Evolution, Gene Duplication, and Expression Pattern Analysis of CrRLK1L Gene Family in Zea mays (L.)
Abstract
:1. Introduction
2. Results
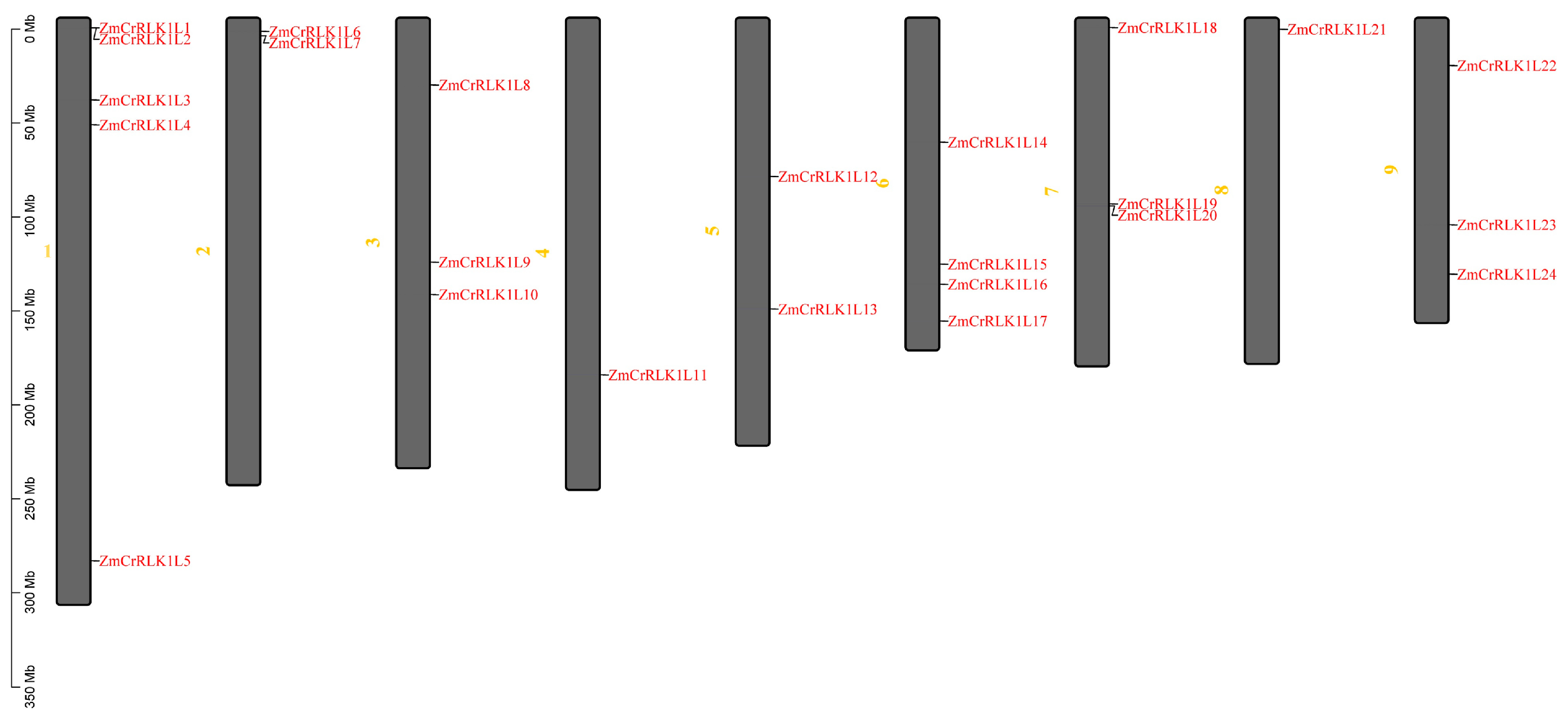
2.1. Identification of ZmCrRLK1L Family Genes
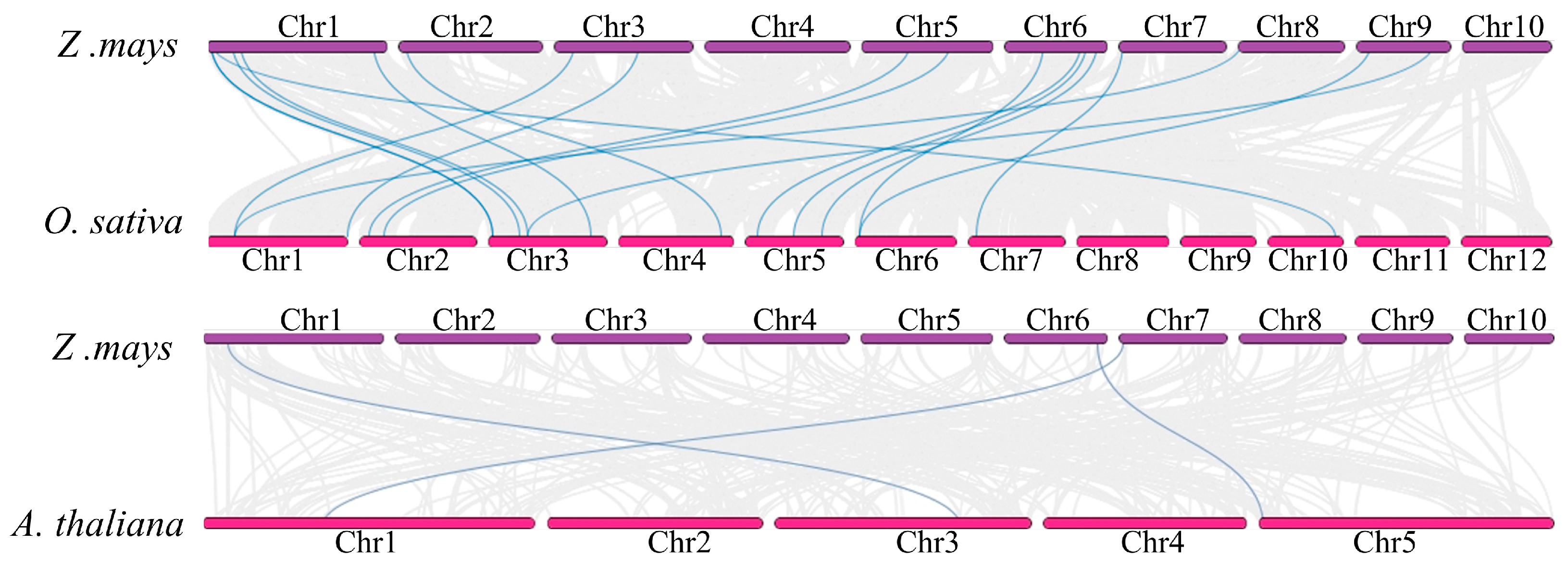
2.2. Phylogenetic Analysis of ZmCrRLK1L, OsCrRLK1L, and AtCrRLK1L Proteins
2.3. Analysis of Gene Structure and Conserved Protein Motifs
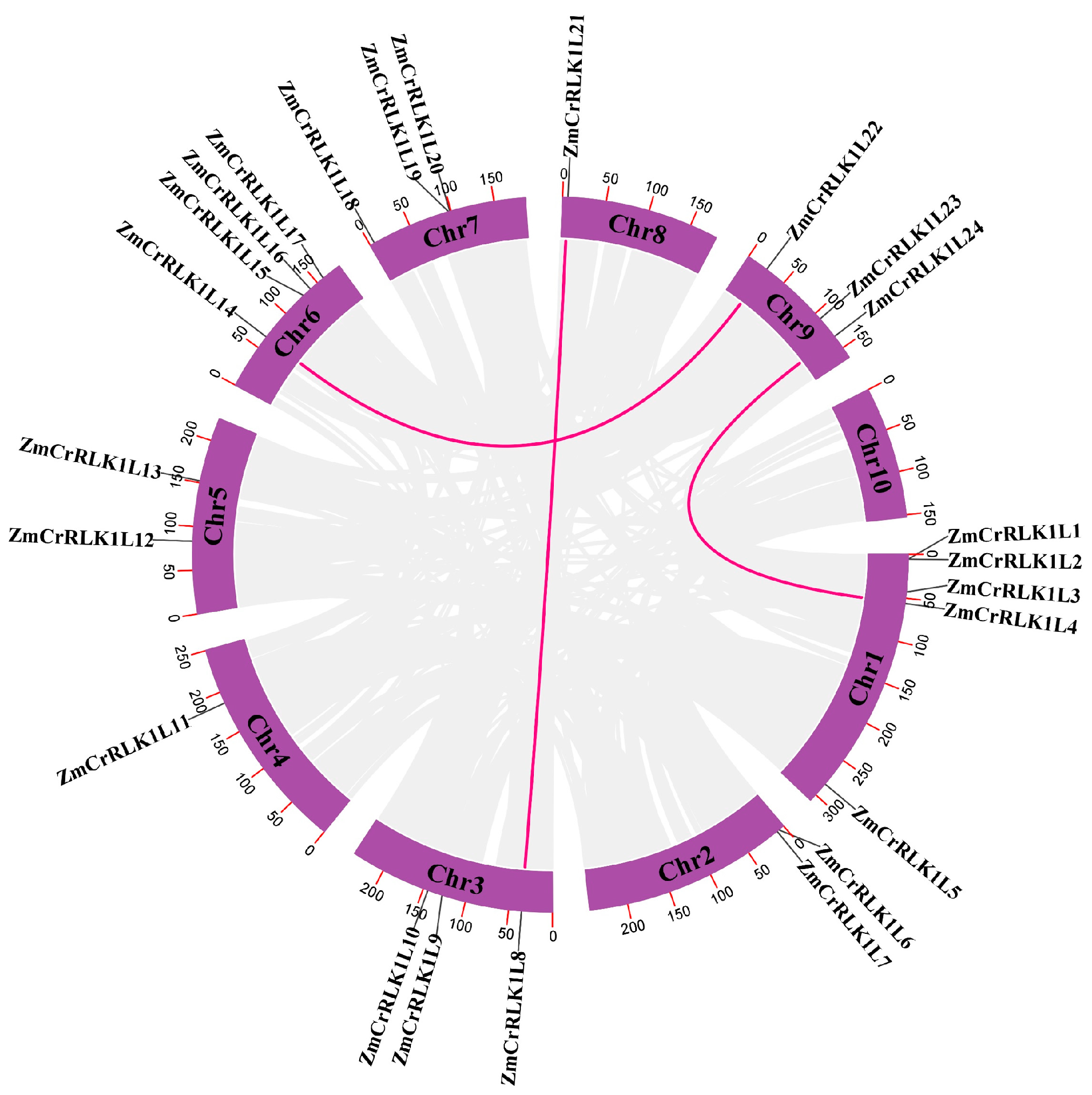
2.4. Replication Event Analysis of the ZmCrRLK1L Gene Family
2.5. Collinearity Relationship Analysis of ZmCrRLK1L Genes
2.6. Analysis of Cis-Acting Elements of ZmCrRLK1L Gene
2.7. Expression Pattern Analysis of ZmCrRLK1L Genes in Different Tissues
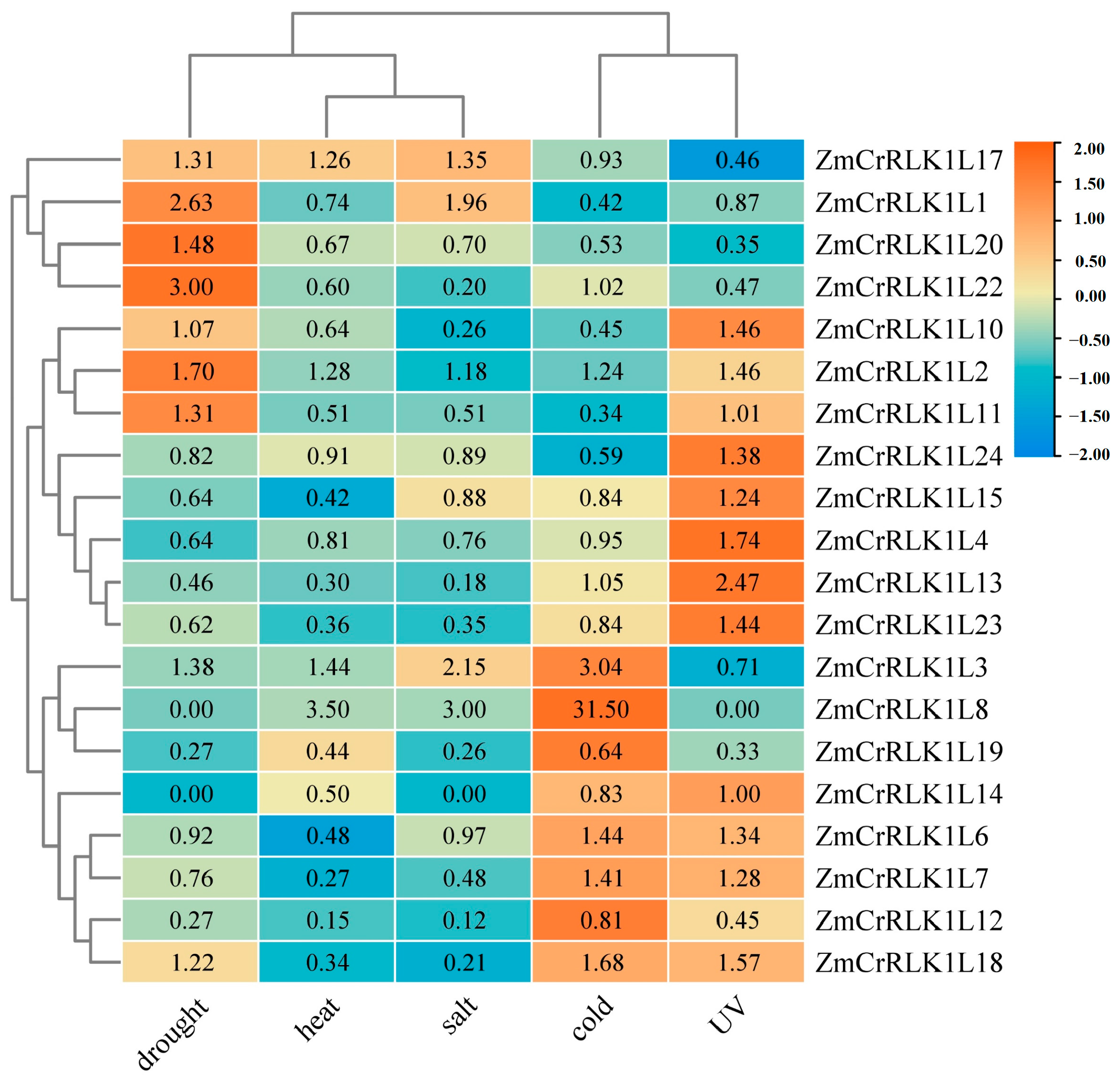
2.8. Expression Patterns Analysis of ZmCrRLK1L Genes under Different Abiotic Stresses
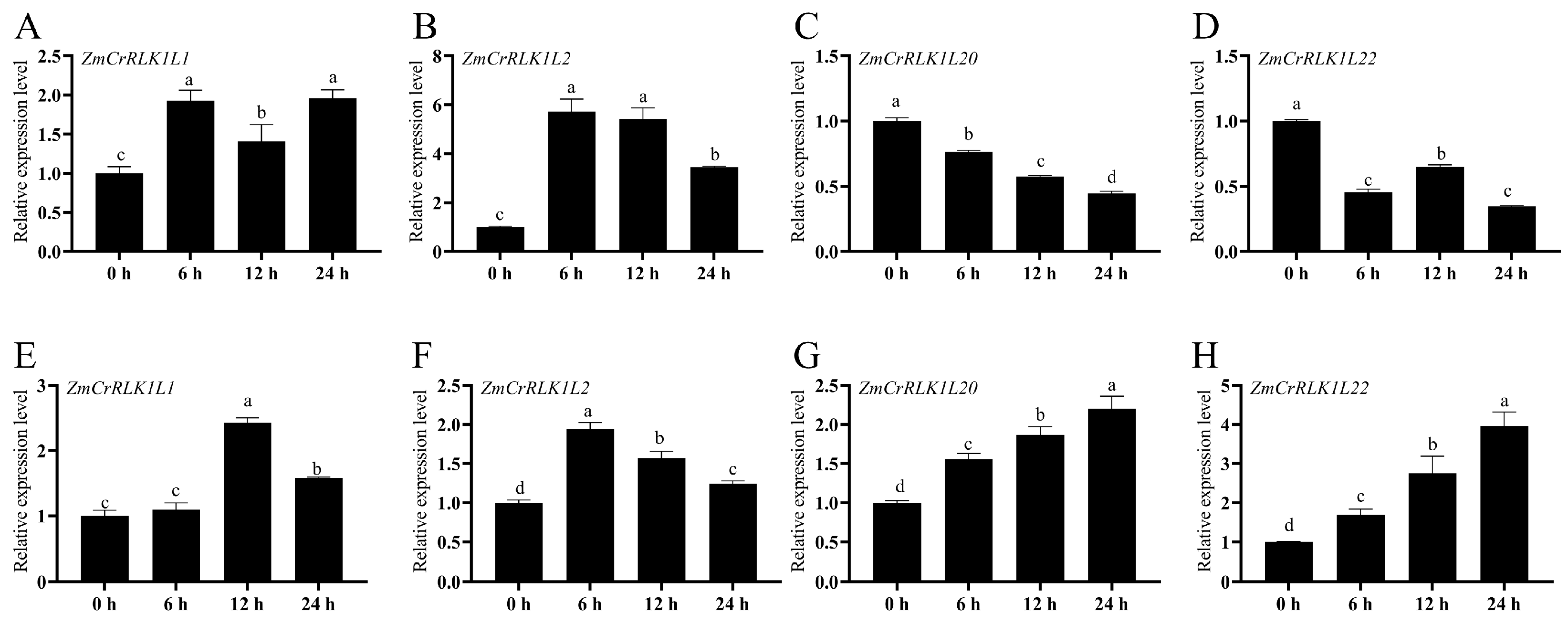
2.9. Expression Pattern Analysis of ZmCrRLK1L1/2/20/22 under PEG and Salt Treatments by RT-qPCR
3. Discussion
4. Materials and Methods
4.1. Identification and Phylogenetic Analysis of ZmCrRLK1L Family Genes in Maize
4.2. Gene Structure, Protein Motif, and Cis-Acting Element Analysis of ZmCrRLK1L
4.3. Orthologous Gene, Paralogous Gene, and Ka/KS Ratio Analysis of ZmCrRLK1L
4.4. The ZmCrRLK1L Gene Expression Patterns Analysis
4.5. Plant Materials, Growth Conditions, and PEG and NaCl Treatments
4.6. Total RNA Extraction and RT-qPCR Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiu, S.H.; Bleecker, A.B. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. USA 2001, 98, 10763–10768. [Google Scholar] [CrossRef]
- Zhu, S.; Fu, Q.; Xu, F.; Zhang, H.P.; Yu, F. New paradigms in cell adaptation: Decades of discoveries on the CrRLK1L receptor kinase signalling network. New Phytol. 2021, 232, 1168–1183. [Google Scholar] [CrossRef] [PubMed]
- Franck, C.M.; Westermann, J. Plant malectin-like receptor kinases: From cell wall integrity to immunity and beyond. Annu. Rev. Plant Biol. 2018, 69, 301–328. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 2018, 28, 666–675. [Google Scholar] [CrossRef]
- Stegmann, M.; Monaghan, J.; Smakowska-Luzan, E.; Rovenich, H.; Lehner, A.; Holton, N.; Belkhadir, Y.; Zipfel, C. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 2017, 355, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Kanaoka, M.M.; Torii, K.U. FERONIA as an upstream receptor kinase for polar cell growth in plants. Proc. Natl. Acad. Sci. USA 2010, 107, 17461–17472. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.J.; Quinto, C. The CrRLK1L subfamily: One of the keys to versatility in plants. Plant Physiol. Biochem. 2021, 166, 88–102. [Google Scholar] [CrossRef]
- Guo, H.; Nolan, T.M.; Song, G.; Liu, S.; Xie, Z.; Chen, J.; Schnable, P.S.; Walley, J.W.; Yin, Y. FERONIA receptor kinase contributes to plant immunity by suppressing jasmonic acid signaling in Arabidopsis thaliana. Curr. Biol. 2018, 28, 3316–3324. [Google Scholar] [CrossRef]
- Mang, H.; Feng, B.; Hu, Z.; Boisson-Dernier, A.; Franck, C.M.; Meng, X.; Huang, Y.; Zhou, J.; Xu, G.; Wang, T.; et al. Differential regulation of two-tiered plant immunity and sexual reproduction by ANXUR receptor-like kinases. Plant Cell 2017, 29, 3140–3156. [Google Scholar] [CrossRef]
- Jing, X.Q.; Shi, P.T.; Zhang, R.; Zhou, M.R.; Shalmani, A.; Wang, G.F.; Liu, W.T.; Li, W.Q.; Chen, K.M. Rice kinase OsMRLK63 contributes to drought tolerance by regulating reactive oxygen species production. Plant Physiol. 2024, 194, 2679–2696. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, W.; Zayed, O.; Liu, X.; Tang, K.; Nie, W.; Li, Y.; Xie, S.; Li, Y.; Long, T.; et al. The LRXs-RALFs-FER module controls plant growth and salt stress responses by modulating multiple plant hormones. Natl. Sci. Rev. 2020, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; De Smet, I. Understanding the RALF family: A tale of many species. Trends Plant Sci. 2014, 19, 664–671. [Google Scholar] [CrossRef]
- Schoenaers, S.; Balcerowicz, D.; Breen, G.; Hill, K.; Zdanio, M.; Mouille, G.; Holman, T.J.; Oh, J.; Wilson, M.H.; Nikonorova, N.; et al. The Auxin-Regulated CrRLK1L Kinase ERULUS Controls Cell Wall Composition during Root Hair Tip Growth. Curr. Biol. 2018, 28, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yeh, F.L.; Cheung, A.Y.; Duan, Q.; Kita, D.; Liu, M.C.; Maman, J.; Luu, E.J.; Wu, B.W.; Gates, L.; et al. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. Elife 2015, 4, e06587. [Google Scholar] [CrossRef]
- Duan, Q.; Kita, D.; Li, C.; Cheung, A.Y.; Wu, H.M. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. USA 2010, 107, 17821–17826. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, Z.; Liu, Y.; Tao, L.Z.; Liu, H. FERONIA regulates auxin-mediated lateral root development and primary root gravitropism. FEBS Lett. 2019, 593, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Martínez, P.J.; Estevez, J.M.; Yu, F. Autocrine regulation of root hair size by the RALF-FERONIA-RSL4 signaling pathway. New Phytol. 2020, 227, 45–49. [Google Scholar] [CrossRef]
- Feng, H.; Liu, C.; Fu, R.; Zhang, M.; Li, H.; Shen, L.; Wei, Q.; Sun, X.; Xu, L.; Ni, B.; et al. LORELEI-LIKE GPI-ANCHORED PROTEINS 2/3 Regulate Pollen Tube Growth as Chaperones and Coreceptors for ANXUR/BUPS Receptor Kinases in Arabidopsis. Mol. Plant 2019, 12, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, L.; Xiao, Y.; Vyshedsky, D.; Peng, C.; Sun, X.; Liu, Z.; Cheng, L.; Zhang, H.; Han, Z.; et al. Pollen PCP-B peptides unlock a stigma peptide-receptor kinase gating mechanism for pollination. Science 2021, 372, 171–175. [Google Scholar] [CrossRef]
- Xiao, Y.; Stegmann, M.; Han, Z.; DeFalco, T.A.; Parys, K.; Xu, L.; Belkhadir, Y.; Zipfel, C.; Chai, J. Mechanisms of RALF peptide perception by a heterotypic receptor complex. Nature 2019, 572, 270–274. [Google Scholar] [CrossRef]
- Song, Y.; Wilson, A.J.; Zhang, X.C.; Thoms, D.; Sohrabi, R.; Song, S.; Geissmann, Q.; Liu, Y.; Walgren, L.; He, S.Y.; et al. FERONIA restricts Pseudomonas in the rhizosphere microbiome via regulation of reactive oxygen species. Nat. Plants 2021, 7, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chu, L.C.; Liang, Y.; Zhang, X.Q.; Chen, L.Q.; Ye, D. The Arabidopsis CrRLK1L protein kinases BUPS1 and BUPS2 are required for normal growth of pollen tubes in the pistil. Plant J. 2018, 95, 474–486. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Hamurcu, M.; Vyhnánek, T.; Zargar, S.M.; Kahraman, A.; Topal, A.; Gezgin, S. Exploring strigolactones for inducing abiotic stress tolerance in plants. Czech J. Genet. Plant Breed. 2024, 60, 55–69. [Google Scholar] [CrossRef]
- Ma, W.; Du, J.; Yu, X.; Chen, K.; Ming, Y.; Jiang, L.; Chen, T.; Ji, D. Genome-Wide Identification and Analysis of Catharanthus roseus Receptor-like Kinase 1-like Proteins in Eggplant. Plants 2023, 12, 3379. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. Tbtools v2.121 (Beijing, China)-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Gawande, N.D.; Sankaranarayanan, S. Genome wide characterization and expression analysis of CrRLK1L gene family in wheat unravels their roles in development and stress-specific responses. Front. Plant Sci. 2024, 15, 1345774. [Google Scholar] [CrossRef]
- Ma, W.; Liu, X.; Chen, K.; Yu, X.; Ji, D. Genome-Wide Re-Identification and Analysis of CrRLK1Ls in Tomato. Int. J. Mol. Sci. 2023, 24, 3142. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Zhao, Y.; Liu, M.C.; Zhou, L.Z.; Wang, L.; Zhong, S.; Hou, S.; Jiang, J.; Liu, T.; Huang, Q.; et al. LLG2/3 Are Co-receptors in BUPS/ANX-RALF Signaling to Regulate Arabidopsis Pollen Tube Integrity. Curr. Biol. 2019, 29, 3256–3265. [Google Scholar] [CrossRef]
- Nguyen, Q.N.; Lee, Y.S.; Cho, L.H.; Jeong, H.J.; An, G.; Jung, K.H. Genome-wide identification and analysis of Catharanthus roseus RLK1-like kinases in rice. Planta 2015, 241, 603–613. [Google Scholar] [CrossRef]
- Lindner, H.; Müller, L.M.; Boisson-Dernier, A.; Grossniklaus, U. CrRLK1L receptor-like kinases: Not just another brick in the wall. Curr. Opin. Plant Biol. 2012, 15, 659–669. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Yu, T.F.; Sun, G.Z.; Zheng, J.C.; Chen, J.; Zhou, Y.B.; Chen, M.; Ma, Y.Z.; Wei, W.L.; Xu, Z.S. Genome-Wide Analysis of the Catharanthus roseus RLK1-Like in Soybean and GmCrRLK1L20 Responds to Drought and Salt Stresses. Front. Plant Sci. 2021, 12, 614909. [Google Scholar] [CrossRef]
- Jiang, W.; Li, C.; Li, L.; Li, Y.; Wang, Z.; Yu, F.; Yi, F.; Zhang, J.; Zhu, J.K.; Zhang, H.; et al. Genome-Wide Analysis of CqCrRLK1L and CqRALF Gene Families in Chenopodium quinoa and Their Roles in Salt Stress Response. Front. Plant Sci. 2022, 13, 918594. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Liu, Q.; Huang, Y.; Liu, C.; Guo, X.; Fan, C.; Zhang, K.; Liu, Y.; Han, F. Non-B-form DNA is associated with centromere stability in newly-formed polyploid wheat. Sci. China Life Sci. 2024, 67, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Yang, M.; Tang, Y.; Zhang, L.; Zhao, H.; Ni, H.; Chen, Q.; Meng, F.; Jiang, J. Dynamics of cis-regulatory sequences and transcriptional divergence of duplicated genes in soybean. Proc. Natl. Acad. Sci. USA 2023, 120, e2303836120. [Google Scholar] [CrossRef] [PubMed]
- Magadum, S.; Banerjee, U.; Murugan, P.; Gangapur, D.; Ravikesavan, R. Gene duplication as a major force in evolution. J. Genet. 2013, 92, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.K.; An, H.; Pires, J.C. Mangroves and multiplications: Influence of genome duplications on salt tolerance. Mol. Ecol. 2023, 32, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Ma, Z.; Mao, H. Duplicate Genes Contribute to Variability in Abiotic Stress Resistance in Allopolyploid Wheat. Plants 2023, 12, 2465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Garcia, H.M.; Berardini, T.Z.; Chen, G.; Crist, D.; Doyle, A.; Huala, E.; Knee, E.; Lambrecht, M.; Miller, N.; Mueller, L.A.; et al. TAIR: A resource for integrated Arabidopsis data. Funct. Integr. Genom. 2002, 2, 239–253. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, H.; Long, Y.; Shu, Y.; Zhai, J. Plant Public RNA-seq Database: A comprehensive online database for expression analysis of ~45,000 plant public RNA-Seq libraries. Plant Biotechnol. J. 2022, 20, 806–808. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, L.; Li, T.; Ruan, Y.; Zhang, A.; Dong, X.; Zhu, Y.; Li, C.; Fan, J. Genome-Wide Investigation and Characterization of SWEET Gene Family with Focus on Their Evolution and Expression during Hormone and Abiotic Stress Response in Maize. Genes 2022, 13, 1682. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Xue, B.; He, Y.; Zhao, H.; Liu, B.; Jiang, W.; Jin, P.; Wang, Y.; Zhang, X.; He, X. Evolution, Gene Duplication, and Expression Pattern Analysis of CrRLK1L Gene Family in Zea mays (L.). Int. J. Mol. Sci. 2024, 25, 10487. https://doi.org/10.3390/ijms251910487
Wang K, Xue B, He Y, Zhao H, Liu B, Jiang W, Jin P, Wang Y, Zhang X, He X. Evolution, Gene Duplication, and Expression Pattern Analysis of CrRLK1L Gene Family in Zea mays (L.). International Journal of Molecular Sciences. 2024; 25(19):10487. https://doi.org/10.3390/ijms251910487
Chicago/Turabian StyleWang, Kai, Baoping Xue, Yan He, Haibin Zhao, Bo Liu, Wenting Jiang, Pengfei Jin, Yanfeng Wang, Xiangqian Zhang, and Xiaolong He. 2024. "Evolution, Gene Duplication, and Expression Pattern Analysis of CrRLK1L Gene Family in Zea mays (L.)" International Journal of Molecular Sciences 25, no. 19: 10487. https://doi.org/10.3390/ijms251910487




