Periostin in Bronchiolitis Obliterans Syndrome after Lung Transplant
Abstract
:1. Introduction
2. Results
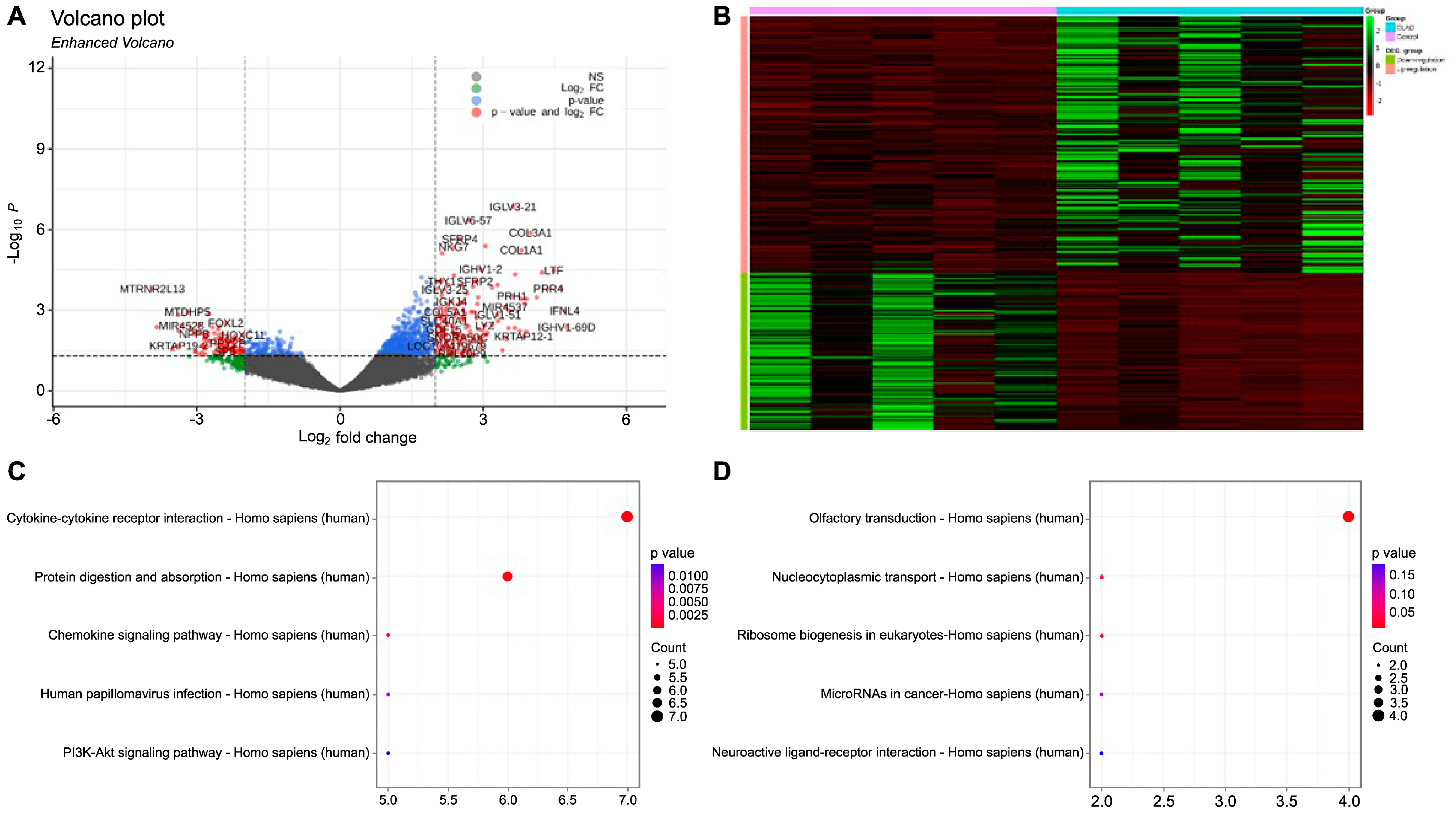
2.1. Gene Expression Profiling and Identification of Differentially Expressed Genes in BOS
2.2. Clinical Characteristics of the Validation Cohorts
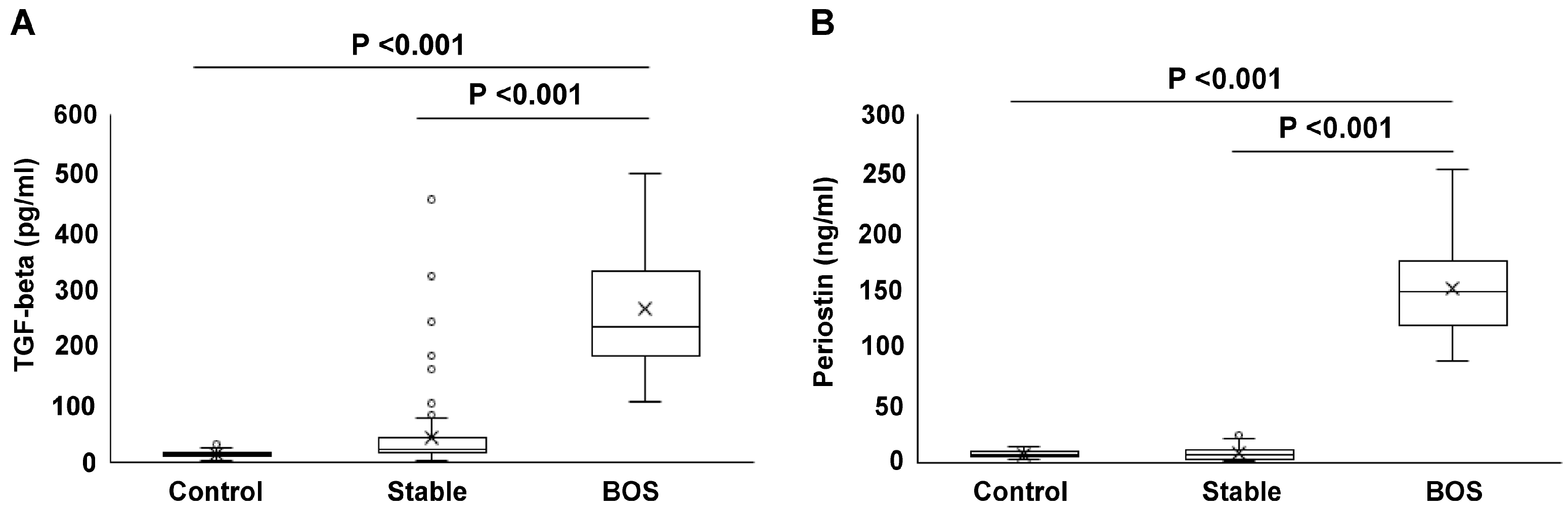
2.3. Serum Concentrations of TGF-Beta and Periostin
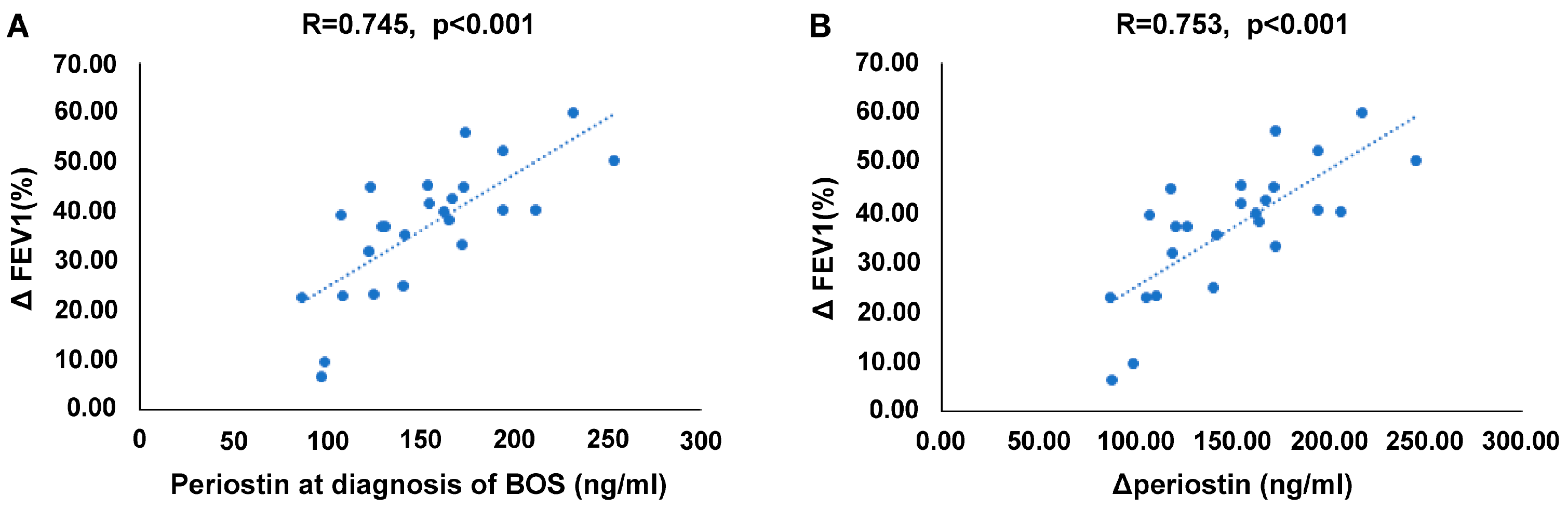
2.4. Correlation between Serum Periostin Levels and Reduction in Lung Function
2.5. Serum Periostin Levels as a Biomarker for BOS after Lung Transplantation
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment and Sample Collection for Differentially Expressed Gene Analysis
4.2. RNA Sequencing Library Construction and Sequencing
4.3. RNA-Seq Data Preprocessing and Analysis
4.4. Study Participants and Controls for Validation
4.5. Periostin and TGF-Beta Detection
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulkarni, H.S.; Cherikh, W.S.; Chambers, D.C.; Garcia, V.C.; Hachem, R.R.; Kreisel, D.; Puri, V.; Kozower, B.D.; Byers, D.E.; Witt, C.A.; et al. Bronchiolitis obliterans syndrome-free survival after lung transplantation: An International Society for Heart and Lung Transplantation Thoracic Transplant Registry analysis. J. Heart. Lung. Transpl. 2019, 38, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kim, Y.H.; Cho, W.H.; Kim, D.; So, M.W.; Son, B.S.; Yeo, H.J. Unique Changes in the Lung Microbiome following the Development of Chronic Lung Allograft Dysfunction. Microorganisms 2024, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Verleden, G.M.; Glanville, A.R.; Lease, E.D.; Fisher, A.J.; Calabrese, F.; Corris, P.A.; Ensor, C.R.; Gottlieb, J.; Hachem, R.R.; Lama, V.; et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J. Heart. Lung. Transpl. 2019, 38, 493–503. [Google Scholar] [CrossRef]
- Rahman, M.; Ravichandran, R.; Bansal, S.; Sanborn, K.; Bowen, S.; Eschbacher, J.; Sureshbabu, A.; Fleming, T.; Bharat, A.; Walia, R.; et al. Novel role for tumor suppressor gene, liver kinase B1, in epithelial-mesenchymal transition leading to chronic lung allograft dysfunction. Am. J. Transpl. 2022, 22, 843–852. [Google Scholar] [CrossRef]
- Hodge, S.; Holmes, M.; Banerjee, B.; Musk, M.; Kicic, A.; Waterer, G.; Reynolds, P.N.; Hodge, G.; Chambers, D.C. Posttrans-plant bronchiolitis obliterans syndrome is associated with bronchial epithelial to mesenchymal transition. Am. J. Transpl. 2009, 9, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Rout-Pitt, N.; Farrow, N.; Parsons, D.; Donnelley, M. Epithelial mesenchymal transition (EMT): A universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir. Res. 2018, 19, 136. [Google Scholar] [CrossRef]
- Willis, B.C.; duBois, R.M.; Borok, Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc. Am. Thorac. Soc. 2006, 3, 377–382. [Google Scholar] [CrossRef]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.; Kudo, A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life. Sci. 2014, 71, 1279–1288. [Google Scholar] [CrossRef]
- Krasilnikova, S.V.; Tush, E.V.; Frolov, P.A.; Ovsyannikov, D.Y.; Terentyeva, A.B.; Kubysheva, N.I.; Eliseeva, T.I. Periostin as a Biomarker of Allergic Inflammation in Atopic Bronchial Asthma and Allergic Rhinitis (a Pilot Study). Sovrem. Tekhnologii. Med. 2021, 12, 37–45. [Google Scholar] [CrossRef]
- Nejman-Gryz, P.; Górska, K.; Paplińska-Goryca, M.; Proboszcz, M.; Krenke, R. Periostin and Thymic Stromal Lymphopoietin-Potential Crosstalk in Obstructive Airway Diseases. J. Clin. Med. 2020, 9, 3667. [Google Scholar] [CrossRef]
- Nanishi, M.; Fujiogi, M.; Freishtat, R.J.; Hoptay, C.E.; Bauer, C.S.; Stevenson, M.D.; Camargo, C.A., Jr.; Hasegawa, K. Serum periostin among infants with severe bronchiolitis and risk of developing asthma: A prospective multicenter cohort study. Allergy 2022, 77, 2121–2130. [Google Scholar] [CrossRef]
- Hwang, J.H.; Yang, S.H.; Kim, Y.C.; Kim, J.H.; An, J.N.; Moon, K.C.; Oh, Y.K.; Park, J.Y.; Kim, D.K.; Kim, Y.S.; et al. Experimental Inhibition of Periostin Attenuates Kidney Fibrosis. Am. J. Nephrol. 2017, 46, 501–517. [Google Scholar] [CrossRef]
- Kumar, P.; Smith, T.; Raeman, R.; Chopyk, D.M.; Brink, H.; Liu, Y.; Sulchek, T.; Anania, F.A. Periostin promotes liver fi-brogenesis by activating lysyl oxidase in hepatic stellate cells. J. Biol. Chem. 2018, 293, 12781–12792. [Google Scholar] [CrossRef]
- Gil, H.; Goldshtein, M.; Etzion, S.; Elyagon, S.; Hadad, U.; Etzion, Y.; Cohen, S. Defining the timeline of periostin up-regulation in cardiac fibrosis following acute myocardial infarction in mice. Sci. Rep. 2022, 12, 21863. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Nygard, K.; Gan, B.S.; O'Gorman, D.B. Periostin induces fibroblast proliferation and myofibroblast persistence in hypertrophic scarring. Exp. Dermatol. 2015, 24, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Renaud-Picard, B.; Vallière, K.; Toussaint, J.; Kreutter, G.; El-Habhab, A.; Kassem, M.; El-Ghazouani, F.; Olland, A.; Hirschi, S.; Porzio, M.; et al. Epithelial-mesenchymal transition and membrane microparticles: Potential implications for bronchiolitis obliterans syndrome after lung transplantation. Transpl. Immunol. 2020, 59, 101273. [Google Scholar] [CrossRef]
- Salton, F.; Volpe, M.C.; Confalonieri, M. Epithelial-Mesenchymal Transition in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Medicina 2019, 55, 83. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Rosmark, O.; Åhrman, E.; Brunnström, H.; Wassilew, K.; Nybom, A.; Michaliková, B.; Larsson, H.; Eriksson, L.T.; Schultz, H.H.; et al. Protein Signatures of Remodeled Airways in Transplanted Lungs with Bronchiolitis Obliterans Syndrome Obtained Using Laser-Capture Microdissection. Am. J. Pathol. 2021, 191, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, M.; Shiraishi, H.; Ohta, S.; Suzuki, S.; Arima, K.; Aoki, S.; Toda, S.; Inagaki, N.; Kurihara, Y.; Hayashida, S.; et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J. Clin. Investig. 2012, 122, 2590–2600. [Google Scholar] [CrossRef] [PubMed]
- Kavvadas, P.; Dussaule, J.C.; Chatziantoniou, C. Searching novel diagnostic markers and targets for therapy of CKD. Kidney. Int. Suppl. 2014, 4, 53–57. [Google Scholar] [CrossRef]
- Greenland, N.Y.; Deiter, F.; Calabrese, D.R.; Hays, S.R.; Kukreja, J.; Leard, L.E.; Kolaitis, N.A.; Golden, J.A.; Singer, J.P.; Greenland, J.R. Inflammation on bronchoalveolar lavage cytology is associated with decreased chronic lung allograft dysfunction-free survival. Clin. Transpl. 2022, 36, e14639. [Google Scholar] [CrossRef] [PubMed]
- Dugger, D.T.; Fung, M.; Hays, S.R.; Singer, J.P.; Kleinhenz, M.E.; Leard, L.E.; Golden, J.A.; Shah, R.J.; Lee, J.S.; Deiter, F.; et al. Chronic lung allograft dysfunction small airways reveal a lymphocytic inflammation gene signature. Am. J. Transpl. 2021, 21, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.C.; Borok, Z. TGF-beta-induced EMT: Mechanisms and implications for fibrotic lung disease. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2007, 293, L525–L534. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Massagué, J. TGF-β in developmental and fibrogenic EMTs. Semin. Cancer. Biol. 2022, 86 Pt 2, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, J.E.; Adjei-Sowah, E.; Korcari, A.; Muscat, S.N.; Nichols, A.E.C.; Buckley, M.R.; Loiselle, A.E. Identification of Periostin as a critical niche for myofibroblast dynamics and fibrosis during tendon healing. bioRxiv 2023, 125, 59–72. [Google Scholar] [CrossRef]
- Ashley, S.L.; Wilke, C.A.; Kim, K.K.; Moore, B.B. Periostin regulates fibrocyte function to promote myofibroblast differentiation and lung fibrosis. Mucosal. Immunol. 2017, 10, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Nanri, Y.; Nunomura, S.; Terasaki, Y.; Yoshihara, T.; Hirano, Y.; Yokosaki, Y.; Yamaguchi, Y.; Feghali-Bostwick, C.; Ajito, K.; Murakami, S.; et al. Cross-Talk between Transforming Growth Factor-β and Periostin Can Be Targeted for Pulmonary Fibrosis. Am. J. Respir. Cell. Mol. Biol. 2020, 62, 204–216. [Google Scholar] [CrossRef]
- Tirunavalli, S.K.; Kuncha, M.; Sistla, R.; Andugulapati, S.B. Targeting TGF-β/periostin signaling by sesamol ameliorates pulmonary fibrosis and improves lung function and survival. J. Nutr. Biochem. 2023, 116, 109294. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Yuan, S.; Innes, A.L.; Kerr, S.; Woodruff, P.G.; Hou, L.; Muller, S.J.; Fahy, J.V. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc. Natl. Acad. Sci. USA. 2010, 107, 14170–14175. [Google Scholar] [CrossRef]
- Takayama, G.; Arima, K.; Kanaji, T.; Toda, S.; Tanaka, H.; Shoji, S.; McKenzie, A.N.; Nagai, H.; Hotokebuchi, T.; Izuhara, K. Periostin: A novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J. Allergy. Clin. Immunol. 2006, 118, 98–104. [Google Scholar] [CrossRef]
- Williams, K.M.; Pavletic, S.Z.; Lee, S.J.; Martin, P.J.; Farthing, D.E.; Hakim, F.T.; Rose, J.; Manning-Geist, B.L.; Gea-Banacloche, J.C.; Comis, L.E.; et al. Prospective Phase II Trial of Montelukast to Treat Bronchiolitis Obliterans Syndrome after Hematopoietic Cell Transplantation and Investigation into Bronchiolitis Obliterans Syndrome Pathogenesis. Transpl. Cell. Ther. 2022, 28, 264.e1–264.e9. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Peng, C.; Lian, W.; Liu, H.; Fu, G. Effect of azithromycin on bronchiolitis obliterans syndrome in posttransplant recipients: A systematic review and meta-analysis. Medicine 2022, 101, e29160. [Google Scholar] [CrossRef] [PubMed]



| Total (n = 97) | |
|---|---|
| Age | 56.0 ± 9.5 |
| Male | 65 (67) |
| BMI, kg/m2 | 21.0 ± 3.8 |
| Type of lung transplantation | |
| Bilateral | 94 (96.9) |
| Primary disease | |
| COPD | 7 (7.2) |
| IPF | 45 (46.4) |
| PPH | 1 (1) |
| Non-IPF IIP | 21 (21.6) |
| Bronchiectasis | 2 (2.1) |
| Bronchiolitis Obliterans | 13 (13.4) |
| Other | 8 (8.2) |
| Pre-existing DSA | 4 (4.1) |
| Previous acute rejection, A3 | 18 (18.6) |
| Donor age | 40.3 ± 12.3 |
| Donor smoking dose | 6.6 ± 10.0 |
| Donor PaO2/FiO2 ratio | 443.2 ± 98.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, H.J.; Kang, J.; Kim, Y.H.; Cho, W.H. Periostin in Bronchiolitis Obliterans Syndrome after Lung Transplant. Int. J. Mol. Sci. 2024, 25, 10423. https://doi.org/10.3390/ijms251910423
Yeo HJ, Kang J, Kim YH, Cho WH. Periostin in Bronchiolitis Obliterans Syndrome after Lung Transplant. International Journal of Molecular Sciences. 2024; 25(19):10423. https://doi.org/10.3390/ijms251910423
Chicago/Turabian StyleYeo, Hye Ju, Junho Kang, Yun Hak Kim, and Woo Hyun Cho. 2024. "Periostin in Bronchiolitis Obliterans Syndrome after Lung Transplant" International Journal of Molecular Sciences 25, no. 19: 10423. https://doi.org/10.3390/ijms251910423






