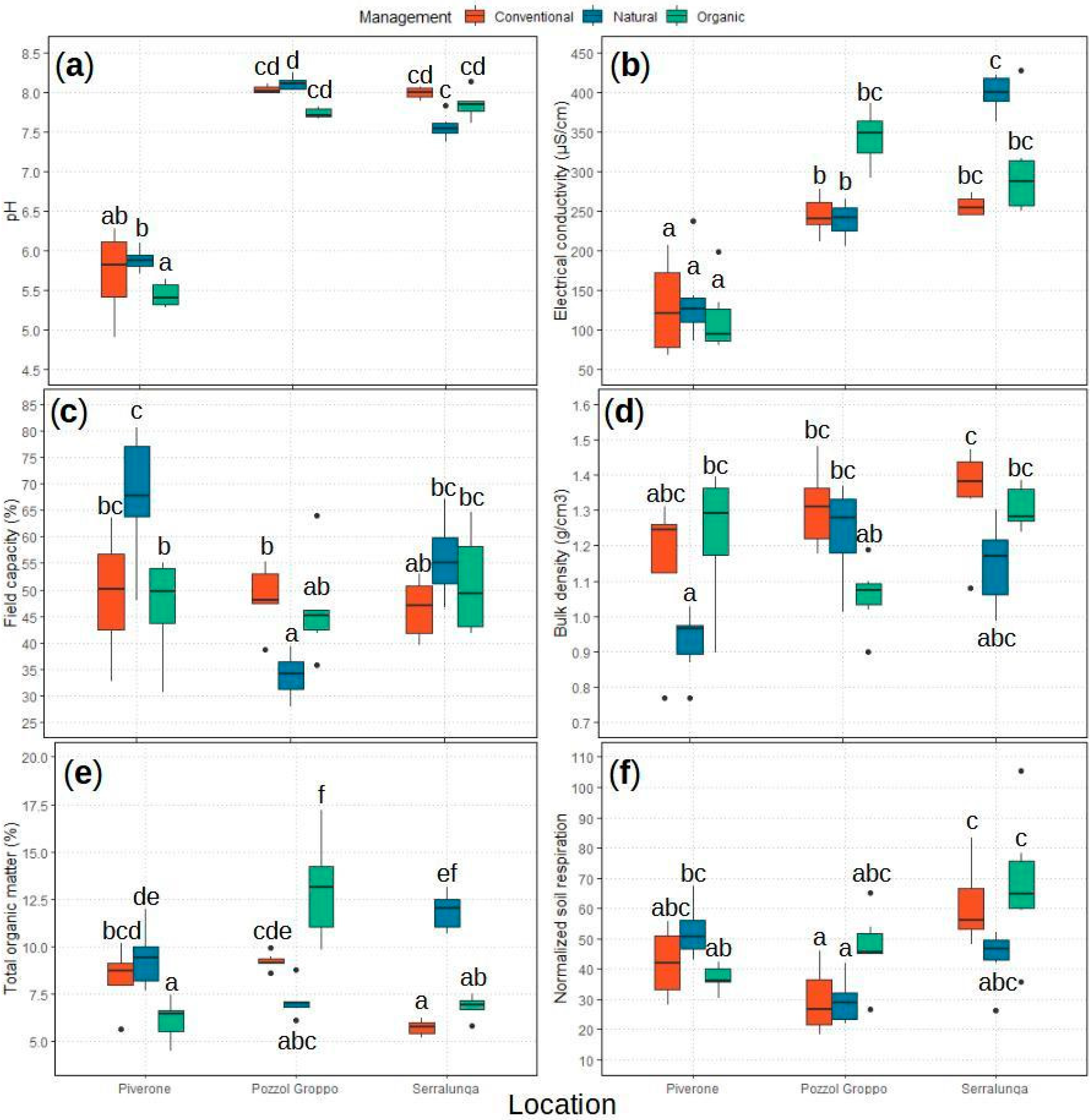
2.1. Soil Sampling Results
2.1.1. Soil’s Main Chemical and Physical Properties
2.1.2. Soil Respiration: bSR and nSR
2.2. Lasius Paralienus Cuticular Hydrocarbon Profile Description and Comparisons
2.2.1. Univariate Analyses on the CHC Profile Variation
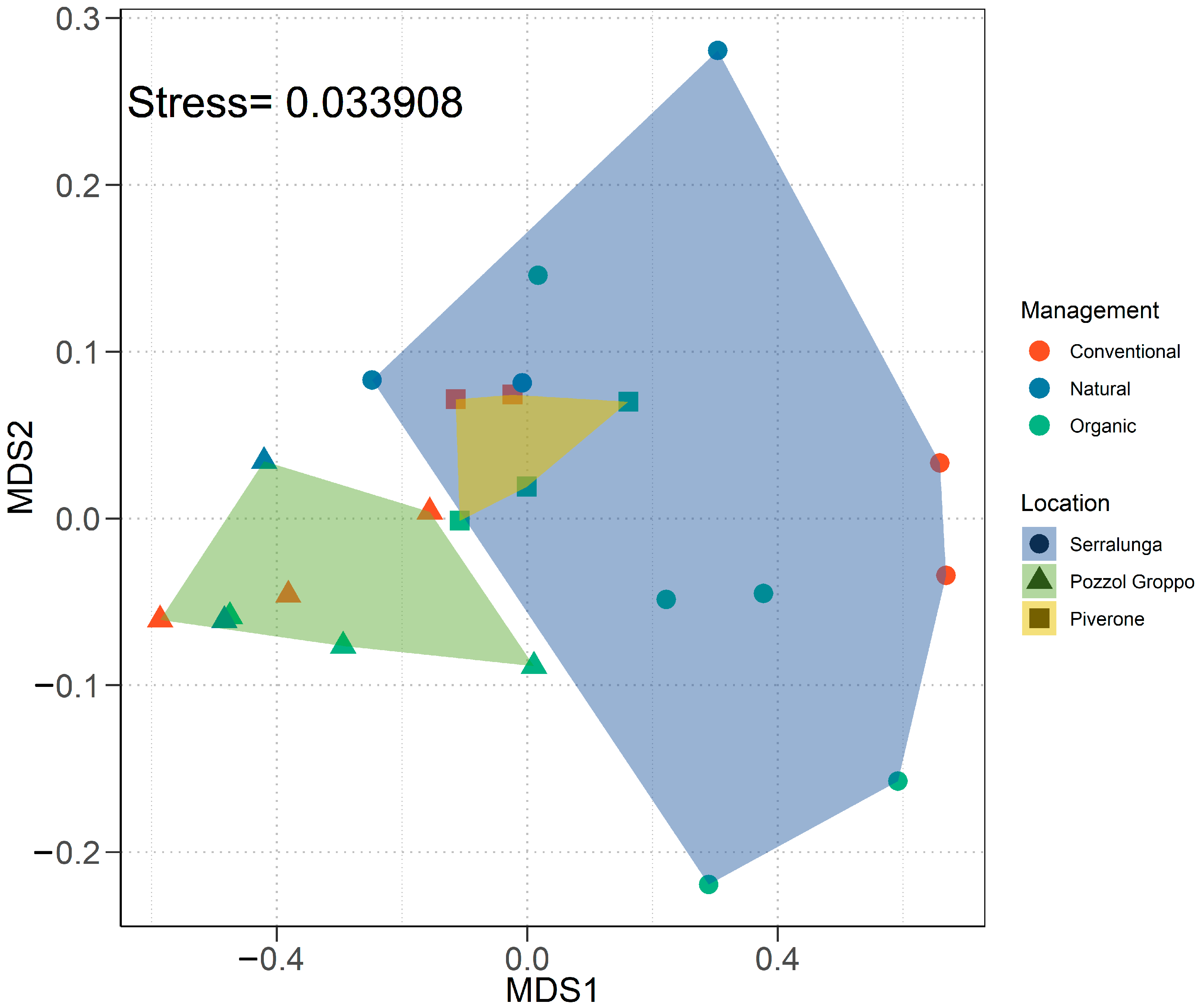
2.2.2. Multivariate Analyses on the CHC Profile
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Chemical Analyses
4.3. Soil Samples Analyses
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, E.O. The sociogenesis of insect colonies. Science 1985, 228, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.M. The organization of work in social insect colonies. Nature 1996, 380, 121–124. [Google Scholar] [CrossRef]
- Casacci, L.P.; Bonelli, S.; Balletto, E.; Barbero, F. Multimodal signaling in myrmecophilous butterflies. Front. Ecol. Evol. 2019, 7, 454. [Google Scholar] [CrossRef]
- Lenoir, A.; D’Ettorre, P.; Errard, C.; Hefetz, A. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 2001, 46, 573–599. [Google Scholar] [CrossRef]
- Hölldobler, B. Multimodal signals in ant communication. J. Comp. Physiol. 1999, 184, 129–141. [Google Scholar] [CrossRef]
- Vander Meer, R.K.; Morel, L. Nestmate recognition in ants. In Pheromone Communication in Social Insects; CRC Press: Boca Raton, FL, USA, 2019; pp. 79–103. [Google Scholar]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef]
- Hepper, P.G. Kin recognition: Functions and mechanisms a review. Biol. Rev. 1986, 61, 63–93. [Google Scholar] [CrossRef]
- van Zweden, J.S.; d’Ettorre, P. Nestmate recognition in social insects and the role of hydrocarbons. In Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Blomquist, G.J., Bagnères, A.-G., Eds.; Cambridge University Press: Cambridge, UK, 2010; Volume 11, pp. 222–243. [Google Scholar]
- Sturgis, S.J.; Gordon, D.M. Nestmate recognition in ants (Hymenoptera: Formicidae): A review. Myrmecol. News 2012, 16, 101–110. [Google Scholar]
- Barbero, F. Cuticular lipids as a cross-talk among ants, plants and butterflies. Int. J. Mol. Sci. 2016, 17, 1966. [Google Scholar] [CrossRef]
- d’Ettorre, P.; Lenoir, A. Nestmate recognition in ants. In Ant Ecology; Lach, L., Parr, C., Abbott, K., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 194–209. [Google Scholar]
- Drijfhout, F.P.; Kather, R.; Martin, S.J. The role of cuticular hydrocarbons in insects. Behav. Chem. Ecol. 2009, 3, 91–114. [Google Scholar]
- Martin, S.; Drijfhout, F. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 2009, 35, 1151–1161. [Google Scholar] [CrossRef]
- Greene, M.J.; Gordon, D.M. Structural complexity of chemical recognition cues affects the perception of group membership in the ants Linephithema humile and Aphaenogaster cockerelli. J. Exp. Biol. 2007, 210, 897–905. [Google Scholar] [CrossRef]
- Ozaki, M.; Wada-Katsumata, A.; Fujikawa, K.; Iwasaki, M.; Yokohari, F.; Satoji, Y.; Nishimura, T.; Yamaoka, R. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 2005, 309, 311–314. [Google Scholar] [CrossRef]
- van Zweden, J.S.; Dreier, S.; d’Ettorre, P. Disentangling environmental and heritable nestmate recognition cues in a carpenter ant. J. Insect Physiol. 2009, 55, 159–164. [Google Scholar] [CrossRef]
- Obin, M.S.; Vander Meer, R.K. Mechanism of template-label matching in fire ant, Solenopsis Invicta buren, nestmate recognition. Anim. Behav. 1989, 38, 430–435. [Google Scholar] [CrossRef]
- Stuart, R.J. Differences in aggression among sympatric, facultatively polygynous Leptothorax ant species. Anim. Behav. 1993, 46, 809–812. [Google Scholar] [CrossRef]
- Menzel, F.; Schmitt, T.; Blüthgen, N. Intraspecific nestmate recognition in two parabiotic ant species: Acquired recognition cues and low inter-colony discrimination. Insectes Sociaux 2009, 56, 251–260. [Google Scholar] [CrossRef]
- Sprenger, P.P.; Menzel, F. Cuticular hydrocarbons in ants (Hymenoptera: Formicidae) and other insects: How and why they differ among individuals, colonies, and species. Myrmecol. News 2020, 30, 1–26. [Google Scholar] [CrossRef]
- Boulay, R.; Katzav-Gozansky, T.; Hefetz, A.; Lenoir, A. Odour convergence and tolerance between nestmates through trophallaxis and grooming in the ant Camponotus fellah (Dalla Torre). Insectes Sociaux 2004, 51, 55–61. [Google Scholar] [CrossRef]
- Lenoir, A.; Hefetz, A.; Simon, T.; Soroker, V. Comparative dynamics of gestalt odour formation in two ant species Camponotus fellah and Aphaenogaster senilis (Hymenoptera: Formicidae). Physiol. Entomol. 2001, 26, 275–283. [Google Scholar] [CrossRef]
- Lockey, K.H. Insect hydrocarbon classes: Implications for chemotaxonomy. Insect Biochem. 1991, 21, 91–97. [Google Scholar] [CrossRef]
- Kather, R.; Martin, S.J. Evolution of cuticular hydrocarbons in the Hymenoptera: A meta-analysis. J. Chem. Ecol. 2015, 41, 871–883. [Google Scholar] [CrossRef]
- Khidr, S.K.; Linforth, R.S.T.; Hardy, I.C.W. Genetic and environmental influences on the cuticular hydrocarbon profiles of Goniozus wasps. Entomol. Exp. Appl. 2013, 147, 175–185. [Google Scholar] [CrossRef]
- Lenoir, A.; Cuvillier-Hot, V.; Devers, S.; Christidès, J.-P.; Montigny, F. Ant cuticles: A trap for atmospheric phthalate contaminants. Sci. Total Environ. 2012, 441, 209–212. [Google Scholar] [CrossRef]
- Viles, H.A.; Goudie, A.S.; Goudie, A.M. Ants as geomorphological agents: A global assessment. Earth-Sci. Rev. 2021, 213, 103469. [Google Scholar] [CrossRef]
- Mazed, M.K.; Afroz, M.; Rahman, M.M. Global decline of insects: A review from agricultural perspective. Agric. Rev. 2022, 43, 334–340. [Google Scholar] [CrossRef]
- Sanford, M.P.; Manley, P.N.; Murphy, D.D. Effects of urban development on ant communities: Implications for ecosystem services and management. Conserv. Biol. 2009, 23, 131–141. [Google Scholar] [CrossRef]
- Folgarait, P.J. Ant biodiversity and its relationship to ecosystem functioning: A review. Biodivers. Conserv. 1998, 7, 1221–1244. [Google Scholar] [CrossRef]
- Philpott, S.M.; Armbrecht, I. Biodiversity in tropical agroforests and the ecological role of ants and ant diversity in predatory function. Ecol. Entomol. 2006, 31, 369–377. [Google Scholar] [CrossRef]
- Menzel, F.; Zumbusch, M.; Feldmeyer, B. How ants acclimate: Impact of climatic conditions on the cuticular hydrocarbon profile. Funct. Ecol. 2018, 32, 657–666. [Google Scholar] [CrossRef]
- Duarte, B.F.; Michelutti, K.B.; Antonialli-Junior, W.F.; Cardoso, C.A. Effect of temperature on survival and cuticular composition of three different ant species. J. Therm. Biol. 2019, 80, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, P.P.; Burkert, L.H.; Abou, B.; Federle, W.; Menzel, F. Coping with the climate: Cuticular hydrocarbon acclimation of ants under constant and fluctuating conditions. J. Exp. Biol. 2018, 221, jeb171488. [Google Scholar] [CrossRef] [PubMed]
- Sguarizi-Antonio, D.; Batista, N.R.; Michelutti, K.B.; Soares, E.R.P.; Solórzano, J.C.J.; Cardoso, C.A.L.; Lima-Júnior, S.E.; Torres, V.d.O.; Antonialli-Júnior, W.F. Anthropic action affects the cuticular chemical profile of social wasps. Papéis Avulsos Zool. 2022, 62, e202262013. [Google Scholar] [CrossRef]
- Seifert, B. A Taxonomic revision of the Palaearctic members of the subgenus Lasius s.str. (Hymenoptera, Formicidae). Soil Org. 2020, 92, 15–86. [Google Scholar] [CrossRef]
- Kruger, E.L.; Pappas, C.D. Geographic variation of cuticular hydrocarbons among fourteen populations of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 1993, 30, 544–548. [Google Scholar] [CrossRef]
- Nowbahari, E.; Lenoir, A.; Clément, J.L.; Lange, C.; Bagneres, A.G.; Joulie, C. Individual, geographical and experimental variation of cuticular hydrocarbons of the ant Cataglyphis cursor (Hymenoptera: Formicidae): Their use in nest and subspecies recognition. Biochem. Syst. Ecol. 1990, 18, 63–73. [Google Scholar] [CrossRef]
- Lampkin, N.; Foster, C.; Padel, S. The Policy and Regulatory Environment for Organic Farming in Europe: Country Reports; Universität Hohenheim: Stuttgart, Germany, 1999. [Google Scholar]
- Helms IV, J.A.; Smith, J.; Clark, S.; Knupp, K.; Haddad, N.M. Ant communities and ecosystem services in organic versus conventional agriculture in the US corn belt. Environ. Entomol. 2021, 50, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Mone, S.; Kusha, K.M.; Jathanna, D.; Ali, M.; Goel, A. Comparison of insect biodiversity between organic and conventional plantations in Kodagu, Karnataka, India. J. Threat. Taxa 2014, 6, 6186–6194. [Google Scholar] [CrossRef]
- Masoni, A.; Frizzi, F.; Brühl, C.; Zocchi, N.; Palchetti, E.; Chelazzi, G.; Santini, G. Management matters: A comparison of ant assemblages in organic and conventional vineyards. Agric. Ecosyst. Environ. 2017, 246, 175–183. [Google Scholar] [CrossRef]
- Csata, E.; Timuş, N.; Witek, M.; Casacci, L.P.; Lucas, C.; Bagnères, A.-G.; Sztencel-Jabłonka, A.; Barbero, F.; Bonelli, S.; Rákosy, L.; et al. Lock-picks: Fungal infection facilitates the intrusion of strangers into ant colonies. Sci. Rep. 2017, 7, 46323. [Google Scholar] [CrossRef]
- Casacci, L.P.; Schönrogge, K.; Thomas, J.A.; Balletto, E.; Bonelli, S.; Barbero, F. Host specificity pattern and chemical deception in a social parasite of ants. Sci. Rep. 2019, 9, 1619. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, A.; Depickère, S.; Devers, S.; Christidès, J.-P.; Detrain, C. Hydrocarbons in the ant Lasius niger: From the cuticle to the nest and home range marking. J. Chem. Ecol. 2009, 35, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Villalta, I.; Rami, L.; Alvarez-Blanco, P.; Angulo, E.; Cerda, X.; Boulay, R. Environmental and genetic constraints on cuticular hydrocarbon composition and nestmate recognition in ants. Anim. Behav. 2020, 159, 105–119. [Google Scholar] [CrossRef]
- Nielsen, J.; Boomsma, J.J.; Oldham, N.J.; Petersen, H.C.; Morgan, E.D. Colony-level and season-specific variation in cuticular hydrocarbon profiles of individual workers in the ant Formica truncorum. Insectes Sociaux 1999, 46, 58–65. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, P.; Angmo, P.; Satpute, S. Total and labile pools of organic carbon in relation to soil biological properties under contrasting land-use systems in a dry mountainous region. Carbon Manag. 2022, 13, 352–371. [Google Scholar] [CrossRef]
- Anderson, J.P.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C.; Aguirre, A.J. Straw management effects on CO2 efflux and C storage in different Mediterranean agricultural soils. Sci. Total Environ. 2013, 465, 233–239. [Google Scholar] [CrossRef]
- Di Salvo, M.; Calcagnile, M.; Talà, A.; Tredici, S.M.; Maffei, M.E.; Schönrogge, K.; Barbero, F.; Alifano, P. The microbiome of the Maculinea-Myrmica host-parasite interaction. Sci. Rep. 2019, 9, 8048. [Google Scholar] [CrossRef]
- Teseo, S.; van Zweden, J.S.; Pontieri, L.; Kooij, P.W.; Sørensen, S.J.; Wenseleers, T.; Poulsen, M.; Boomsma, J.J.; Sapountzis, P. The scent of symbiosis: Gut bacteria may affect social interactions in leaf-cutting ants. Anim. Behav. 2019, 150, 239–254. [Google Scholar] [CrossRef]
- Dosmann, A.; Bahet, N.; Gordon, D.M. Experimental modulation of external microbiome affects nestmate recognition in harvester ants (Pogonomyrmex barbatus). PeerJ 2016, 4, e1566. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M.; Gunapala, N.; Graham, K.J. Determinants of Soil Microbial Communities: Effects of Agricultural Management, Season, and Soil Type on Phospholipid Fatty Acid Profiles. Microb. Ecol. 1998, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bos, N.; Grinsted, L.; Holman, L. Wax on, wax off: Nest soil facilitates indirect transfer of recognition cues between ant nestmates. PLoS ONE 2011, 6, e19435. [Google Scholar] [CrossRef] [PubMed]
- Frouz, J.; Holec, M.; Kalčík, J. The effect of Lasius niger (Hymenoptera, Formicidae) ant nest on selected soil chemical properties. Pedobiologia 2003, 47, 205–212. [Google Scholar] [CrossRef]
- Holec, M.; Frouz, J. The effect of two ant species Lasius niger and Lasius flavus on soil properties in two contrasting habitats. Eur. J. Soil Biol. 2006, 42, S213–S217. [Google Scholar] [CrossRef]
- Boulton, A.M.; Jaffee, B.A.; Scow, K.M. Effects of a Common Harvester Ant (Messor Andrei) on Richness and Abundance of Soil Biota. Appl. Soil Ecol. 2003, 23, 257–265. [Google Scholar] [CrossRef]
- Lindström, S.; Timonen, S.; Sundström, L.; Johansson, H. Ants Reign over a Distinct Microbiome in Forest Soil. Soil Biol. Biochem. 2019, 139, 107529. [Google Scholar] [CrossRef]
- Travanty, N.V.; Vargo, E.L.; Apperson, C.S.; Ponnusamy, L. Colonization by the Red Imported Fire Ant, Solenopsis Invicta, Modifies Soil Bacterial Communities. Microb. Ecol. 2022, 84, 240–256. [Google Scholar] [CrossRef]
- Botella-Cruz, M.; Pallarés, S.; Millán, A.; Velasco, J. Role of cuticle hydrocarbons composition in the salinity tolerance of aquatic beetles. J. Insect Physiol. 2019, 117, 103899. [Google Scholar] [CrossRef]
- Barbosa, R.; Braga, M.; Blomquist, G.; Queiroz, M. Cuticular hydrocarbon profiles as a chemotaxonomic tool for three blowfly species (Diptera: Calliphoridae) of forensic interest. J. Nat. Hist. 2017, 51, 1491–1498. [Google Scholar] [CrossRef]
- Srour, H.A. Desiccation intolerance of the red palm weevil, Rhynchophorus ferrugineus (oliv) adults in relation to their cuticular hydrocarbons. Egypt. Acad. J. Biol. Sci. A Entomol. 2009, 2, 47–53. [Google Scholar]
- Menzel, F.; Morsbach, S.; Martens, J.H.; Räder, P.; Hadjaje, S.; Poizat, M.; Abou, B. Communication versus waterproofing: The physics of insect cuticular hydrocarbons. J. Exp. Biol. 2019, 222, jeb210807. [Google Scholar] [CrossRef]
- Brooks, L.; Brunelli, M.; Pattison, P.; Jones, G.; Fitch, A. Crystal structures of eight mono-methyl alkanes (C26–C32) via single-crystal and powder diffraction and DFT-D optimization. IUCrJ 2015, 2, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.; Pomonis, J.G. Physical properties of insect cuticular hydrocarbons: The effects of chain length, methyl-branching and unsaturation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 243–249. [Google Scholar] [CrossRef]
- Gibbs, A.G.; Rajpurohit, S. Cuticular lipids and water balance. In Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Blomquist, G.J., Bagnères, A.-G., Eds.; Cambridge University Press: Cambridge, UK, 2010; Volume 11, pp. 100–120. [Google Scholar]
- Buellesbach, J.; Whyte, B.A.; Cash, E.; Gibson, J.D.; Scheckel, K.J.; Sandidge, R.; Tsutsui, N.D. Desiccation resistance and micro-climate adaptation: Cuticular hydrocarbon signatures of different argentine ant supercolonies across California. J. Chem. Ecol. 2018, 44, 1101–1114. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Liu, D.; Pei, X.; Khoso, A.G. Rapid changes in composition and contents of cuticular hydrocarbons in Sitobion avenae (Hemiptera: Aphididae) clones adapting to desiccation stress. J. Econ. Entomol. 2022, 115, 508–518. [Google Scholar] [CrossRef]
- Cordonnier, M.; Kaufmann, B.; Simon, L.; Escarguel, G.; Mondy, N. Discrimination of conspecifics from heterospecifics in a hybrid zone: Behavioral and chemical cues in ants. Insect Sci. 2022, 29, 276–288. [Google Scholar] [CrossRef]
- Bos, N.; Dreier, S.; Jørgensen, C.G.; Nielsen, J.; Guerrieri, F.J.; d’Ettorre, P. Learning and perceptual similarity among cuticular hydrocarbons in ants. J. Insect Physiol. 2012, 58, 138–146. [Google Scholar] [CrossRef]
- Wang, Z.; Receveur, J.P.; Pu, J.; Cong, H.; Richards, C.; Liang, M.; Chung, H. Desiccation resistance differences in Drosophila species can be largely explained by variations in cuticular hydrocarbons. eLife 2022, 11, e80859. [Google Scholar] [CrossRef]
- Lorenzi, M.C.; Sledge, M.F.; Laiolo, P.; Sturlini, E.; Turillazzi, S. Cuticular hydrocarbon dynamics in young adult Polistes dominulus (Hymenoptera: Vespidae) and the role of linear hydrocarbons in nestmate recognition systems. J. Insect Physiol. 2004, 50, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Dani, F.R.; Jones, G.R.; Destri, S.; Spencer, S.H.; Turillazzi, S. Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Anim. Behav. 2001, 62, 165–171. [Google Scholar] [CrossRef]
- Nooten, S.; Schultheiss, P.; Rowe, R.C.; Facey, S.L.; Cook, J.M. Habitat complexity affects functional traits and diversity of ant assemblages in urban green spaces (Hymenoptera: Formicidae). Myrmecol. News 2019, 29, 67–77. [Google Scholar] [CrossRef]
- Blanco-Moure, N.; Gracia Ballarín, R.; Bielsa Aced, A.; López Sánchez, M.V. Long-term effect of no-tillage on soil organic matter fractions in rainfed Aragon (NE Spain). Span. J. Soil Sci. 2011, 1, 1–10. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Part 2. Chemical and microbiological properties. In Methods of Soil Analysis, 2nd ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Alfaro-Leranoz, A.; Badia-Villas, D.; Marti-Dalmau, C.; Emran, M.; Conte-Dominguez, A.P.; Ortiz-Perpiña, O. Long-term evolution of shrub prescribed burning effects on topsoil organic matter and biological activity in the Central Pyrenees (NE-Spain). Sci. Total Environ. 2023, 888, 163994. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C. Effect of simulated fire on organic matter and selected microbiological properties of two contrasting soils. Arid Land Res. Manag. 2003, 17, 55–69. [Google Scholar] [CrossRef]


| Profile Features | Linear Alkane | Linear Alkene | Methylated Linear Alkane | |||
|---|---|---|---|---|---|---|
| Monomethyl- Alkanes | Dimethyl- Alkanes | Trimethyl- Alkanes | Methylated Linear Alkane Total | |||
| CHC number | 5 | 1 | 45 | 69 | 15 | 129 |
| CHC compound class (%) | 3.70 | 0.74 | 33.34 | 51.11 | 11.11 | 95.56 |
| Number of peaks | 4 1 | 0 2 | 11 3 | 17 3 | 9 3 | 43 1,2,3 |
| Peak compound class (%) | 8.16 | 0 | 22.45 | 34.69 | 18.37 | 87.76 |
| Peak Correspondence | |||||
|---|---|---|---|---|---|
| n | Peak Identified Compounds | n | Peak Identified Compounds | n | Peak Identified Compounds |
| 1 | n-C28 | 18 | 5,15-, 5,13-, 5,9-diMeC31 | 35 | 5,9,15-, 5,7,15-triMeC33 |
| 2 | n-C29 | 19 | 7,11,15-triMeC31 | 36 | 17-, 16-, 15-, 14-, 13-, 12-MeC34 |
| 3 | 15-, 13-, 11-MeC29 | 20 | 5,9,15-triMeC31+ 5,7,15-, 5,7,13-, 5,7,11-triMeC31 | 37 | 8,16-diMeC34 |
| 4 | 3-MeC29 | 21 | 16-, 15-, 14-, 13-, 12-, 11-, 10-MeC32 | 38 | 8,12,16-triMeC34 |
| 5 | 5,15-, 5,13-, 5,9-diMeC29 | 22 | 9-MeC32+8,16-, 8,14-, 8,12-diMeC32 | 39 | 6,16-, 6,14-, 6,12-diMeC34 |
| 6 | n-C30 | 23 | 6-MeC32 | 40 | 4,16-, 4,14-, 4,12-diMeC34 |
| 7 | 5,9,11-triMeC29 | 24 | 4-MeC32+8,12,15-triMeC32+8,16, 8,14-, 8,12-diMeC32 | 41 | 17-, 15-, 13-MeC35 |
| 8 | 15-, 14-, 13-, 12-, 11-MeC30+9,12-diMeC30 | 25 | 6,16-, 6,14-, 6,12-, 6,10-diMeC32+5,15-, 5,13-diMeC32 | 42 | 13,21-diMeC35 |
| 9 | 4-MeC30+7,14-diMeC30 | 26 | 4,16-, 4,14-, 4,12, 4,10-, 4,8-diMeC32 | 43 | 7,27-, 7,25-, 7,23-, 7,21-, 7,19-, 7,17-diMeC35 |
| 10 | X-C31:1+5,15-diMeC30+6,12-diMeC30 | 27 | n-C33+6,10,16-triMeC32 | 44 | 5,19-, 5,17-, 5,15-, 5,13, 5,9-diMeC35 |
| 11 | 4,14-, 4,12-, 4,10-, 4,8-diMeC30 | 28 | 4,8,16-triMeC32 | 45 | 5,9,15-triMeC35 |
| 12 | n-C31 | 29 | 17-, 15-, 13-, 11-MeC33 | 46 | 8,18-diMeC36 |
| 13 | 4,8,14-triMeC30 | 30 | 7-MeC33 | 47 | 14,22-diMeC36+12,24-diMeC36 |
| 14 | 15-, 13-, 11-, 9-MeC31 | 31 | 5-MeC33+13,21-diMeC33+11,17-diMeC33+9,17-diMeC33 | 48 | 19-, 17-, 15-, 13-MeC37 |
| 15 | 5-MeC31 | 32 | 7,25-, 7,21-, 7,19-, 7,17-diMeC33 | 49 | 13,23-diMeC37 |
| 16 | 9,15-, 9,13-diMeC31 | 33 | 5,17-, 5,15-, 5,13-, 5,9-diMeC33 | ||
| 17 | 7,23-, 7-19-diMeC31+3-MeC31 | 34 | 7,11,15-triMeC33 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hais, A.; Casacci, L.P.; d’Ettorre, P.; Badía-Villas, D.; Leroy, C.; Barbero, F. Variation in Ants’ Chemical Recognition Signals across Vineyard Agroecosystems. Int. J. Mol. Sci. 2024, 25, 10407. https://doi.org/10.3390/ijms251910407
Hais A, Casacci LP, d’Ettorre P, Badía-Villas D, Leroy C, Barbero F. Variation in Ants’ Chemical Recognition Signals across Vineyard Agroecosystems. International Journal of Molecular Sciences. 2024; 25(19):10407. https://doi.org/10.3390/ijms251910407
Chicago/Turabian StyleHais, Arthur, Luca Pietro Casacci, Patrizia d’Ettorre, David Badía-Villas, Chloé Leroy, and Francesca Barbero. 2024. "Variation in Ants’ Chemical Recognition Signals across Vineyard Agroecosystems" International Journal of Molecular Sciences 25, no. 19: 10407. https://doi.org/10.3390/ijms251910407








