Biotransformation of Xanthohumol by Entomopathogenic Filamentous Fungi
Abstract
:1. Introduction
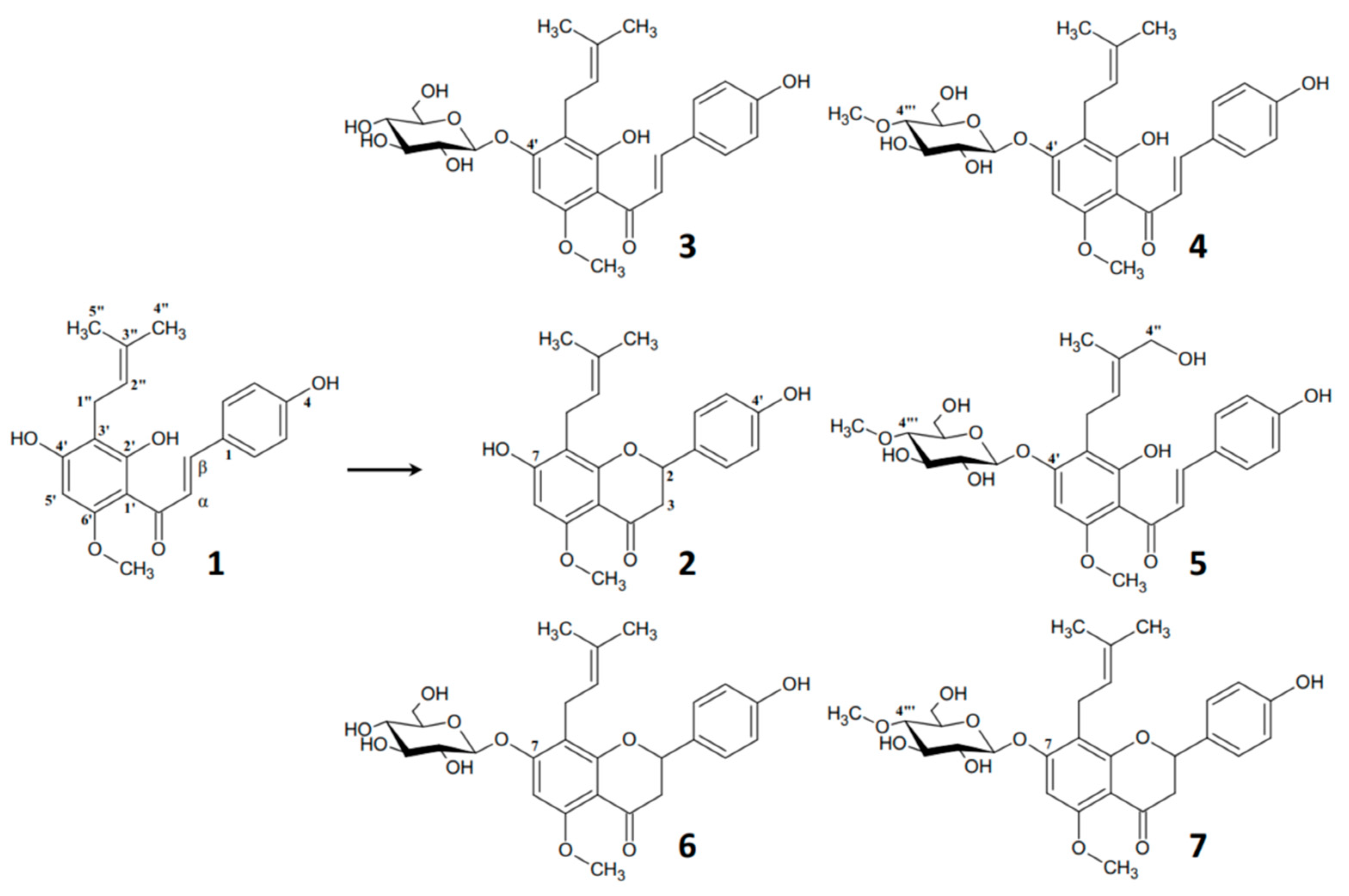
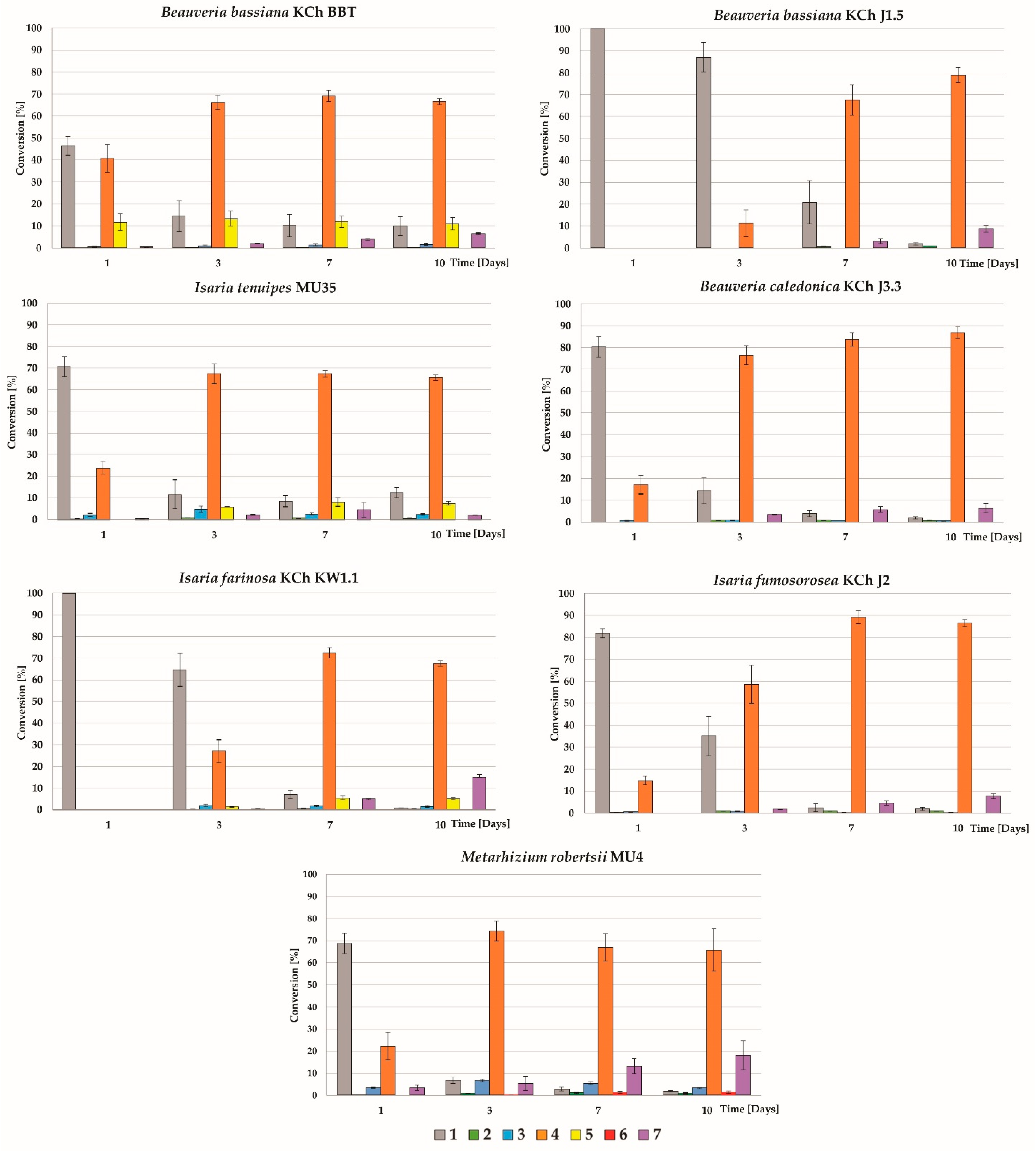
2. Results and Discussion
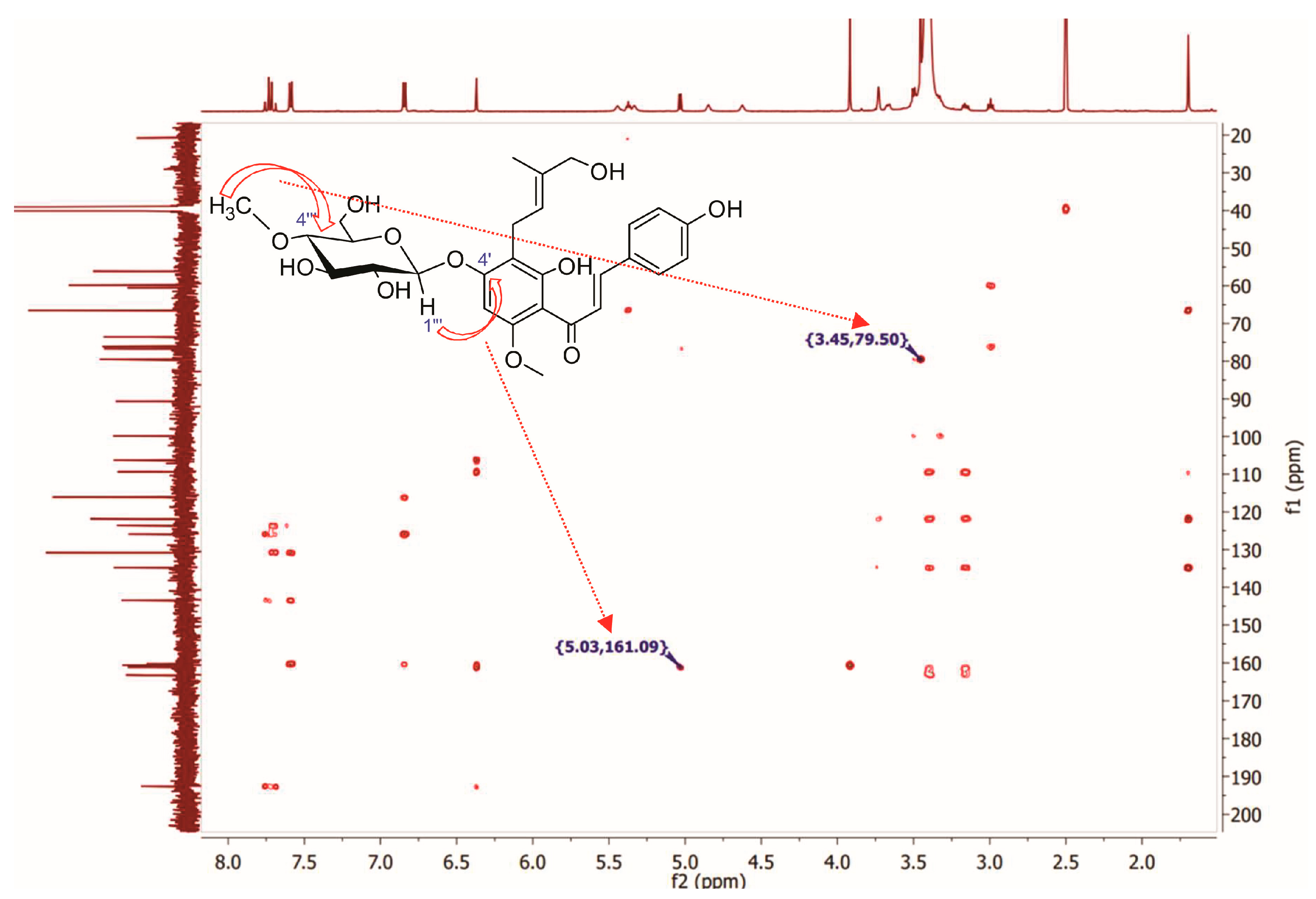
Identification of (2″E)-4″-Hydroxyxanthohumol 4′-O-β-D-(4‴-O-Methyl)-Glucopyranoside (5)
3. Materials and Methods
3.1. General Experimental Methods
3.2. Microorganisms
3.3. Conditions for Biotransformation
3.3.1. Study of the Biotransformation Process over Time
3.3.2. Scale-Up Biotransformation
3.4. Purification of (2″E)-4″-Hydroxyxanthohumol 4′-O-β-D-(4‴-O-Methyl)-Glucopyranoside (5)
3.5. (2″E)-4″-Hydroxyxanthohumol 4′-O-β-D-(4‴-O-Methyl)-Glucopiranoside (5)
3.6. Ultra High-Performance Liquid Chromatography
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Gerhäuser, C. Broad spectrum antiinfective potential of xanthohumol from hop (Humulus lupulus L.) in comparison with activities of other hop constituents and xanthohumol metabolites. Mol. Nutr. Food Res. 2005, 49, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hansen, P.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological Profile of Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus). Molecules 2015, 20, 754. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Sun, T.-L.; Xiang, D.-X.; Wei, S.-S.; Li, W.-Q. Anticancer activity and mechanism of xanthohumol: A prenylated flavonoid from hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Samuels, J.S.; Shashidharamurthy, R.; Rayalam, S. Novel anti-obesity effects of beer hops compound xanthohumol: Role of AMPK signaling pathway. Nutr. Metab. 2018, 15, 42. [Google Scholar] [CrossRef]
- Chen, X.; Mukwaya, E.; Wong, M.-S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Dong, L.P.; Wang, S.C.; Wang, Q. The first total synthesis of sophoflavescenol, flavenochromane C, and citrusinol. Eur. J. Org. Chem. 2015, 2015, 2297–2302. [Google Scholar] [CrossRef]
- Shi, S.; Li, J.; Zhao, X.; Liu, Q.; Song, S.-J. A comprehensive review: Biological activity, modification and synthetic methodologies of prenylated flavonoids. Phytochemistry 2021, 191, 112895. [Google Scholar] [CrossRef]
- Nowak, B.; Poźniak, B.; Popłoński, J.; Bobak, Ł.; Matuszewska, A.; Kwiatkowska, J.; Dziewiszek, W.; Huszcza, E.; Szeląg, A. Pharmacokinetics of xanthohumol in rats of both sexes after oral and intravenous administration of pure xanthohumol and prenylflavonoid extract. Adv. Clin. Exp. Med. 2020, 29, 1101–1109. [Google Scholar] [CrossRef]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet flavonoids”: Glycosidase-catalyzed modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of flavonoid O-glycoside, C-glycoside and their aglycones on antioxidant capacity and metabolism during in vitro digestion and in vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Stopa, J.D.; Neuberg, D.; Puligandla, M.; Furie, B.; Flaumenhaft, R.; Zwicker, J.I. Protein disulfide isomerase inhibition blocks thrombin generation in humans by interfering with platelet factor V activation. JCI Insight 2017, 2, e89373. [Google Scholar] [CrossRef] [PubMed]
- Mbikay, M.; Chrétien, M. Isoquercetin as an anti-COVID-19 medication: A potential to realize. Front. Pharmacol. 2022, 13, 830205. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Narayanan, S.; Chang, K.-O. Inhibition of influenza virus replication by plant-derived isoquercetin. Antivir. Res. 2010, 88, 227–235. [Google Scholar] [CrossRef]
- Khodzhaieva, R.S.; Gladkov, E.S.; Kyrychenko, A.; Roshal, A.D. Progress and achievements in glycosylation of flavonoids. Front. Chem. 2021, 9, 637994. [Google Scholar] [CrossRef]
- Lairson, L.; Henrissat, B.; Davies, G.; Withers, S. Glycosyltransferases: Structures, functions, and mechanisms. Annu. Rev. Biochem. 2008, 77, 521–555. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, L.; Wang, C.; Wang, X.; Xu, Y.-M.; Yu, H.; Wu, P.; Li, S.; Han, L.; Gunatilaka, A.L. Methylglucosylation of aromatic amino and phenolic moieties of drug-like biosynthons by combinatorial biosynthesis. Proc. Natl. Acad. Sci. USA 2018, 115, E4980–E4989. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, L.; Bai, J.; Yue, Q.; Zhang, M.; Li, J.; Wang, C.; Xu, Y. Methylglucosylation of phenolic compounds by fungal glycosyltransferase-methyltransferase functional modules. J. Agric. Food Chem. 2019, 67, 8573–8580. [Google Scholar] [CrossRef]
- Tronina, T.; Bartmańska, A.; Milczarek, M.; Wietrzyk, J.; Popłoński, J.; Rój, E.; Huszcza, E. Antioxidant and antiproliferative activity of glycosides obtained by biotransformation of xanthohumol. Bioorg. Med. Chem. Lett. 2013, 23, 1957–1960. [Google Scholar] [CrossRef]
- Bartmańska, A.; Tronina, T.; Popłoński, J. Biotransformation of a major beer prenylflavonoid–isoxanthohumol. Z. Für Naturforschung C 2019, 74, 1–7. [Google Scholar] [CrossRef]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Glycosylation of 3-Hydroxyflavone, 3-Methoxyflavone, Quercetin and Baicalein in Fungal Cultures of the Genus Isaria. Molecules 2018, 23, 2477. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, B.; Qiao, M.; Li, J.; Xu, H.; Zhang, L.; Zhang, X. Advances on the in vivo and in vitro glycosylations of flavonoids. Appl. Microbiol. Biotechnol. 2020, 104, 6587–6600. [Google Scholar] [CrossRef] [PubMed]
- Huszcza, E.; Bartmańska, A.; Tronina, T. Glycosylation of xanthohumol by fungi. Z. Für Naturforschung C 2008, 63, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Herath, W.; Mikell, J.R.; Hale, A.L.; Ferreira, D.; Khan, I.A. Microbial metabolism. Part 6. Metabolites of 3-and 7-hydroxyflavones. Chem. Pharm. Bull. 2006, 54, 320–324. [Google Scholar] [CrossRef]
- Costa, E.M.D.; Pimenta, F.C.; Luz, W.C.; Oliveira, V.D. Selection of filamentous fungi of the Beauveria genus able to metabolize quercetin like mammalian cells. Braz. J. Microbiol. 2008, 39, 405–408. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Zhan, J.; Leslie Gunatilaka, A. Selective 4′-O-methylglycosylation of the pentahydroxy-flavonoid quercetin by Beauveria bassiana ATCC 7159. Biocatal. Biotransform. 2006, 24, 396–399. [Google Scholar] [CrossRef]
- Ren, J.; Jackson, K.; Barton, C.D.; Huang, Y.; Zhan, J. Enhancing the physicochemical properties and bioactivities of 2′-hydroxyflavanone through fungal biotransformation. J. Biosci. Bioeng. 2024, 138, 144–152. [Google Scholar] [CrossRef]
- Zienecker, M. Deglycosylation of Phenolic Glucosides by the Bark Beetle Entomopathogenic Fungus Beauveria bassiana. Bachelor’s Thesis, Friedrich Schiller University Jena, Jena, Germany, 2024. [Google Scholar]
- Tronina, T.; Łużny, M.; Dymarska, M.; Urbaniak, M.; Kozłowska, E.; Piegza, M.; Stępień, Ł.; Janeczko, T. Glycosylation of quercetin by selected entomopathogenic Filamentous Fungi and prediction of its products’ bioactivity. Int. J. Mol. Sci. 2023, 24, 11857. [Google Scholar] [CrossRef]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Biotransformations of flavones and an isoflavone (daidzein) in cultures of entomopathogenic filamentous fungi. Molecules 2018, 23, 1356. [Google Scholar] [CrossRef]
- Hollman, P.C.; Bijsman, M.N.; Van Gameren, Y.; Cnossen, E.P.; De Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free. Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, I.-S. Microbial metabolism of the prenylated chalcone xanthohumol. J. Nat. Prod. 2006, 69, 1522–1524. [Google Scholar] [CrossRef] [PubMed]
- Żołnierczyk, A.K.; Mączka, W.K.; Grabarczyk, M.; Wińska, K.; Woźniak, E.; Anioł, M. Isoxanthohumol—Biologically active hop flavonoid. Fitoterapia 2015, 103, 71–82. [Google Scholar] [CrossRef]
- Seliger, J.M.; Misuri, L.; Maser, E.; Hintzpeter, J. The hop-derived compounds xanthohumol, isoxanthohumol and 8-prenylnaringenin are tight-binding inhibitors of human aldo-keto reductases 1B1 and 1B10. J. Enzym. Inhib. Med. Chem. 2018, 33, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Becker, T.; Qian, F.; Ring, J. Beer and beer compounds: Physiological effects on skin health. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 142–150. [Google Scholar] [CrossRef]
- Meinert, H.; Yi, D.; Zirpel, B.; Schuiten, E.; Geißler, T.; Gross, E.; Brückner, S.I.; Hartmann, B.; Roettger, C.; Ley, J.P. Discovery of Novel Bacterial Chalcone Isomerases by a Sequence-Structure-Function-Evolution Strategy for Enzymatic Synthesis of (S)-Flavanones. Angew. Chem. Int. Ed. 2021, 60, 16874–16879. [Google Scholar] [CrossRef]
- Furumura, S.; Ozaki, T.; Sugawara, A.; Morishita, Y.; Tsukada, K.; Ikuta, T.; Inoue, A.; Asai, T. Identification and functional characterization of fungal chalcone synthase and chalcone isomerase. J. Nat. Prod. 2023, 86, 398–405. [Google Scholar] [CrossRef]
- Tronina, T.; Bartmańska, A.; Popłoński, J.; Huszcza, E. Microbial transformation of xanthohumol by Aspergillus ochraceus. J. Basic Microbiol. 2012, 54, 66–71. [Google Scholar] [CrossRef]
- Tueting, D.R.; Echavarren, A.M.; Stille, J. Palladium catalyzed coupling of organostannanes with vinyl epoxides. Tetrahedron 1989, 45, 979–992. [Google Scholar] [CrossRef]
- Mao, Y.; Zhai, X.; Khan, A.; Cheng, J.; Wu, X.; Zhang, Y.J. Cross-coupling of vinylethylene carbonates with arylboronic acids catalyzed by in situ generated palladium nanoparticles in water. Tetrahedron Lett. 2016, 57, 3268–3271. [Google Scholar] [CrossRef]
- Nowak, B.; Matuszewska, A.; Popłoński, J.; Nikodem, A.; Filipiak, J.; Tomanik, M.; Dziewiszek, W.; Danielewski, M.; Belowska-Bień, K.; Kłobucki, M. Prenylflavonoids counteract ovariectomy-induced disturbances in rats. J. Funct. Foods 2021, 86, 104742. [Google Scholar] [CrossRef]
| Strain Tested | Capability of Transforming Xanthohumol (1) * |
|---|---|
| Beauveria bassiana KCh J1.5 | ++ |
| Beauveria bassiana KCh BBT | + |
| Beauveria caledonica KCh J3.3 | ++ |
| Beauveria feline ENC3 | − |
| Isaria fumosorosea KCh J2 | ++ |
| Isaria farinosa KCh KW1.1 | ++ |
| Isaria tenuipes MU35 | + |
| Metarhizium robertsii MU4 | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łój, D.; Janeczko, T.; Bartmańska, A.; Huszcza, E.; Tronina, T. Biotransformation of Xanthohumol by Entomopathogenic Filamentous Fungi. Int. J. Mol. Sci. 2024, 25, 10433. https://doi.org/10.3390/ijms251910433
Łój D, Janeczko T, Bartmańska A, Huszcza E, Tronina T. Biotransformation of Xanthohumol by Entomopathogenic Filamentous Fungi. International Journal of Molecular Sciences. 2024; 25(19):10433. https://doi.org/10.3390/ijms251910433
Chicago/Turabian StyleŁój, Daniel, Tomasz Janeczko, Agnieszka Bartmańska, Ewa Huszcza, and Tomasz Tronina. 2024. "Biotransformation of Xanthohumol by Entomopathogenic Filamentous Fungi" International Journal of Molecular Sciences 25, no. 19: 10433. https://doi.org/10.3390/ijms251910433






