Comparative Analysis of Cystamine and Cysteamine as Radioprotectors and Antioxidants: Insights from Monte Carlo Chemical Modeling under High Linear Energy Transfer Radiation and High Dose Rates
Abstract
:1. Introduction
1.1. Radiolysis of Water: Effects of Radiation Quality and Dose Rate on the Formation of Radical and Molecular Products
1.2. Assessing the Radioprotective, Free-Radical Scavenging, and Antioxidant Properties of Cystamine and Cysteamine Using the Fricke Dosimeter: Influence of Proton Energy Variability and Dose Rate
2. Results and Discussion
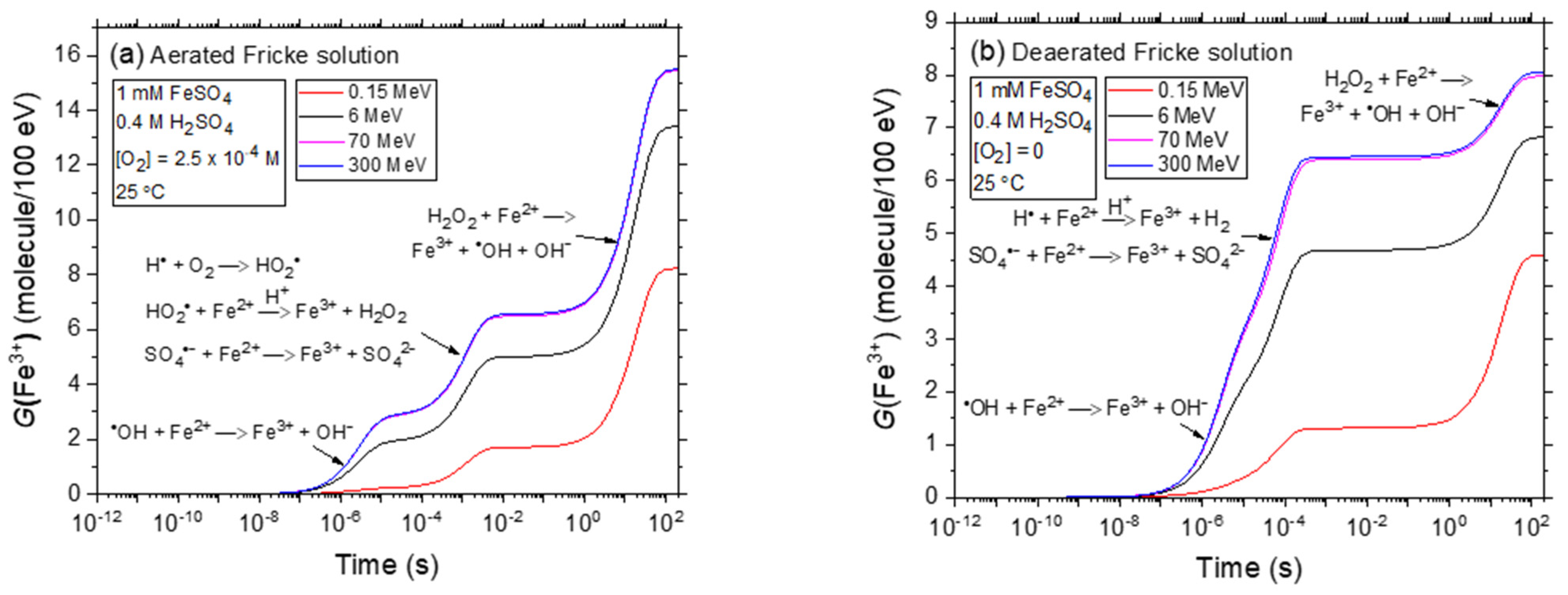
2.1. Kinetics of Fe3+ Formation in Aerated and Deaerated Fricke Solutions Exposed to 0.15–300 MeV (~72–0.3 keV/μm) Proton Irradiation without Added Cystamine or Cysteamine, in the Absence of Dose-Rate Effects
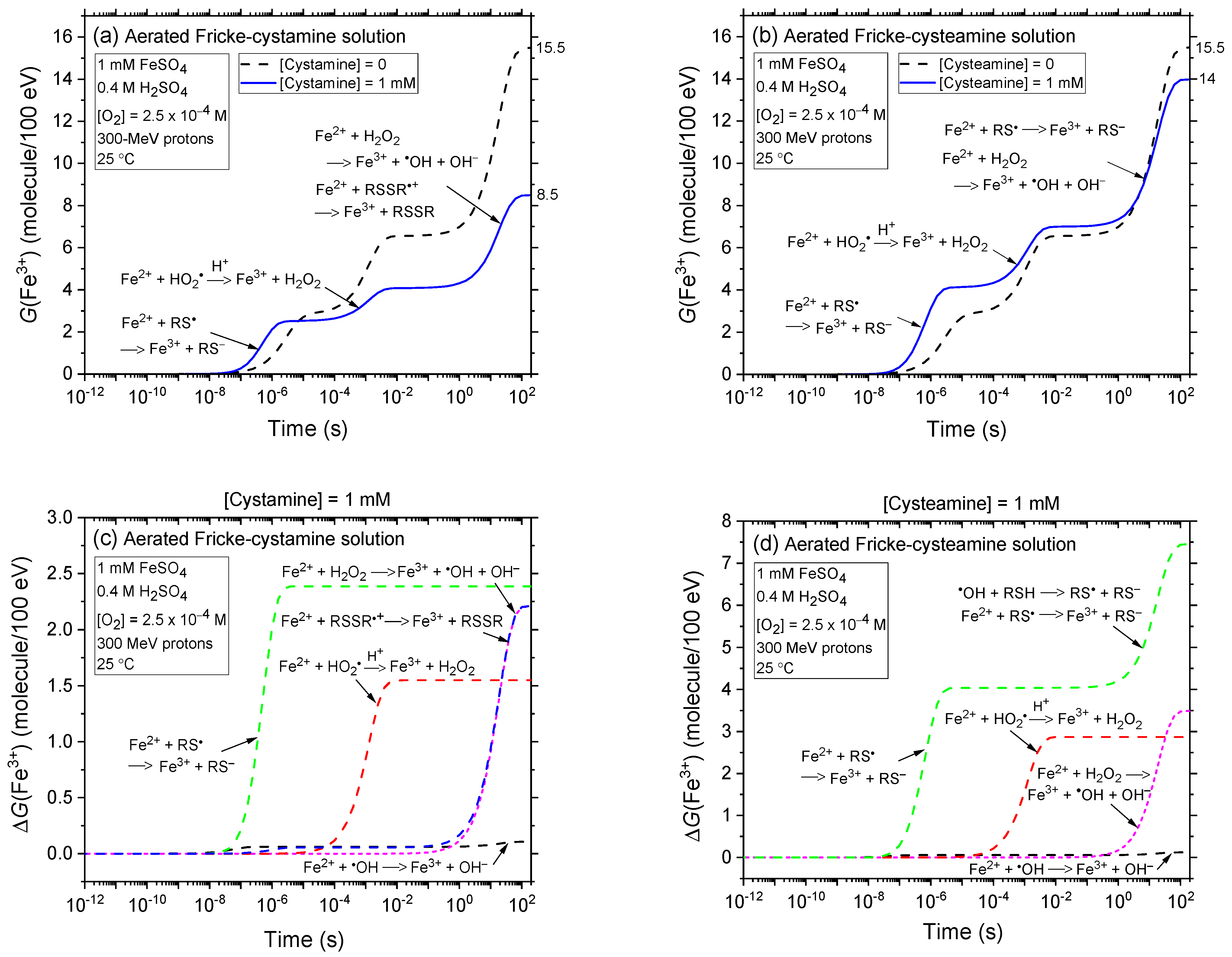
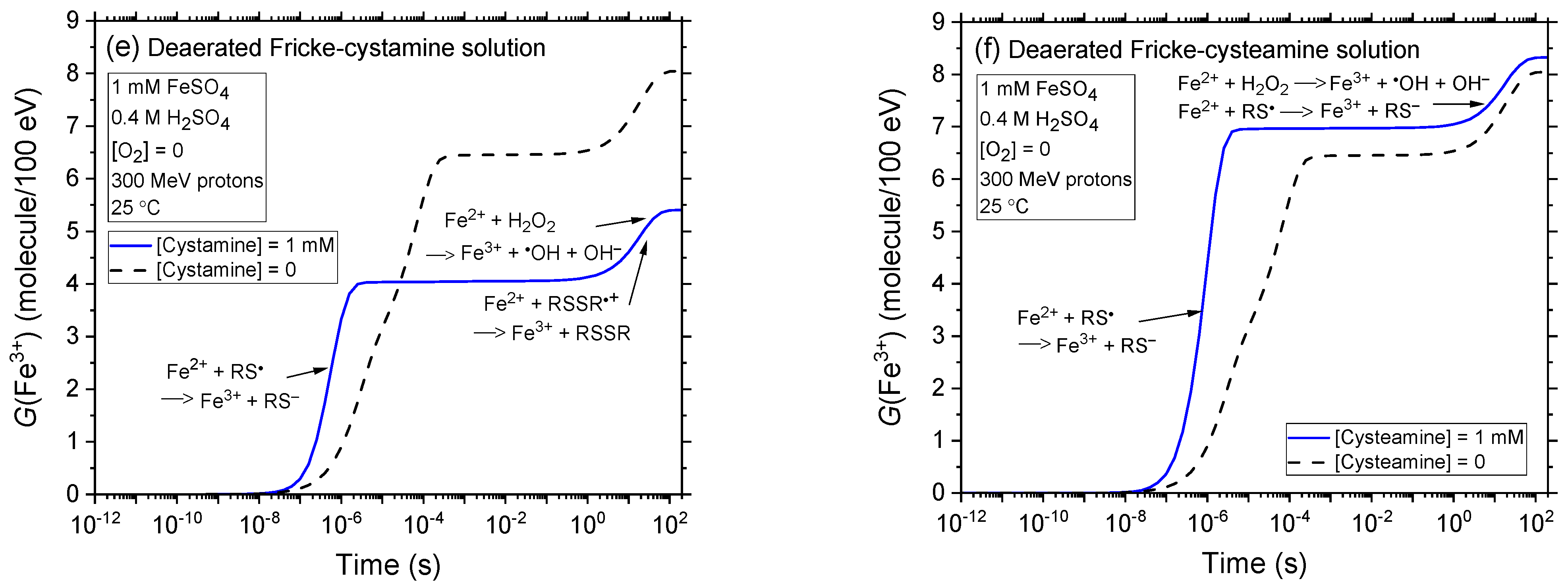
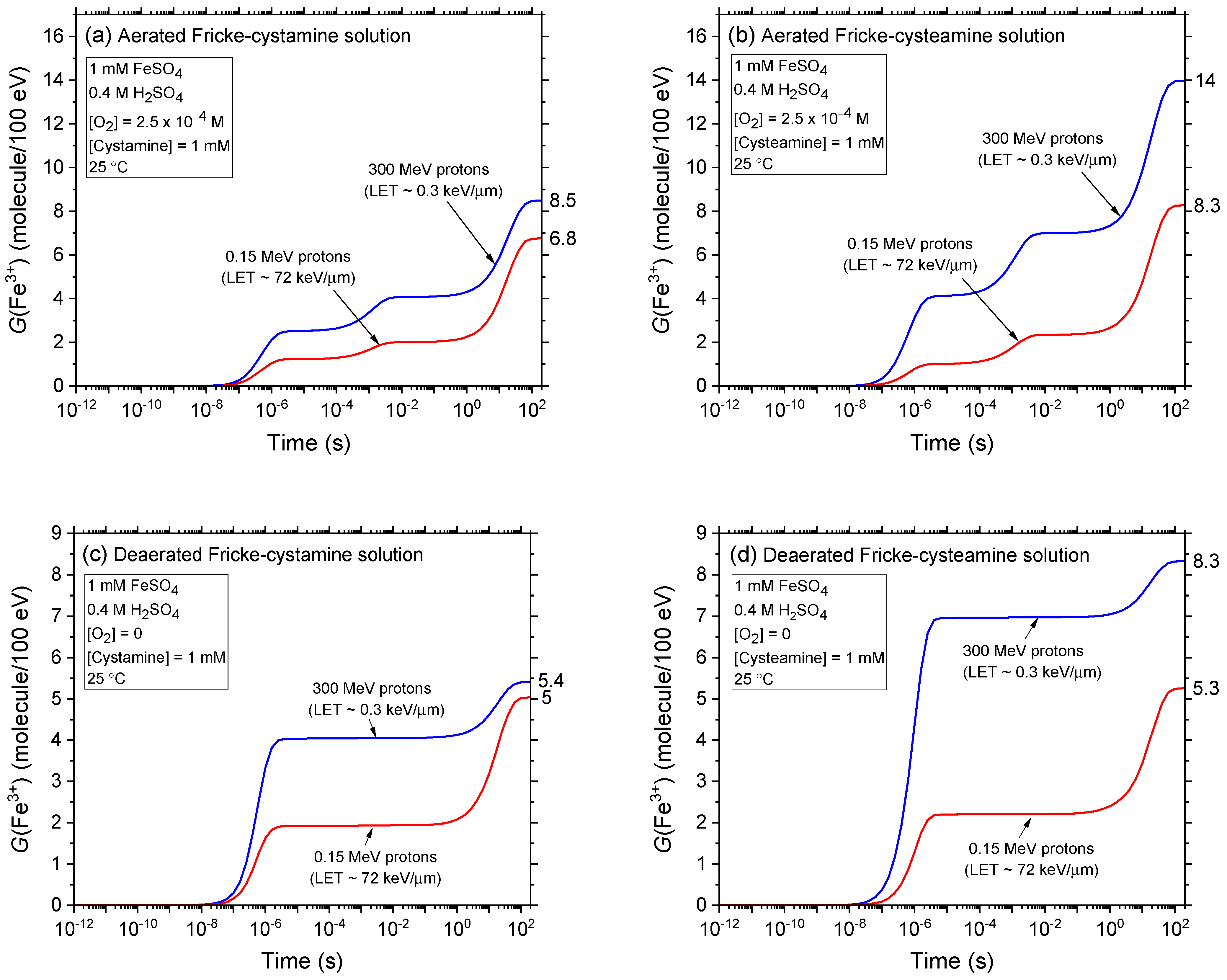
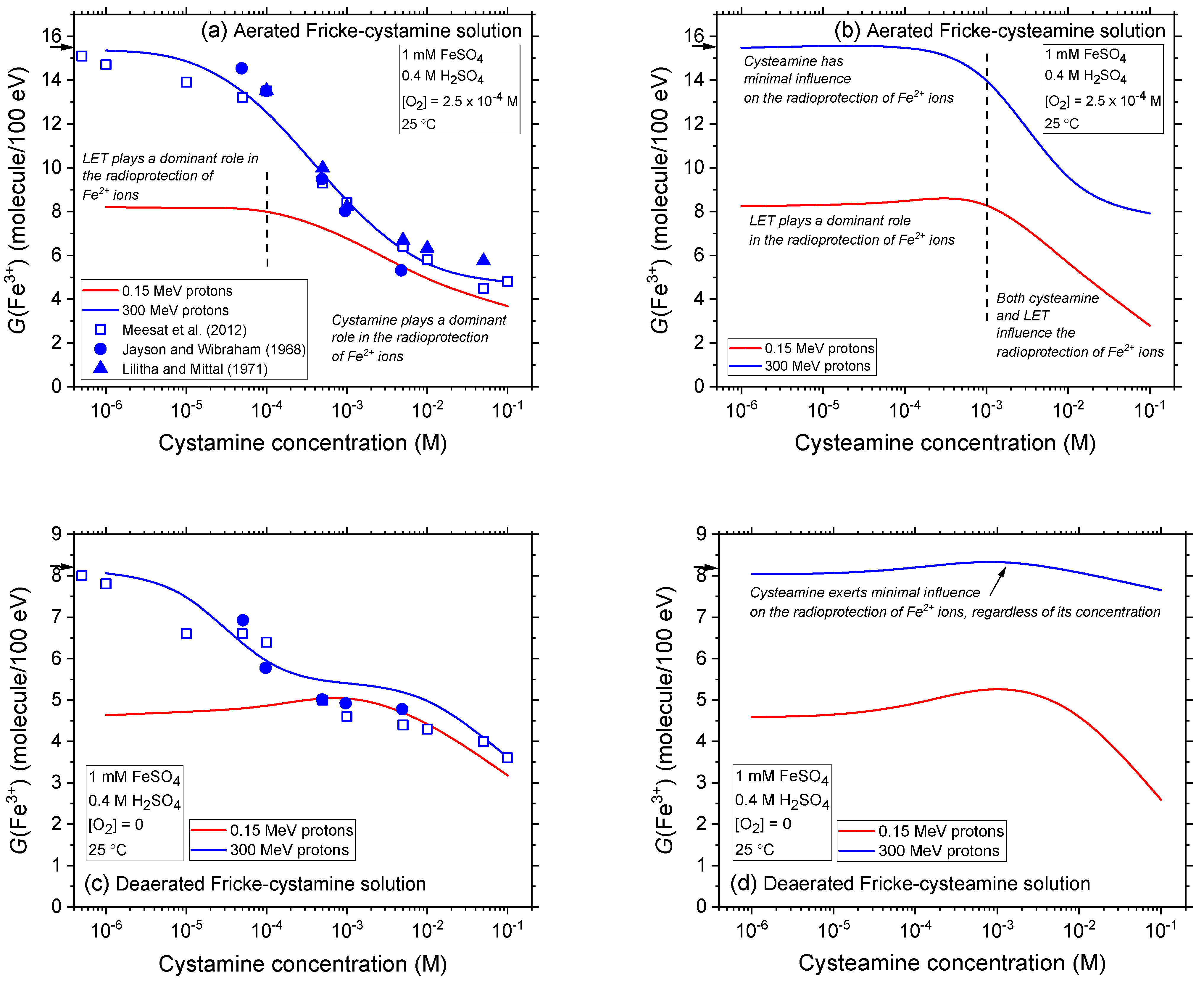
2.2. Comparison of Fe3+ Ion Yields in Aerated and Deaerated Fricke-Cystamine and Fricke-Cysteamine Solutions Exposed to 0.15–300 MeV (~72–0.3 keV/μm) Proton Irradiation, in the Absence of Dose-Rate Effects
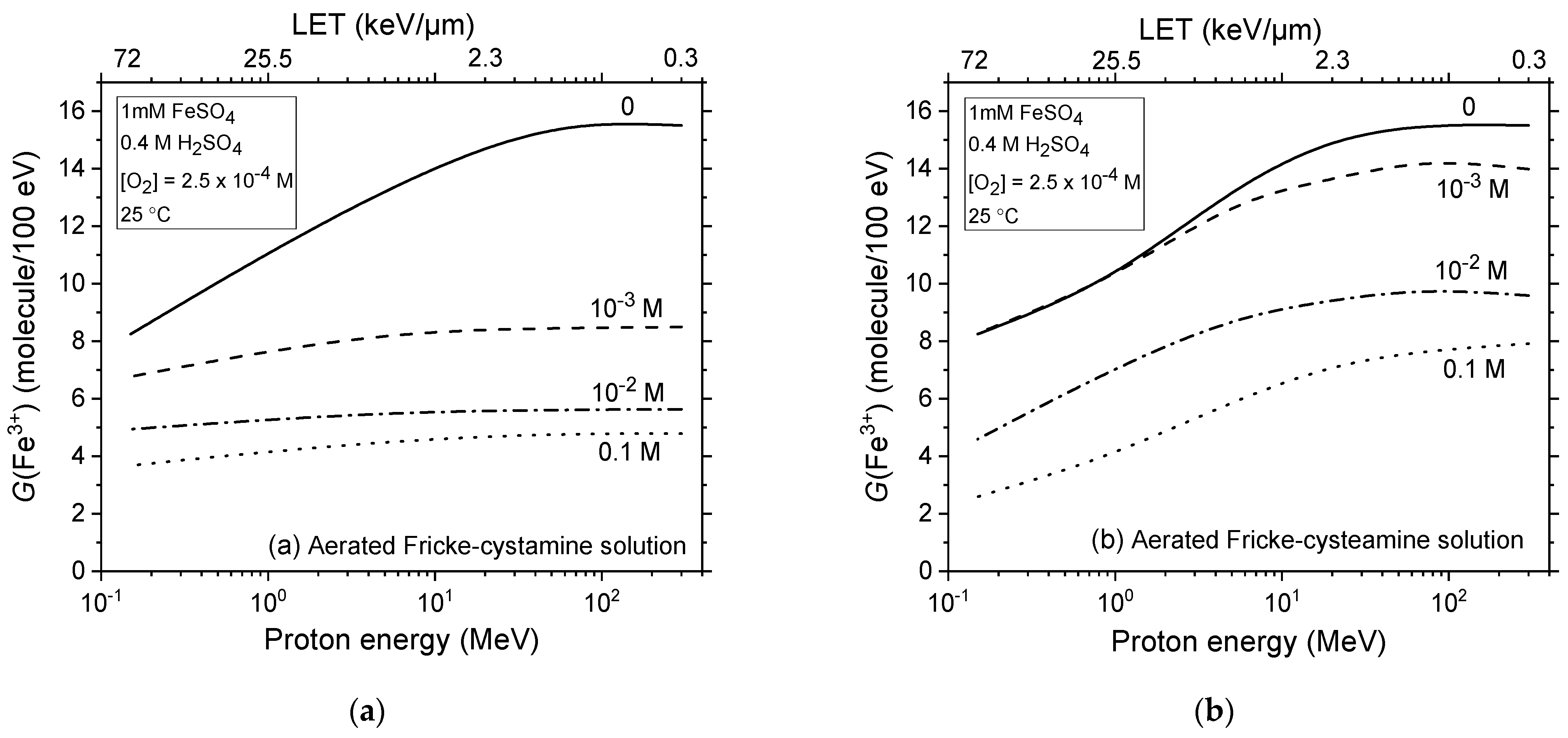
2.3. Impact of Cystamine and Cysteamine Concentrations on Fricke Yield across Proton Energies from 300 to 0.15 MeV without Dose-Rate Effects
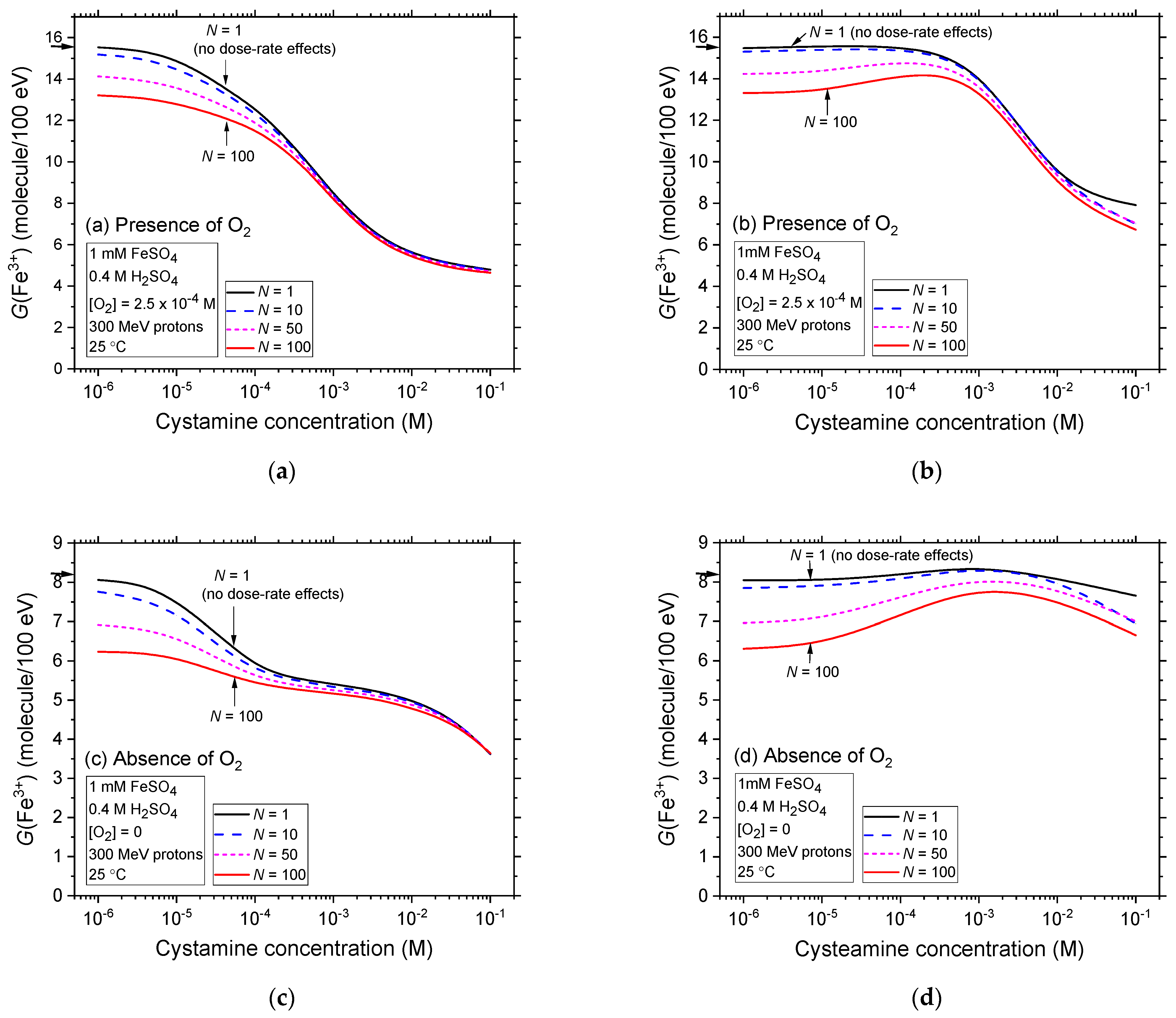
2.4. Comparison of Fe3+ Ion Yields in Aerated and Deaerated Fricke-Cystamine and Fricke-Cysteamine Solutions Exposed to 300 MeV (~0.3 keV/μm) Proton Irradiation, in the Presence of Dose-Rate Effects
3. Materials and Methods
3.1. The Aqueous Ferrous Sulfate (Fricke) Chemical Dosimeter
3.2. Monte Carlo Chemical Modeling of Radiolysis in Fricke-Cystamine and Fricke-Cysteamine Solutions: Chemical Reaction Scheme and Effects of LET and Dose Rate
3.2.1. The IONLYS-IRT Simulation Code and the Chemical Reaction Scheme
3.2.2. Assessing the Effect of LET with Protons of Various Energies
3.2.3. Modeling Dose-Rate Effects Using the “Instantaneous Pulse” (Dirac) Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A.; Kirkpatrick, D.R.; Smith, S.; Smith, T.K.; Pearson, T.; Kailasam, A.; Herrmann, K.Z.; Schubert, J.; Agrawal, D.K. Radioprotective agents to prevent cellular damage due to ionizing radiation. J. Transl. Med. 2017, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 8th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2019; pp. 126–134. [Google Scholar]
- Maier, P.; Wenz, F.; Herskind, C. Radioprotection of normal tissue cells. Strahlenther. Onkol. 2014, 190, 745–752. [Google Scholar] [CrossRef]
- Vasin, M.V. Comments on the mechanisms of action of radiation protective agents: Basis components and their polyvalence. SpringerPlus 2014, 3, 414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Li, Z.; Wu, H.; Zou, B.; Xu, Y. Exploring natural products as radioprotective agents for cancer therapy: Mechanisms, challenges, and opportunities. Cancers 2023, 15, 3585. [Google Scholar] [CrossRef] [PubMed]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Favaudon, V.; Fouillade, C.; Vozenin, M.-C. Radiothérapie « FLASH » à très haut débit de dose: Un moyen d’augmenter l’indice thérapeutique par minimisation des dommages aux tissus sains? Ultrahigh dose-rate, “FLASH” irradiation minimizes the side-effects of radiotherapy. Cancer Radiother. 2015, 19, 526–531. [Google Scholar] [CrossRef]
- Esplen, N.; Mendonca, M.S.; Bazalova-Carter, M. Physics and biology of ultrahigh dose-rate (FLASH) radiotherapy: A topical review. Phys. Med. Biol. 2020, 65, 23TR03. [Google Scholar] [CrossRef]
- Bogaerts, E.; Macaeva, E.; Isebaert, S.; Haustermans, K. Potential molecular mechanisms behind the ultra-high dose rate “FLASH” effect. Int. J. Mol. Sci. 2022, 23, 12109. [Google Scholar] [CrossRef]
- Chow, J.C.L.; Ruda, H.E. FLASH radiotherapy: Innovative cancer treatment. Encyclopedia 2023, 3, 808–823. [Google Scholar] [CrossRef]
- Hughes, J.R.; Parsons, J.L. FLASH radiotherapy: Current knowledge and future insights using proton-beam therapy. Int. J. Mol. Sci. 2020, 21, 6492. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.L.; Ruda, H.E. Mechanisms of action in FLASH radiotherapy: A comprehensive review of physicochemical and biological processes on cancerous and normal cells. Cells 2024, 13, 835. [Google Scholar] [CrossRef] [PubMed]
- Borghini, A.; Labate, L.; Piccinini, S.; Panaino, C.M.V.; Andreassi, M.G.; Gizzi, L.A. FLASH radiotherapy: Expectations, challenges, and current knowledge. Int. J. Mol. Sci. 2024, 25, 2546. [Google Scholar] [CrossRef] [PubMed]
- Conklin, J.J.; Walker, R.I. (Eds.) Military Radiobiology; Academic Press: Orlando, FL, USA, 1987. [Google Scholar]
- Bump, E.A.; Malaker, K. (Eds.) Radioprotectors: Chemical, Biological, and Clinical Perspectives; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Dziegielewski, J.; Goetz, W.; Baulch, J.E. Heavy ions, radioprotectors and genomic instability: Implications for human space exploration. Radiat. Environ. Biophys. 2010, 49, 303–316. [Google Scholar] [CrossRef]
- Nukala, U.; Thakkar, S.; Krager, K.J.; Breen, P.J.; Compadre, C.M.; Aykin-Burns, N. Antioxidant tocols as radiation countermeasures (challenges to be addressed to use tocols as radiation countermeasures in humans). Antioxidants 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair: A Chemical Perspective; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Buxton, G.V. Radiation chemistry of the liquid state: (1) Water and homogeneous aqueous solutions. In Radiation Chemistry: Principles and Applications; Farhataziz, Rodgers, M.A.J., Eds.; VCH: New York, NY, USA, 1987; pp. 321–349. [Google Scholar]
- Spinks, J.W.T.; Woods, R.J. An Introduction to Radiation Chemistry, 3rd ed.; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Ferradini, C.; Jay-Gerin, J.-P. La radiolyse de l’eau et des solutions aqueuses: Historique et actualité. Can. J. Chem. 1999, 77, 1542–1575. [Google Scholar] [CrossRef]
- Elliot, A.J.; Bartels, D.M. The Reaction Set, Rate Constants and g-Values for the Simulation of the Radiolysis of Light. Water over the Range 20 to 350 °C Based on Information Available in 2008; Report No. 153-127160-450-001; Atomic Energy of Canada Limited: Mississauga, ON, Canada, 2009. [Google Scholar]
- Klassen, N.V. Primary species in irradiated water. J. Chim. Phys. 1991, 88, 747–757. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- International Commission on Radiation Units and Measurements. Linear Energy Transfer; ICRU Report No. 16; International Commission on Radiation Units and Measurements: Washington, DC, USA, 1970. [Google Scholar]
- Magee, J.L. Radiation chemistry. Annu. Rev. Nucl. Sci. 1953, 3, 171–192. [Google Scholar] [CrossRef]
- Freeman, G.R. Basics of radiation chemistry. In The Study of Fast Processes and Transient Species by Electron Pulse Radiolysis: Proceedings of the NATO Advanced Study Institute Held at Capri, Italy, 7–18 September 1981; Baxendale, J.H., Busi, F., Eds.; Reidel Publishing: Dordrecht, The Netherlands, 1982; pp. 19–34. [Google Scholar] [CrossRef]
- Mozumder, A. Fundamentals of Radiation Chemistry; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- LaVerne, J.A. Radiation chemical effects of heavy ions. In Charged Particle and Photon Interactions with Matter: Chemical, Physicochemical, and Biological Consequences with Applications; Mozumder, A., Hatano, Y., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 403–429. [Google Scholar]
- Meesungnoen, J.; Jay-Gerin, J.-P. Radiation chemistry of liquid water with heavy ions: Monte Carlo simulation studies. In Charged Particle and Photon Interactions with Matter: Recent Advances, Applications, and Interfaces; Hatano, Y., Katsumura, Y., Mozumder, A., Eds.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2011; pp. 355–400. [Google Scholar]
- Alanazi, A.; Meesungnoen, J.; Jay-Gerin, J.-P. A computer modeling study of water radiolysis at high dose rates. Relevance to FLASH radiotherapy. Radiat. Res. 2021, 195, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Kuppermann, A. Diffusion kinetics in radiation chemistry. In Actions Chimiques et Biologiques des Radiations; Haïssinsky, M., Ed.; Masson: Paris, France, 1961; Volume 5, pp. 85–166. [Google Scholar]
- Bepari, M.I.; Meesungnoen, J.; Jay-Gerin, J.-P. Early and transient formation of highly acidic pH spikes in water radiolysis under the combined effect of high dose rate and high linear energy transfer. Radiation 2023, 3, 165–182. [Google Scholar] [CrossRef]
- Sepulveda, E.; Sanguanmith, S.; Meesungnoen, J.; Jay-Gerin, J.-P. Evaluation of the radioprotective ability of cystamine for 150 keV–500 MeV proton irradiation: A Monte Carlo track chemistry simulation study. Can. J. Chem. 2019, 97, 100–111. [Google Scholar] [CrossRef]
- Patt, H.M.; Tyree, E.B.; Straube, R.L.; Smith, D.E. Cysteine protection against X irradiation. Science 1949, 110, 213–214. [Google Scholar] [CrossRef]
- Bacq, Z.-M.; Alexander, P. Principes de Radiobiologie; Masson: Paris, France, 1955; pp. 361–407. [Google Scholar]
- Bacq, Z.-M.; Beaumariage, M.L. Action radioprotectrice de la cystéamine et de la cystamine chez la souris en fonction du temps séparant l’injection du protecteur du début de l’irradiation par rayons X. Arch. Int. Pharmacodyn. Ther. 1965, 153, 457–459. [Google Scholar] [PubMed]
- Hollaender, A.; Doherty, D.G. Radiation Damage and Sulfhydryl Compounds; International Atomic Energy Agency: Vienna, Austria, 1969. [Google Scholar]
- Johnke, R.M.; Sattler, J.A.; Allison, R.R. Radioprotective agents for radiation therapy: Future trends. Future Oncol. 2014, 10, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. Chemical aspects of DNA radioprotection. In Radioprotectors and Anticarcinogens; Nygaard, O.F., Simić, M.G., Eds.; Academic Press: New York, NY, USA, 1983; pp. 73–85. [Google Scholar]
- Pinto, J.T.; Van Raamsdonk, J.M.; Leavitt, B.R.; Hayden, M.R.; Jeitner, T.M.; Thaler, H.T.; Krasnikov, B.F.; Cooper, A.J. Treatment of YAC128 mice and their wild-type littermates with cystamine does not lead to its accumulation in plasma or brain: Implications for the treatment of Huntington disease. J. Neurochem. 2005, 94, 1087–1101. [Google Scholar] [CrossRef]
- Jeitner, T.M.; Pinto, J.T.; Cooper, A.J.L. Cystamine and cysteamine as inhibitors of transglutaminase activity in vivo. Biosci. Rep. 2018, 38, BSR20180691. [Google Scholar] [CrossRef]
- Bousquet, M.; Gibrat, C.; Ouellet, M.; Rouillard, C.; Calon, F.; Cicchetti, F. Cystamine metabolism and brain transport properties: Clinical implications for neurodegenerative diseases. J. Neurochem. 2010, 114, 1651–1658. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. Therapeutic applications of cysteamine and cystamine in neurodegenerative and neuropsychiatric diseases. Front. Neurol. 2019, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Gibrat, C.; Cicchetti, F. Potential of cystamine and cysteamine in the treatment of neurodegenerative diseases. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 380–389. [Google Scholar] [CrossRef]
- Toohey, J.I. Sulfur metabolism in AIDS: Cystamine as an anti-HIV agent. AIDS Res. Hum. Retroviruses 2009, 25, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.G.; Wilbraham, A.C. The utilisation of the Fricke dosimeter for evaluating the biological radiation-protective potential of water-soluble organic compounds. Chem. Commun. 1968, 641–642. [Google Scholar] [CrossRef]
- Lalitha, B.; Mittal, J.P. Electron transfer reaction in the radiation chemistry of some biologically important disulphide compounds. Radiat. Eff. 1971, 7, 159–162. [Google Scholar] [CrossRef]
- Meesat, R.; Jay-Gerin, J.-P.; Khalil, A.; Lepage, M. Evaluation of the radiation-sensitizer/protector and/or antioxidant efficiencies using Fricke and PAG dosimeters. J. Phys. Conf. Ser. 2009, 164, 012006. [Google Scholar] [CrossRef]
- Meesat, R.; Sanguanmith, S.; Meesungnoen, J.; Lepage, M.; Khalil, A.; Jay-Gerin, J.-P. Utilization of the ferrous sulfate (Fricke) dosimeter for evaluating the radioprotective potential of cystamine: Experiment and Monte Carlo simulation. Radiat. Res. 2012, 177, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Penabeï, S.; Meesungnoen, J.; Jay-Gerin, J.-P. Assessment of cystamine’s radioprotective/antioxidant ability under high-dose-rate irradiation: A Monte Carlo multi-track chemistry simulation study. Antioxidants 2023, 12, 776. [Google Scholar] [CrossRef] [PubMed]
- Penabeï, S.; Sepulveda, E.; Zakaria, A.M.; Meesungnoen, J.; Jay-Gerin, J.-P. Effect of linear energy transfer on cystamine’s radioprotective activity: A study using the Fricke dosimeter with 6–500 MeV per nucleon carbon ions—Implication for carbon ion hadrontherapy. Molecules 2023, 28, 8144. [Google Scholar] [CrossRef]
- Fricke, H.; Morse, S. The chemical action of roentgen rays on dilute ferrosulphate solutions as a measure of dose. Am. J. Roentgenol. Radium Ther. 1927, 18, 430–432. [Google Scholar]
- Fricke, H.; Hart, E.J. Chemical dosimetry. In Radiation Dosimetry, 2nd ed.; Attix, F.H., Roesch, W.C., Eds.; Academic Press: New York, NY, USA, 1966; Volume II, pp. 167–239. [Google Scholar]
- Dewhurst, H.A. Effect of organic substances on the γ-ray oxidation of ferrous sulfate. J. Chem. Phys. 1951, 19, 129. [Google Scholar] [CrossRef]
- Guczi, L. Étude de l’effet d’addition de diverses substances sur l’oxydation des ions ferreux en solution aqueuse. J. Chim. Phys. 1962, 59, 795–796. [Google Scholar] [CrossRef]
- Das, R.C. Radiation chemistry of aqueous aerated ferrous sulphate solution. Radiat. Res. Rev. 1971, 3, 121–139. [Google Scholar]
- Matthews, R.W. Aqueous chemical dosimetry. Int. J. Appl. Radiat. Isot. 1982, 33, 1159–1170. [Google Scholar] [CrossRef]
- Schardt, D.; Elsässer, T.; Schulz-Ertner, D. Heavy-ion tumor therapy: Physical and radiobiological benefits. Rev. Mod. Phys. 2010, 82, 383–425. [Google Scholar] [CrossRef]
- Klassen, N.V.; Shortt, K.R.; Seuntjens, J.; Ross, C.K. Fricke dosimetry: The difference between G(Fe3+) for 60Co γ-rays and high-energy X-rays. Phys. Med. Biol. 1999, 44, 1609–1624. [Google Scholar] [CrossRef]
- McEwen, M.; El Gamal, I.; Mainegra-Hing, E.; Cojocaru, C. Determination of the Radiation Chemical Yield (G) for the Fricke Chemical Dosimetry System in Photon and Electron Beams; Report NRC-PIRS-1980; National Research Council Canada: Ottawa, ON, Canada, 2014. [Google Scholar] [CrossRef]
- International Commission on Radiation Units and Measurements. The Dosimetry of Pulsed Radiation; ICRU Report No. 34; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 1982. [Google Scholar]
- Allen, A.O. The Radiation Chemistry of Water and Aqueous Solutions; D. Van Nostrand Co.: Princeton, NJ, USA, 1961. [Google Scholar]
- International Commission on Radiation Units and Measurements. Radiation Dosimetry: X rays and Gamma Rays with Maximum Photon Energies Between 0.6 and 50 MeV; ICRU Report No. 14; International Commission on Radiation Units and Measurements: Washington, DC, USA, 1969. [Google Scholar]
- Autsavapromporn, N.; Meesungnoen, J.; Plante, I.; Jay-Gerin, J.-P. Monte Carlo simulation study of the effects of acidity and LET on the primary free-radical and molecular yields of water radiolysis—Application to the Fricke dosimeter. Can. J. Chem. 2007, 85, 214–229. [Google Scholar] [CrossRef]
- Tippayamontri, T.; Sanguanmith, S.; Meesungnoen, J.; Sunaryo, G.R.; Jay-Gerin, J.-P. Fast neutron radiolysis of the ferrous sulfate (Fricke) dosimeter: Monte Carlo simulations. In Recent Research Developments in Physical Chemistry; Pandalai, S.G., Ed.; Transworld Research Network: Trivandrum, India, 2009; Volume 10, pp. 143–211. [Google Scholar]
- Zakaria, A.M.; Lertnaisat, P.; Islam, M.M.; Meesungnoen, J.; Katsumura, Y.; Jay-Gerin, J.-P. Yield of the Fricke dosimeter irradiated with the recoil α and Li ions of the 10B(n,α)7Li nuclear reaction: Effects of multiple ionization and temperature. Can. J. Chem. 2021, 99, 425–435. [Google Scholar] [CrossRef]
- Christman, E.A.; Appleby, A.; Jayko, M. Radiation chemistry of high-energy carbon, neon, and argon ions: Integral yields from ferrous sulfate solutions. Radiat. Res. 1981, 85, 443–457. [Google Scholar] [CrossRef]
- LaVerne, J.A.; Schuler, R.H. Track effects in water radiolysis: Yields of the Fricke dosimeter for carbon ions with energies up to 1700 MeV. J. Phys. Chem. 1994, 98, 4043–4049. [Google Scholar] [CrossRef]
- LaVerne, J.A. Track effects of heavy ions in liquid water. Radiat. Res. 2000, 153, 487–496. [Google Scholar] [CrossRef]
- Pimblott, S.M.; LaVerne, J.A. Effects of track structure on the ion radiolysis of the Fricke dosimeter. J. Phys. Chem. A 2002, 106, 9420–9427. [Google Scholar] [CrossRef]
- Barendsen, G.W.; Walter, H.M.D. Effects of different ionizing radiations on human cells in tissue culture: IV. Modification of radiation damage. Radiat. Res. 1964, 21, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Salvador, R.; Villaescusa, J.I.; Soriano, J.M.; Estrela, J.M.; Montoro, A. Radioprotection and radiomitigation: From the bench to clinical practice. Biomedicines 2020, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Bĕgusová, M.; Pimblott, S.M. Stochastic simulation of γ radiolysis of acidic ferrous sulfate solution at elevated temperatures. Radiat. Prot. Dosim. 2002, 99, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Ferradini, C.; Jay-Gerin, J.-P. The effect of pH on water radiolysis: A still open question—A minireview. Res. Chem. Intermed. 2000, 26, 549–565. [Google Scholar] [CrossRef]
- Cobut, V.; Frongillo, Y.; Patau, J.P.; Goulet, T.; Fraser, M.-J.; Jay-Gerin, J.-P. Monte Carlo simulation of fast electron and proton tracks in liquid water—I. Physical and physicochemical aspects. Radiat. Phys. Chem. 1998, 51, 229–243. [Google Scholar] [CrossRef]
- Frongillo, Y.; Goulet, T.; Fraser, M.-J.; Cobut, V.; Patau, J.P.; Jay-Gerin, J.-P. Monte Carlo simulation of fast electron and proton tracks in liquid water—II. Nonhomogeneous chemistry. Radiat. Phys. Chem. 1998, 51, 245–254. [Google Scholar] [CrossRef]
- Tachiya, M. Theory of diffusion-controlled reactions: Formulation of the bulk reaction rate in terms of the pair probability. Radiat. Phys. Chem. 1983, 21, 167–175. [Google Scholar] [CrossRef]
- Pimblott, S.M.; Pilling, M.J.; Green, N.J.B. Stochastic models of spur kinetics in water. Radiat. Phys. Chem. 1991, 37, 377–388. [Google Scholar] [CrossRef]
- Pimblott, S.M.; Green, N.J.B. Recent advances in the kinetics of radiolytic processes. In Research in Chemical Kinetics; Compton, R.G., Hancock, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 3, pp. 117–174. [Google Scholar] [CrossRef]
- Plante, I. Développement de Codes de Simulation Monte Carlo de la Radiolyse de l’Eau par des Électrons, Ions Lourds, Photons et Neutrons. Applications à Divers Sujets d’Intérêt Expérimental. Ph.D. Thesis, Université de Sherbrooke, Sherbrooke, QC, Canada, 2009. [Google Scholar]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Schmidt, K.H.; Bartels, D.M. Lack of ionic strength effect in the recombination of hydrated electrons: (e−)aq + (e−)aq → 2(OH−) + H2. Chem. Phys. 1995, 190, 145–152. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Salimi-Amiri, M.; Karimi, F.; Khalilzadeh, M.A.; Baghayeri, M. A voltametric sensor based on NiO nanoparticle-modified carbon-paste electrode for determination of cysteamine in the presence of high concentration of tryptophan. J. Chem. 2013, 2013, 946230. [Google Scholar] [CrossRef]
- Watt, D.E. Quantities for Dosimetry of Ionizing Radiations in Liquid Water; Taylor & Francis: London, UK, 1996. [Google Scholar]
- International Commission on Radiation Units and Measurements. Stopping Powers and Ranges for Protons and Alpha Particles; ICRU Report No. 49; International Commission on Radiation Units and Measurements: Bethesda, DC, USA, 1993. [Google Scholar]
- Sultana, A.; Alanazi, A.; Meesungnoen, J.; Jay-Gerin, J.-P. Generation of ultrafast, transient, highly acidic pH spikes in the radiolysis of water at very high dose rates: Relevance for FLASH radiotherapy. Can. J. Chem. 2022, 100, 272–279. [Google Scholar] [CrossRef]
- Zakaria, A.M.; Colangelo, N.W.; Meesungnoen, J.; Jay-Gerin, J.-P. Transient hypoxia in water irradiated by swift carbon ions at ultra-high dose rates: Implication for FLASH carbon-ion therapy. Can. J. Chem. 2021, 99, 842–849. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penabeï, S.; Meesungnoen, J.; Jay-Gerin, J.-P. Comparative Analysis of Cystamine and Cysteamine as Radioprotectors and Antioxidants: Insights from Monte Carlo Chemical Modeling under High Linear Energy Transfer Radiation and High Dose Rates. Int. J. Mol. Sci. 2024, 25, 10490. https://doi.org/10.3390/ijms251910490
Penabeï S, Meesungnoen J, Jay-Gerin J-P. Comparative Analysis of Cystamine and Cysteamine as Radioprotectors and Antioxidants: Insights from Monte Carlo Chemical Modeling under High Linear Energy Transfer Radiation and High Dose Rates. International Journal of Molecular Sciences. 2024; 25(19):10490. https://doi.org/10.3390/ijms251910490
Chicago/Turabian StylePenabeï, Samafou, Jintana Meesungnoen, and Jean-Paul Jay-Gerin. 2024. "Comparative Analysis of Cystamine and Cysteamine as Radioprotectors and Antioxidants: Insights from Monte Carlo Chemical Modeling under High Linear Energy Transfer Radiation and High Dose Rates" International Journal of Molecular Sciences 25, no. 19: 10490. https://doi.org/10.3390/ijms251910490





