Impact of Proton Irradiation Depending on Breast Cancer Subtype in Patient-Derived Cell Lines
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Obtaining a Biological Model Consisting of Primary Cell Cultures
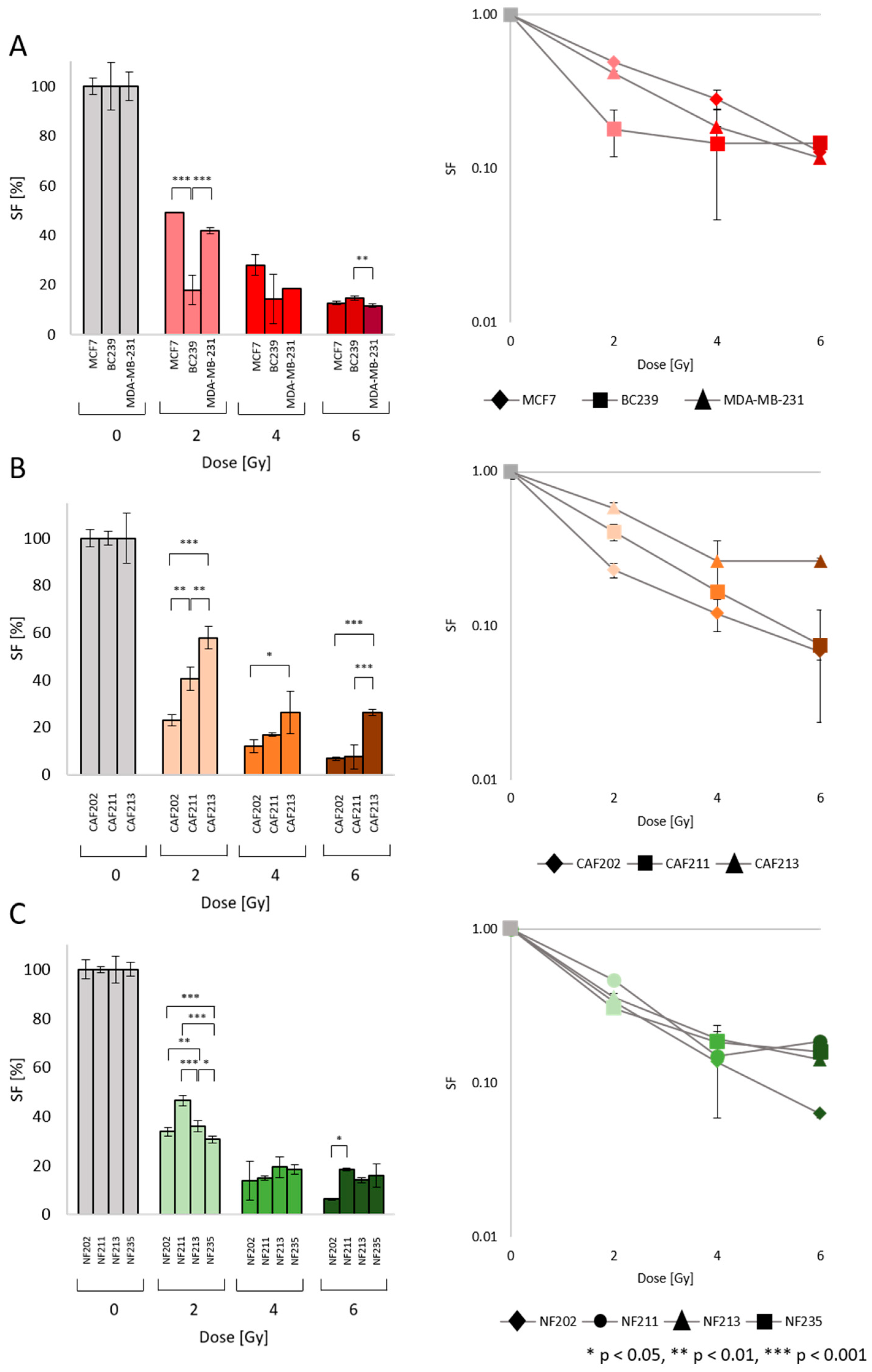
2.2. Survival Fraction (SF): Heterogenic Radiobiological Response
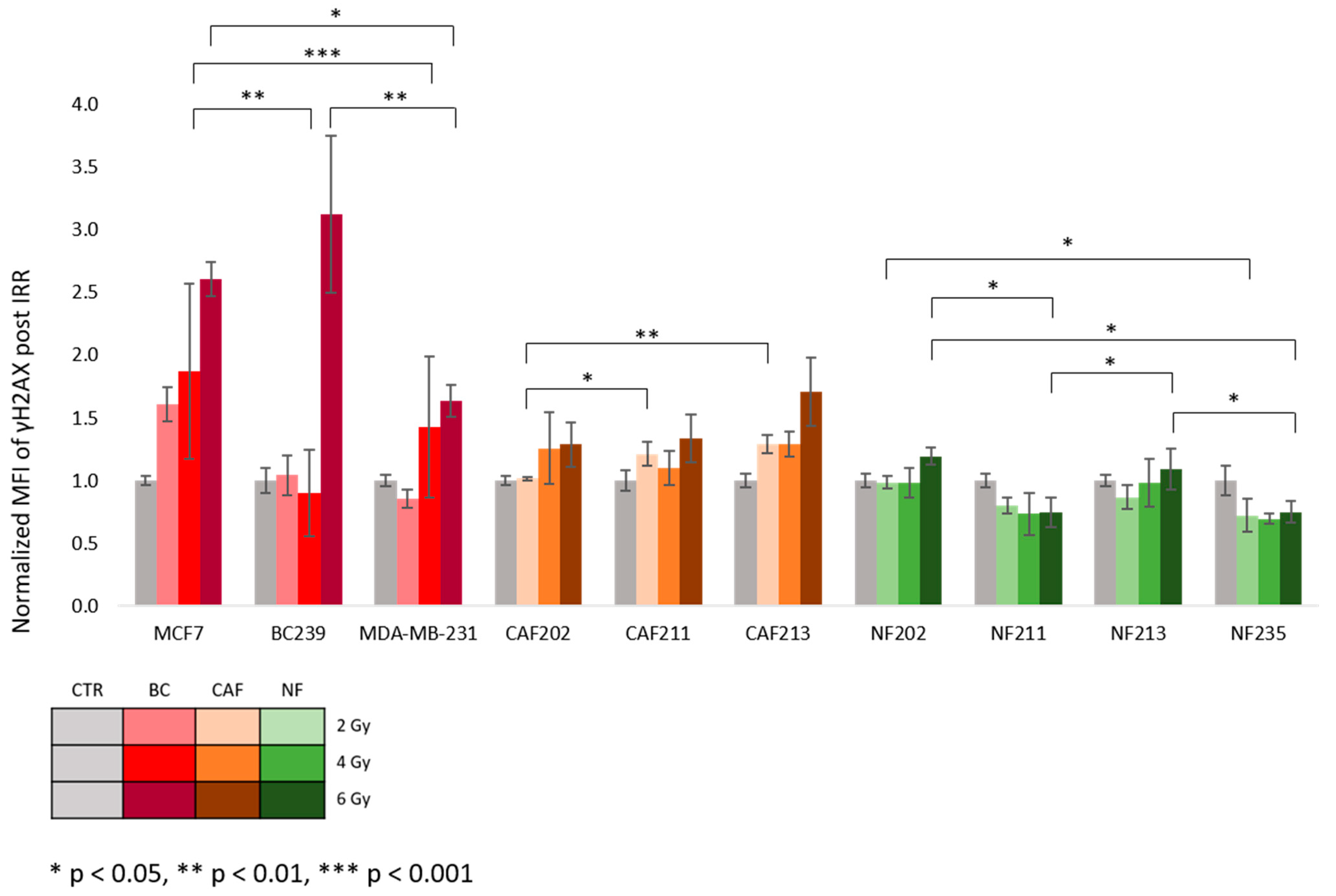
2.3. The DNA Double-Strand Breaks and p53 Expression after Proton IRR
3. Discussion
4. Methods
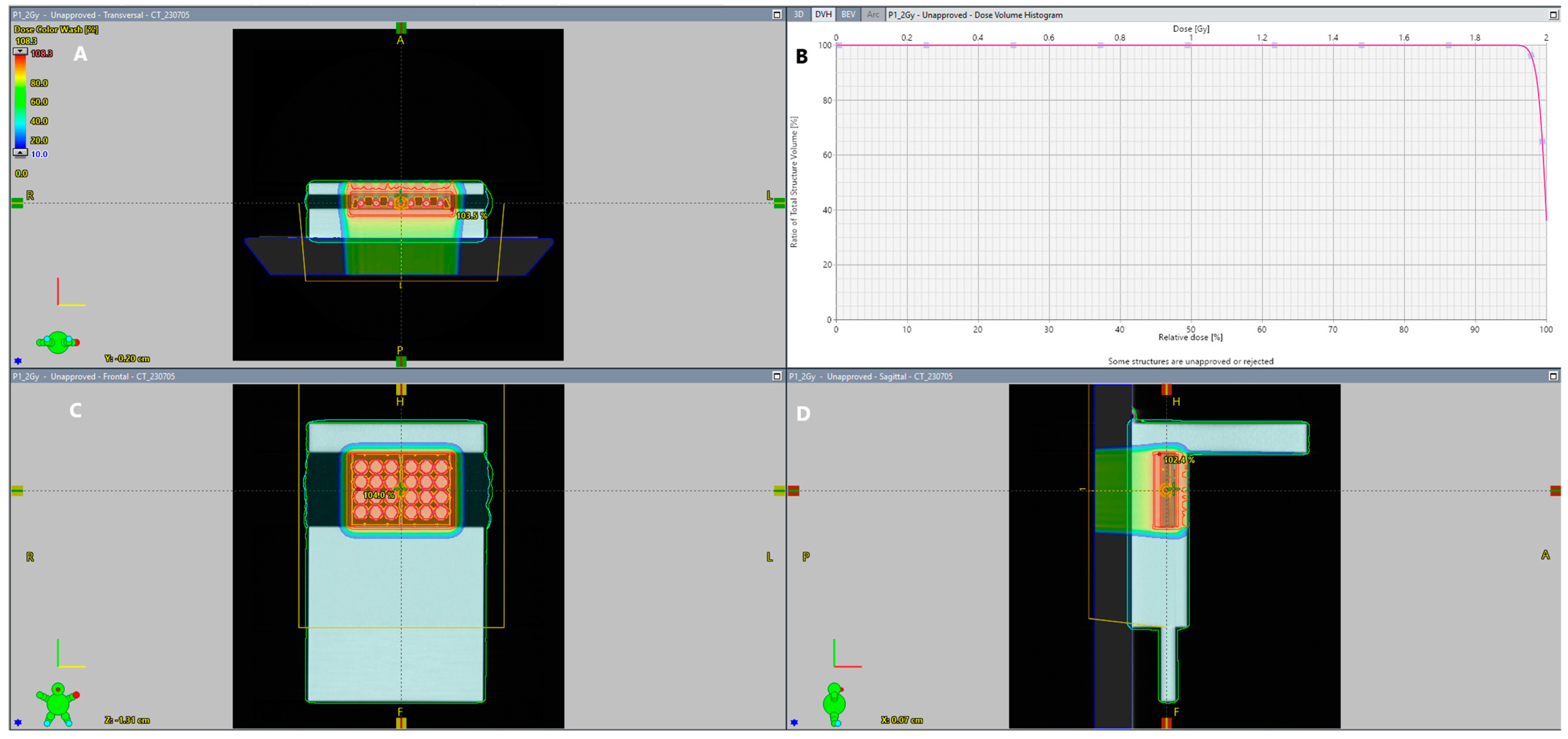
4.1. IRR of Tested Cells
4.2. Patients’ Material and Isolation
4.3. Cell Culture
4.4. Primary Cell Line Phenotyping
4.5. Clonogenic Assay
4.6. Flow Cytometry Analysis after IRR
4.7. Quantitative PCR
4.8. Western Blot
4.9. Statistical Analysis
5. Conclusions
6. Ethics Approval and Consent to Participate
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haussmann, J.; Corradini, S.; Nestle-Kraemling, C.; Bölke, E.; Njanang, F.J.D.; Tamaskovics, B.; Orth, K.; Ruckhaeberle, E.; Fehm, T.; Mohrmann, S.; et al. Recent Advances in Radiotherapy of Breast Cancer. Radiat. Oncol. 2020, 15, 71. [Google Scholar] [CrossRef]
- Mohan, R.; Grosshans, D. Proton Therapy—Present and Future. Adv. Drug Deliv. Rev. 2017, 109, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Musielak, M.; Suchorska, W.M.; Fundowicz, M.; Milecki, P.; Malicki, J. Future Perspectives of Proton Therapy in Minimizing the Toxicity of Breast Cancer Radiotherapy. J. Pers. Med. 2021, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Soumarová, R.; Rušinová, L. Cardiotoxicity of Breast Cancer Radiotherapy—Overview of Current Results. Rep. Pract. Oncol. Radiother. 2020, 25, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun. Signal 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, H.; Fu, Y.; Kuang, J.; Zhao, B.; Zhang, L.; Lin, J.; Lin, S.; Wu, D.; Xie, G. Cancer-Associated Fibroblasts Induce Growth and Radioresistance of Breast Cancer Cells through Paracrine IL-6. Cell Death Discov. 2023, 9, 6. [Google Scholar] [CrossRef]
- Hu, D.; Li, Z.; Zheng, B.; Lin, X.; Pan, Y.; Gong, P.; Zhuo, W.; Hu, Y.; Chen, C.; Chen, L.; et al. Cancer-associated Fibroblasts in Breast Cancer: Challenges and Opportunities. Cancer Commun. 2022, 42, 401–434. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Luo, H.; Tu, G.; Liu, Z.; Liu, M. Cancer-Associated Fibroblasts: A Multifaceted Driver of Breast Cancer Progression. Cancer Lett. 2015, 361, 155–163. [Google Scholar] [CrossRef]
- Mackay, A.; Weigelt, B.; Grigoriadis, A.; Kreike, B.; Natrajan, R.; A’Hern, R.; Tan, D.S.P.; Dowsett, M.; Ashworth, A.; Reis-Filho, J.S. Microarray-Based Class Discovery for Molecular Classification of Breast Cancer: Analysis of Interobserver Agreement. JNCI J. Natl. Cancer Inst. 2011, 103, 662–673. [Google Scholar] [CrossRef]
- Morgan, M.M.; Johnson, B.P.; Livingston, M.K.; Schuler, L.A.; Alarid, E.T.; Sung, K.E.; Beebe, D.J. Personalized in Vitro Cancer Models to Predict Therapeutic Response: Challenges and a Framework for Improvement. Pharmacol. Ther. 2016, 165, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Musielak, M.; Piwocka, O.; Kulcenty, K.; Ampuła, K.; Adamczyk, B.; Piotrowski, I.; Fundowicz, M.; Kruszyna-Mochalska, M.; Suchorska, W.M.; Malicki, J. Biological Heterogeneity of Primary Cancer-Associated Fibroblasts Determines the Breast Cancer Microenvironment. Am. J. Cancer Res. 2022, 12, 4411–4427. [Google Scholar] [PubMed]
- He, L.; Lv, Y.; Song, Y.; Zhang, B. The Prognosis Comparison of Different Molecular Subtypes of Breast Tumors after Radiotherapy and the Intrinsic Reasons for Their Distinct Radiosensitivity. Cancer Manag. Res. 2019, 11, 5765–5775. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Wang, L. In Vitro Human Cell Line Models to Predict Clinical Response to Anticancer Drugs. Pharmacogenomics 2015, 16, 273–285. [Google Scholar] [CrossRef]
- Richter, M.; Piwocka, O.; Musielak, M.; Piotrowski, I.; Suchorska, W.M.; Trzeciak, T. From Donor to the Lab: A Fascinating Journey of Primary Cell Lines. Front. Cell Dev. Biol. 2021, 9, 711381. [Google Scholar] [CrossRef]
- Piwocka, O.; Musielak, M.; Ampuła, K.; Piotrowski, I.; Adamczyk, B.; Fundowicz, M.; Suchorska, W.M.; Malicki, J. Navigating Challenges: Optimising Methods for Primary Cell Culture Isolation. Cancer Cell Int. 2024, 24, 28. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Cremers, N.; Kroese, F.; Orend, G.; Chiquet-Ehrismann, R.; Uede, T.; Yagita, H.; Sleeman, J.P. CD24 Expression Causes the Acquisition of Multiple Cellular Properties Associated with Tumor Growth and Metastasis. Cancer Res. 2005, 65, 10783–10793. [Google Scholar] [CrossRef]
- Kinugasa, Y.; Matsui, T.; Takakura, N. CD44 Expressed on Cancer-Associated Fibroblasts Is a Functional Molecule Supporting the Stemness and Drug Resistance of Malignant Cancer Cells in the Tumor Microenvironment. Stem Cells 2014, 32, 145–156. [Google Scholar] [CrossRef]
- Zeltz, C.; Primac, I.; Erusappan, P.; Alam, J.; Noel, A.; Gullberg, D. Cancer-Associated Fibroblasts in Desmoplastic Tumors: Emerging Role of Integrins. Semin. Cancer Biol. 2020, 62, 166–181. [Google Scholar] [CrossRef]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479.e10. [Google Scholar] [CrossRef] [PubMed]
- Barron, L.; Gharib, S.A.; Duffield, J.S. Lung Pericytes and Resident Fibroblasts. Am. J. Pathol. 2016, 186, 2519–2531. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Gires, O. EpCAM (CD326) Finding Its Role in Cancer. Br. J. Cancer 2007, 96, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Piwocka, O.; Musielak, M.; Piotrowski, I.; Kulcenty, K.; Adamczyk, B.; Fundowicz, M.; Suchorska, W.M.; Malicki, J. Primary Cancer-Associated Fibroblasts Exhibit High Heterogeneity among Breast Cancer Subtypes. Rep. Pract. Oncol. Radiother. 2023, 28, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Jeng, M.-H.; Shupnik, M.A.; Bender, T.P.; Westin, E.H.; Bandyopadhyay, D.; Kumar, R.; Masamura, S.; Santen, R.J. Estrogen Receptor Expression and Function in Long-Term Estrogen-Deprived Human Breast Cancer Cells. Endocrinology 1998, 139, 4164–4174. [Google Scholar] [CrossRef] [PubMed]
- Bush, D.A.; Do, S.; Lum, S.; Garberoglio, C.; Mirshahidi, H.; Patyal, B.; Grove, R.; Slater, J.D. Partial Breast Radiation Therapy with Proton Beam: 5-Year Results with Cosmetic Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 501–505. [Google Scholar] [CrossRef]
- Cammarata, F.P.; Forte, G.I.; Broggi, G.; Bravatà, V.; Minafra, L.; Pisciotta, P.; Calvaruso, M.; Tringali, R.; Tomasello, B.; Torrisi, F.; et al. Molecular Investigation on a Triple Negative Breast Cancer Xenograft Model Exposed to Proton Beams. Int. J. Mol. Sci. 2020, 21, 6337. [Google Scholar] [CrossRef]
- Wang, B.; Liu, W.; Liu, C.; Du, K.; Guo, Z.; Zhang, G.; Huang, Z.; Lin, S.; Cen, B.; Tian, Y.; et al. Cancer-Associated Fibroblasts Promote Radioresistance of Breast Cancer Cells via the HGF/c-Met Signaling Pathway. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Girdhani, S.; Sachs, R.; Hlatky, L. Biological Effects of Proton Radiation: What We Know and Don’t Know. Radiat. Res. 2013, 179, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, F.; Campa, A.; Esposito, G.; Giardullo, P.; Belli, M.; Dini, V.; Meschini, S.; Simone, G.; Sorrentino, E.; Gerardi, S.; et al. Induction and Repair of DNA DSB as Revealed by H2AX Phosphorylation Foci in Human Fibroblasts Exposed to Low- and High-LET Radiation: Relationship with Early and Delayed Reproductive Cell Death. Radiat. Res. 2015, 183, 417–431. [Google Scholar] [CrossRef]
- Lee, Y.; Wang, Q.; Shuryak, I.; Brenner, D.J.; Turner, H.C. Development of a high-throughput γ-H2AX assay based on imaging flow cytometry. Radiat. Oncol. 2019, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, L.G.; Pirovano, G.; Savage, K.I.; Ghita, M.; Ottolenghi, A.; Prise, K.M.; Schettino, G. Use of the γ-H2AX assay to investigate DNA repair dynamics following multiple radiation exposures. PLoS ONE 2013, 8, e79541. [Google Scholar] [CrossRef] [PubMed]
- Alan Mitteer, R.; Wang, Y.; Shah, J.; Gordon, S.; Fager, M.; Butter, P.-P.; Jun Kim, H.; Guardiola-Salmeron, C.; Carabe-Fernandez, A.; Fan, Y. Proton Beam Radiation Induces DNA Damage and Cell Apoptosis in Glioma Stem Cells through Reactive Oxygen Species. Sci. Rep. 2015, 5, 13961. [Google Scholar] [CrossRef] [PubMed]
- Loewer, A.; Karanam, K.; Mock, C.; Lahav, G. The P53 Response in Single Cells Is Linearly Correlated to the Number of DNA Breaks without a Distinct Threshold. BMC Biol. 2013, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kong, Y.; Yang, Z.; Liu, Y.; Liu, R.; Geng, Y.; Luo, H.; Zhang, H.; Li, H.; Feng, S.; et al. Preliminary study on radiosensitivity to carbon ions in human breast cancer. J. Radiat. Res. 2020, 61, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Dibra, D.; Moyer, S.M.; El-Naggar, A.K.; Qi, Y.; Su, X.; Lozano, G. Triple-negative breast tumors are dependent on mutant p53 for growth and survival. Proc. Natl. Acad. Sci. USA 2023, 120, e2308807120. [Google Scholar] [CrossRef] [PubMed]
- Varna, M.; Bousquet, G.; Plassa, L.F.; Bertheau, P.; Janin, A. TP53 status and response to treatment in breast cancers. J. Biomed. Biotechnol. 2011, 2011, 284584. [Google Scholar] [CrossRef] [PubMed]
- Stewart-Ornstein, J.; Iwamoto, Y.; Miller, M.A.; Prytyskach, M.A.; Ferretti, S.; Holzer, P.; Kallen, J.; Furet, P.; Jambhekar, A.; Forrester, W.C.; et al. p53 dynamics vary between tissues and are linked with radiation sensitivity. Nat. Commun. 2021, 12, 898. [Google Scholar] [CrossRef]
- Ghaleb, A.; Yallowitz, A.; Marchenko, N. Irradiation induces p53 loss of heterozygosity in breast cancer expressing mutant p53. Commun. Biol. 2019, 2, 436. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Absalon, M.J.; McLure, K.G.; Kastan, M.B. Regulation of P53 Translation and Induction after DNA Damage by Ribosomal Protein L26 and Nucleolin. Cell 2005, 123, 49–63. [Google Scholar] [CrossRef]
- Brooks, C.L.; Gu, W. P53 Ubiquitination: Mdm2 and Beyond. Mol. Cell 2006, 21, 307–315. [Google Scholar] [CrossRef]
- Oeck, S.; Szymonowicz, K.; Wiel, G.; Krysztofiak, A.; Lambert, J.; Koska, B.; Iliakis, G.; Timmermann, B.; Jendrossek, V. Relating Linear Energy Transfer to the Formation and Resolution of DNA Repair Foci After Irradiation with Equal Doses of X-ray Photons, Plateau, or Bragg-Peak Protons. Int. J. Mol. Sci. 2018, 19, 3779. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, G.; Xiao, J.; Deng, H. Role and Potential Therapeutic Strategies of Matrix Mechanics for Optimizing Tumor Radiotherapy. Mechanobiol. Med. 2024, 2, 100037. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, L.; Yang, M.; Wu, X.; Wang, X.; Huang, W.; Yuan, L.; Pan, H.; Wang, Y.; Wang, Z.; et al. Cancer-Associated Fibroblasts Promote the Survival of Irradiated Nasopharyngeal Carcinoma Cells via the NF-κB Pathway. J. Exp. Clin. Cancer Res. 2021, 40, 87. [Google Scholar] [CrossRef]
- Aristei, C.; Perrucci, E.; Alì, E.; Marazzi, F.; Masiello, V.; Saldi, S.; Ingrosso, G. Personalization in Modern Radiation Oncology: Methods, Results and Pitfalls. Personalized Interventions and Breast Cancer. Front. Oncol. 2021, 11, 616042. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McNutt, T.R.; Benedict, S.H.; Low, D.A.; Moore, K.; Shpitser, I.; Jiang, W.; Lakshminarayanan, P.; Cheng, Z.; Han, P.; Hui, X.; et al. Using Big Data Analytics to Advance Precision Radiation Oncology. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, Y.; Mackin, D.; Li, H.; Poenisch, F.; Lee, A.; Mahajan, A.; Frank, S.; Gillin, M.; Sahoo, N.; et al. Towards Effective and Efficient Patient-Specific Quality Assurance for Spot Scanning Proton Therapy. Cancers 2015, 7, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Janik, K.; Popeda, M.; Peciak, J.; Rosiak, K.; Smolarz, M.; Treda, C.; Rieske, P.; Stoczynska-Fidelus, E.; Ksiazkiewicz, M. Efficient and Simple Approach to in Vitro Culture of Primary Epithelial Cancer Cells. Biosci. Rep. 2016, 36, e00423. [Google Scholar] [CrossRef]
- Michalak, M.; Lach, M.S.; Antoszczak, M.; Huczyński, A.; Suchorska, W.M. Overcoming Resistance to Platinum-Based Drugs in Ovarian Cancer by Salinomycin and Its Derivatives—An In Vitro Study. Molecules 2020, 25, 537. [Google Scholar] [CrossRef]

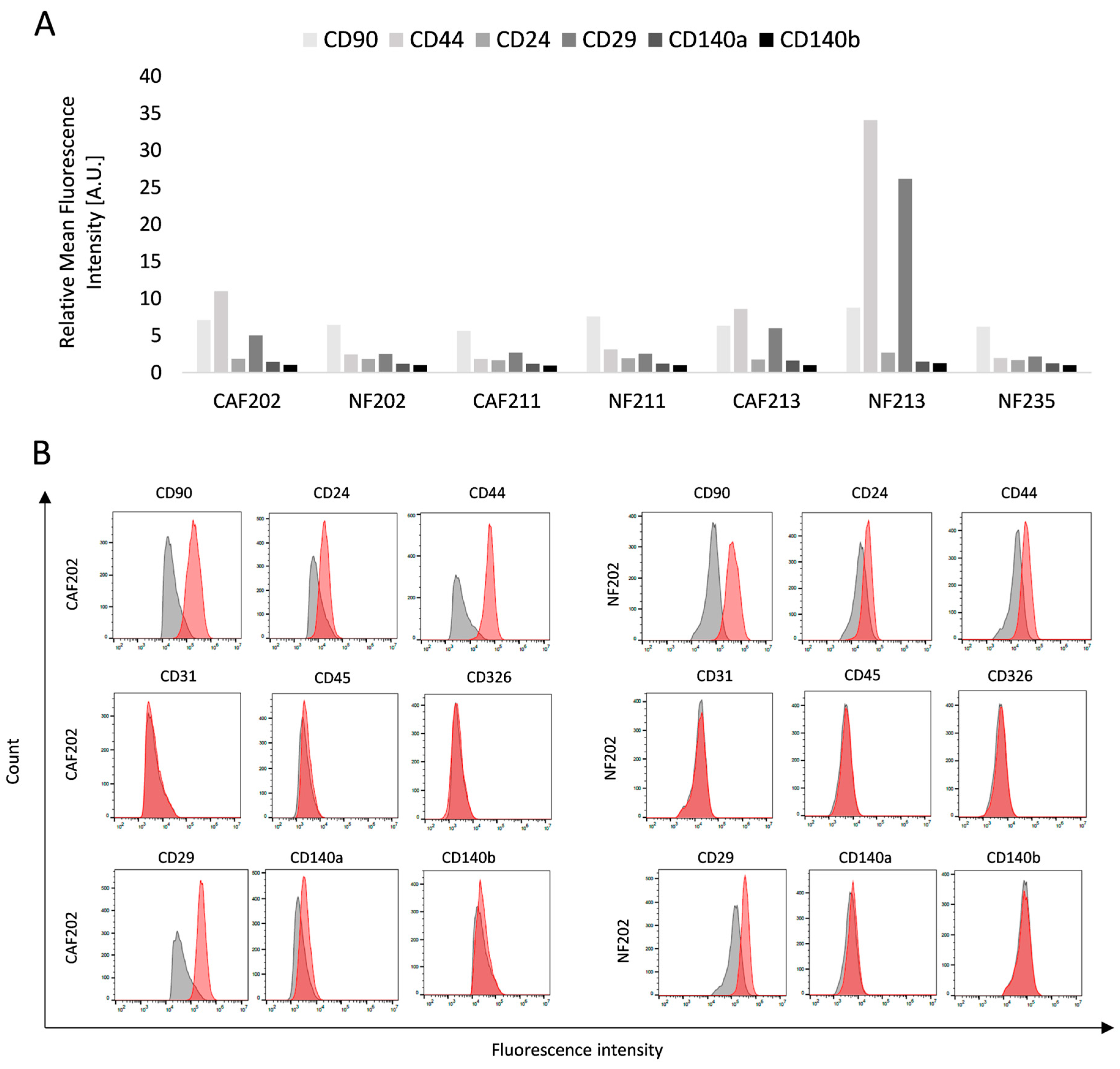



| Cell Line | Grade | Age | Side | ER | PR | HER2+ | Ki67 | BC Subtype |
|---|---|---|---|---|---|---|---|---|
| CAF202 NF202 | NHG2 | 79 | Right | + | + | − | 10% | Luminal A |
| CAF211 NF211 | NHG2 | 54 | Left | + | + | − | 10% | Luminal A |
| BC239 | NHG1 | 72 | Right | + | + | − | 10% | Luminal A |
| CAF213 NF213 | NHG3 | 89 | Right | − | − | − | 70% | TNBC |
| NF235 | NHG3 | 71 | Right | − | − | − | 80% | TNBC |
| Gene | Probe | Forward | Reverse |
|---|---|---|---|
| GAPDH | n/a | TCCACTGGCGTCTTCACC | GGCAGAGATGATGACCCTTTT |
| CD24 | 68 | AGCCTACTGCAAATCCAAACA | GAAGCTCTGAGAATTACTCTGCTG |
| NANOG | 31 | ATGCCTCACACGGAGACTGT | AAGTGGGTTGTTTGCCTTTG |
| COL1A2 | 18 | CACTCCTGGCACTGATGGT | CATTCCCTGAAGACCTGGAG |
| SNAIL1 | 62 | GCTGCAGGACTCTAATCCAGA | ATCTCCGGAGGTGGGATG |
| MMP2 | 67 | ATGCCGCCTTTAACTGGAG | GGAAGCCAGGATCCATTTTC |
| VIM | 24 | GGGACCTCTACGAGGAGGAG | CTTTGTCGTTGGTTAGCTGGT |
| CD44 | 31 | AGCCTACTGCAAATCCAAACA | GAAGCTCTGAGAATTACTCTGCTG |
| CDH1 | 77 | AAGTTTTCCACCAAAGTCACG | TGCTTGGATTCCAGAAACG |
| TP53 | 71 | CTTTCCACGACGGTGACA | TCCTCCATGGCAGTGACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musielak, M.; Graczyk, K.; Liszka, M.; Christou, A.; Rosochowicz, M.A.; Lach, M.S.; Adamczyk, B.; Suchorska, W.M.; Piotrowski, T.; Stenerlöw, B.; et al. Impact of Proton Irradiation Depending on Breast Cancer Subtype in Patient-Derived Cell Lines. Int. J. Mol. Sci. 2024, 25, 10494. https://doi.org/10.3390/ijms251910494
Musielak M, Graczyk K, Liszka M, Christou A, Rosochowicz MA, Lach MS, Adamczyk B, Suchorska WM, Piotrowski T, Stenerlöw B, et al. Impact of Proton Irradiation Depending on Breast Cancer Subtype in Patient-Derived Cell Lines. International Journal of Molecular Sciences. 2024; 25(19):10494. https://doi.org/10.3390/ijms251910494
Chicago/Turabian StyleMusielak, Marika, Kinga Graczyk, Małgorzata Liszka, Athanasia Christou, Monika A. Rosochowicz, Michał S. Lach, Beata Adamczyk, Wiktoria M. Suchorska, Tomasz Piotrowski, Bo Stenerlöw, and et al. 2024. "Impact of Proton Irradiation Depending on Breast Cancer Subtype in Patient-Derived Cell Lines" International Journal of Molecular Sciences 25, no. 19: 10494. https://doi.org/10.3390/ijms251910494






