Relationship between Protein, MicroRNA Expression in Extracellular Vesicles and Rice Seed Vigor
Abstract
:1. Introduction
2. Results
2.1. Rice Seed Germination
2.2. Extracellular Vesicles Extracted from Rice Seeds
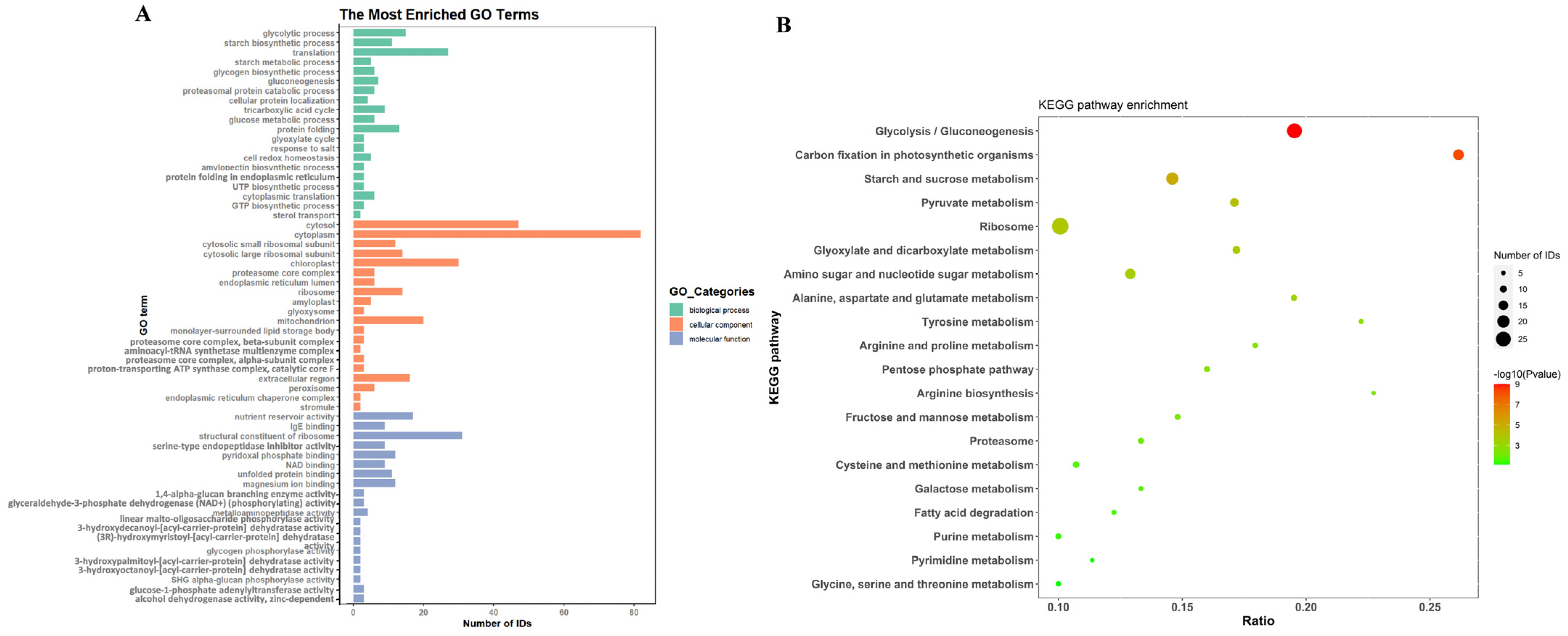
2.3. Proteins in EVs Derived from Rice Seeds
2.4. MicroRNA in EVs and Differential Expression Analysis
3. Discussion
3.1. The Role of EVs in Seed Vigor
3.2. Proteins Associated with Seed Vigor from EVs
3.3. Regulation of Seed Vigor by Four miRNA Families in EVs
4. Materials and Methods
4.1. Seed Germination Test
4.2. EVs Extraction from Rice Seeds
4.3. EVs Protein Extraction from Rice Seed
4.4. EVs RNA Extraction and miRNA Sequencing Analysis of Rice Seeds
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welsh, J.A.; Goberdhan, D.C.I.; O’DRiscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Xu, R.; Chen, L. Plant-derived exosomes: Research progress. J. Int. Pharm. Res. 2020, 47, 614–618. [Google Scholar]
- Zhao, M.; Li, S.M.; Zhang, L.; Cong, M.H.; Hu, L.H.; Qiao, H.Z. Research progress of plant-derived vesicles and their biomedical applications. Acta Pharm. Sin. 2021, 56, 2039−2047. [Google Scholar]
- Cao, M.; Diao, N.; Cai, X.; Chen, X.; Xiao, Y.; Guo, C.; Chen, D.; Zhang, X. Plant exosome nanovesicles (PENs): Green delivery platforms. Mater. Horizons 2023, 10, 3879–3894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, Y.; Zhang, Y.; Li, X. Advances in Plant Extracellular Vesicles and Analysis Techniques. Biotechnol. Bull. 2023, 39, 32–43. [Google Scholar]
- Feng, J.; Xiu, Q.; Huang, Y.; Troyer, Z.; Li, B.; Zheng, L. Plant-Derived Vesicle-Like Nanoparticles as Promising Biotherapeutic Tools: Present and Future. Adv. Mater. 2023, 35, e2207826. [Google Scholar] [CrossRef]
- Li, W.; Xie, R.; Yang, S.; Ren, L.; Zhao, R.; Wang, W. Research Progress of Plant Exosome-like Nanovesicles. Chin. J. Mod. Appl. Pharm. 2023, 40, 3459–3466. [Google Scholar]
- Akao, Y.; Kuranaga, Y.; Heishima, K.; Sugito, N.; Morikawa, K.; Ito, Y.; Soga, T.; Ito, T. Plant hvu-MIR168-3p enhances expression of glucose transporter 1 (SLC2A1) in human cells by silencing genes related to mitochondrial electron transport chain complex I. J. Nutr. Biochem. 2022, 101, 108922. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Voinnet, O. Origin, Biogenesis, and Activity of Plant MicroRNAs. Cell 2009, 136, 669–687. [Google Scholar] [CrossRef]
- Tang, G. siRNA and miRNA: An insight into RISCs. Trends Biochem. Sci. 2005, 30, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-W.; Meng, J.; Cui, J.; Luan, Y.-S. Characterization and Function of MicroRNA*s in Plants. Front. Plant Sci. 2017, 8, 2200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, J.; Xu, X.; Xiong, F. miRNA in Regulating Seed Development and the Response to Abiotic Stress in Plant: A Review. Chin. Agric. Sci. Bull. 2023, 39, 86–92. [Google Scholar]
- Jodder, J. miRNA-mediated regulation of auxin signaling pathway during plant development and stress responses. J. Biosci. 2020, 45, 91. [Google Scholar] [CrossRef]
- Vakilian, K.A. Machine learning improves our knowledge about miRNA functions towards plant abiotic stresses. Sci. Rep. 2020, 10, 3041. [Google Scholar] [CrossRef]
- Vashisht, I.; Mishra, P.; Pal, T.; Chanumolu, S.; Singh, T.R.; Chauhan, R.S. Mining NGS transcriptomes for miRNAs and dissecting their role in regulating growth, development, and secondary metabolites production in different organs of a medicinal herb, Picrorhiza kurroa. Planta 2015, 241, 1255–1268. [Google Scholar] [CrossRef]
- Middleton, H.; Yergeau, É.; Monard, C.; Combier, J.-P.; El Amrani, A. Rhizospheric Plant–Microbe Interactions: miRNAs as a Key Mediator. Trends Plant Sci. 2020, 26, 132–141. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, W.; Zhang, H.; Lian, Z.; Chen, H. Advances of miRNA-mediated regulatory roles in plant-microbe interaction. Chin. J. Biotech. 2022, 38, 1695–1705. [Google Scholar]
- Meng, G.; Liu, B.; Li, Y.; Ma, Y.; Wang, X.; Ma, Y.; Cai, X. Advances in Cross-kingdom Regulation of Gene Expression Via miRNA in Animal and Plant. Anim. Husb. Feed. Sci. 2022, 43, 7–13. [Google Scholar]
- Sun, Q.; Wang, J.-H.; Sun, B.-Q. Advances on Seed Vigor Physiological and Genetic Mechanisms. Agric. Sci. China 2007, 40, 48–53. [Google Scholar] [CrossRef]
- Gao, H.; Jing, L.; Chen, L.; Ju, J.; Wang, Y.; Zhu, J.; Yang, L.; Wang, Y. Effects of elevated atmospheric CO2 and temperature on seed vigor of rice under open-air field conditions. Chin. J. Rice Sci. 2016, 30, 371–379. [Google Scholar]
- Fang, Y.; Song, M. Research progress of seed vigor. Seed Sci. Technol. 2006, 24, 33–36. [Google Scholar]
- Liu, Y.; Wang, T. Research progress of seed vigor. J. Maize Sci. 2012, 20, 90–94. [Google Scholar]
- Zhang, H.; Hu, J. Seed Science; Science Press: Beijing, China, 2010. [Google Scholar]
- Mcdonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 77–237. [Google Scholar]
- Chen, X.; Zhang, A.; Han, Z.; Lu, H.; Ye, Y.; Zhou, Q. Effects of Drying Temperature and Drying Time on Seed Moisture Content and Seed Vigor and Its Correlation Analysis at Different Havest Period in Rice. Southwest China J. Agric. Sci. 2014, 27, 2331–2338. [Google Scholar]
- Liao, L.; Xia, S.; Dong, X.; Lu, H.; Liu, R. Effects of different storage conditions on seed germination ability of rice. China Seed Ind. 2013, 6, 48–50. [Google Scholar]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Liu, X.; Yan, F.; Zhou, Z. Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef]
- Mcdonald, M.B.J.; Nelson, C.J. Physiology of seed deterioration. Plant Growth Regul. 1986, 9, 86–87. [Google Scholar]
- Roberts, E.H. Storage Environment and the Control of Viability; Springer: Amsterdam, The Netherlands, 1972. [Google Scholar] [CrossRef]
- Wang, S.; He, B.; Wu, H.; Cai, Q.; Ramírez-Sánchez, O.; Abreu-Goodger, C.; Birch, P.R.; Jin, H. Plant mRNAs move into a fungal pathogen via extracellular vesicles to reduce infection. Cell Host Microbe 2024, 10, 93–105.e6. [Google Scholar] [CrossRef]
- De Palma, M.; Ambrosone, A.; Leone, A.; Del Gaudio, P.; Ruocco, M.; Turiák, L.; Bokka, R.; Fiume, I.; Tucci, M.; Pocsfalvi, G. Plant Roots Release Small Extracellular Vesicles with Antifungal Activity. Plants 2020, 9, 1777. [Google Scholar] [CrossRef]
- Ma, S.; Li, J.; Peng, Z. Study on the Variation of MDA Content in the Seeds of Pinus flexilis James During Artificial Aging Course. Seed 2011, 30, 9–14. [Google Scholar]
- Liu, N.-J.; Wang, N.; Bao, J.-J.; Zhu, H.-X.; Wang, L.-J.; Chen, X.-Y. Lipidomic Analysis Reveals the Importance of GIPCs in Arabidopsis Leaf Extracellular Vesicles. Mol. Plant 2020, 13, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Fafián-Labora, J.A.; Rodríguez-Navarro, J.A.; O’lOghlen, A. Small Extracellular Vesicles Have GST Activity and Ameliorate Senescence-Related Tissue Damage. Cell Metab. 2020, 7, 71–86.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Miller, D.P.; Kumar, A.; Teng, Y.; Sayed, M.; Mu, J.; Lei, C.; Sriwastva, M.K.; Zhang, L.; Yan, J.; et al. Plant-Derived Exosomal Nanoparticles Inhibit Pathogenicity of Porphyromonas gingivalis. iScience 2019, 21, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-Like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- van de Wakker, S.I.; Bauzá-Martinez, J.; Arceo, C.R.; Manjikian, H.; Blok, C.J.B.S.; Roefs, M.T.; Willms, E.; Maas, R.G.C.; Pronker, M.F.; de Jong, O.G.; et al. Size matters: Functional differences of small extracellular vesicle subpopulations in cardiac repair responses. J. Extracell. Vesicles 2023, 13, e12396. [Google Scholar] [CrossRef]
- Caponnetto, F.; Manini, I.; Skrap, M.; Palmai-Pallag, T.; Di Loreto, C.; Beltrami, A.P.; Cesselli, D.; Ferrari, E. Size-dependent cellular uptake of exosomes. Nanomedicine 2016, 13, 1011–1020. [Google Scholar] [CrossRef]
- Devaiah, S.P.; Pan, X.; Hong, Y.; Roth, M.; Welti, R.; Wang, X. Enhancing seed quality and viability by suppressing phospholipase D in Arabidopsis. Plant J. 2007, 50, 950–957. [Google Scholar] [CrossRef]
- Shin, J.-H.; Kim, S.-R.; An, G. Rice Aldehyde Dehydrogenase7 Is Needed for Seed Maturation and Viability. Plant Physiol. 2009, 149, 905–915. [Google Scholar] [CrossRef]
- Peng, L.; Sun, S.; Yang, B.; Zhao, J.; Li, W.; Huang, Z.; Li, Z.; He, Y.; Wang, Z. Genome-wide association study reveals that the cupin domain protein OsCDP3.10 regulates seed vigour in rice. Plant Biotechnol. J. 2022, 20, 485–498. [Google Scholar] [CrossRef]
- Yasuda, H.; Hirose, S.; Kawakatsu, T.; Wakasa, Y.; Takaiwa, F. Overexpression of BiP has Inhibitory Effects on the Accumulation of Seed Storage Proteins in Endosperm Cells of Rice. Plant Cell Physiol. 2015, 50, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, S.; Craig, E. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Debeaujon, I. Seed longevity: Survival and maintenance of high germination ability of dry seeds. Comptes Rendus Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef]
- Wakasa, Y.; Yasuda, H.; Oono, Y.; Kawakatsu, T.; Hirose, S.; Takahashi, H.; Hayashi, S.; Yang, L.; Takaiwa, F. Expression of ER quality control-related genes in response to changes in BiP1 levels in developing rice endosperm. Plant J. 2011, 65, 675–689. [Google Scholar] [CrossRef]
- Lu, D.-P.; Christopher, D.A. Endoplasmic reticulum stress activates the expression of a sub-group of protein disulfide isomerase genes and AtbZIP60 modulates the response in Arabidopsis thaliana. Mol. Genet. Genom. 2008, 280, 199–210. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yeu, S.Y.; Park, B.S.; Koh, H.J.; Song, J.T.; Seo, H.S. Protein disulfi de isomerase-like protein 1-1 controls endosperm development through regulation of the amount and composition of seed proteins in rice. PLoS ONE. 2012, 7, e44493. [Google Scholar]
- Zhang, X.; Tao, L.; Qiao, S.; Du, B.; Guo, C. Roles of Glutathione s-transferase in plant tolerance to abiotic stresses. China Biotechnol. 2017, 37, 92–98. [Google Scholar]
- Li, Y.; Wang, Y.; He, Y.-Q.; Ye, T.-T.; Huang, X.; Wu, H.; Ma, T.-X.; Pritchard, H.W.; Wang, X.-F.; Xue, H. Glutathionylation of a glycolytic enzyme promotes cell death and vigor loss during aging of elm seeds. Plant Physiol. 2024, 195, 2596–2616. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, S.; Wang, L.; Wu, D.; Cheng, H.; Du, X.; Mao, D.; Zhang, C.; Jiang, X. miR164c and miR168a regulate seed vigor in rice. J. Integr. Plant Biol. 2019, 62, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Mou, G.; Ji, C.; Xu, D.; Zhou, G. Advances in plant miR164 family. Chin. Bull. Life Sci. 2013, 25, 532–538. [Google Scholar]
- Huang, K.R. Elucidation of the miR164c-Guided Gene/Protein Interaction Network Controlling Seed Anti-Aging Ability in Rice. Master’s Thesis, Hunan Normal University, Changsha, China, 2021. [Google Scholar]
- Jiang, X.C. Study on Correlation of miRNA and Rice (Oryza sativa L.) Seed Vigor. Master’s Thesis, Hunan Normal University, Changsha, China, 2011. [Google Scholar]
- Ma, M.; Hou, C.; Wang, Q.; Zhang, C.; Liu, J.; Weng, X. Current progress on endoxylanase inhibitors in cereals. China Biotechnol. 2011, 31, 129–133. [Google Scholar]
- Liu, X.; Xie, X.; Zhu, D.; Chen, H. Interaction between xylanase and XIP-type xylanase inhibitor protein: A review. Microbiol. China 2020, 47, 2300–2308. [Google Scholar]
- Saqib, A.; Scheller, H.V.; Fredslund, F.; Welner, D.H. Molecular characteristics of plant UDP-arabinopyranose mutases. Glycobiology 2019, 29, 839–846. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, M.; Ye, J.; Hu, D.; Zhang, Y.; Li, Z.; Liu, J.; Sun, Y.; Wang, S.; Yuan, X.; et al. Brittle culm 25, which encodes an UDP-xylose synthase, affects cell wall properties in rice. Crop J. 2023, 11, 733–743. [Google Scholar] [CrossRef]
- Macovei, A.; Tuteja, N. microRNAs targeting DEAD-box helicases are involved in salinity stress response in rice (Oryza sativa L.). BMC Plant Biol. 2012, 12, 183. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, D.X.; Chen, X.; Li, D.H.; Zhu, L.Y.; Zhang, Y.J.; Li, J.; Bian, Z.; Liang, X.Y.; Cai, X.; et al. Exogenous plant miR168a specifically targets mammalian LDLRAPI: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef]
- Chen, Z.; Cai, J. Study on osa-miR168a-5p Targeted Regulation of Human ADD1 and E2F2 Gene Expression. Med. Inf. Jun 2020, 33, 64–66. [Google Scholar]
- Sun, Y. Effects and the Relevant Proteomics of Exogenous miR168a on Seed Vigor of Hybrid Rice. Master’s Thesis, Hunan Normal University, Changsha, China, 2019. [Google Scholar]
- Zhou, J.; Zhang, R.; Jia, X.; Tang, X.; Guo, Y.; Yang, H.; Zheng, X.; Qian, Q.; Qi, Y.; Zhang, Y. CRISPR-Cas9 mediated OsMIR168a knockout reveals its pleiotropy in rice. Plant Biotechnol. J. 2022, 20, 310–322. [Google Scholar] [CrossRef]
- Ma, X.; Nicole, M.-C.; Meteignier, L.-V.; Hong, N.; Wang, G.; Moffett, P. Different roles for RNA silencing and RNA processing components in virus recovery and virus-induced gene silencing in plants. J. Exp. Bot. 2015, 66, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, H.; Carbonell, A.; Hoyer, J.S.; Fahlgren, N.; Gilbert, K.B.; Takeda, A.; Giampetruzzi, A.; Ruiz, M.T.G.; McGinn, M.G.; Lowery, N.; et al. Roles and Programming of Arabidopsis ARGONAUTE Proteins during Turnip Mosaic Virus Infection. PLOS Pathog. 2015, 11, e1004755. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z. Origin of the Isocitratr Lyase (ICL) Gene in Plants and Functional Characterization of the Rice OsICL Gene. Master’s Thesis, Yangzhou University, Yangzhou, China, 2021. [Google Scholar]
- Hao, Z.; Yuan, J.; Liu, Y. Role of isocitrate dehydrogenase on oxidative stress in plants. Biotechnol. Bull. 2012, 6, 32–35. [Google Scholar]
- Wang, W.; Wang, J. CRISPR/Cas9-mediated editing of the rice nsLTPs family gene OsLTPL166. Mol. Plant Breed. 2022. Available online: https://kns.cnki.net/kcms/detail/46.1068.s.20220412.1651.022.html (accessed on 8 May 2024).
- Ma, Y.; Yu, B. nsLTPs Genes Involved in Plant Response to Stress: Research Progress. Chin. Agric. Sci. Bull. 2021, 37, 95–101. [Google Scholar]
- Boutrot, F.; Chantret, N.; Gautier, M.-F. Genome-wide analysis of the rice and arabidopsis non-specific lipid transfer protein (nsLtp) gene families and identification of wheat nsLtp genes by EST data mining. BMC Genom. 2008, 9, 86. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, W.; Lu, Z.; Ouyang, Y.; Chol, S.O.; Yao, J. A lipid transfer protein, OsLTPL36, is essential for seed development and seed quality in rice. Plant Sci. 2015, 239, 200–208. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, W.; Wei, J.; Jia, Y. Rice lipid transfer protein, OsLTPL23, controls seed germination by regulating starch-sugar conversion and ABA homeostasis. Front. Genet. 2023, 14, 1111318. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; Cui, Y.; Yan, X.; Ouyang, J.; Li, S. Lipid transfer protein, OsLTPL18, is essential for grain weight and seed germination in rice. Gene 2023, 883, 147671. [Google Scholar] [CrossRef]
- Zhou, Y.; He, N.; Wei, Q.; Wang, W.; Xue, X.; Zhang, S. Research Progress in HD-Zip Protein. Hubei Agric. Sci. 2010, 49, 2914–2917. [Google Scholar]
- Todkar, K.; Chikhi, L.; Desjardins, V.; El-Mortada, F.; Pépin, G.; Germain, M. Selective packaging of mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs. Nat. Commun. 2021, 12, 1971. [Google Scholar] [CrossRef]
- Liang, W.; Sagar, S.; Ravindran, R.; Najo, R.H.; Quiles, J.M.; Chi, L.; Diao, R.Y.; Woodall, B.P.; Leon, L.J.; Zumaya, E. Mitochondria are secreted in extracellular vesicles when lysosomal function is impaired. Nat. Commun. 2023, 14, 5031. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y. Research progress of mitophagy in plants. Hubei Agric. Sci. 2021, 60, 12–16,27. [Google Scholar]
- Parkhey, S.; Naithani, S.; Keshavkant, S. ROS production and lipid catabolism in desiccating Shorea robusta seeds during aging. Plant Physiol. Biochem. 2012, 57, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Xue, H.; Pritchard, H.W.; Wang, X. Changes in the mitochondrial protein profile due to ROS eruption during ageing of elm (Ulmus pumila L.) seeds. Plant Physiol. Biochem. 2017, 114, 72–87. [Google Scholar] [CrossRef]


| Rice Seed | Mean Diameter/nm | Concentration (Particles/mL) | Diameter/nm | Peak Concentration (Particles/mL) |
|---|---|---|---|---|
| H | 157.7 ± 68.8 | 1.60 × 10−11 | 160.4 | 5.50 × 10−6 |
| L | 152.4 ± 56.3 | 5.00 × 10−11 | 138.4 | 5.50 × 10−6 |
| M | 156 ± 70.2 | 4.20 × 10−11 | 143.3 | 5.20 × 10−6 |
| Accession | Description | Note |
|---|---|---|
| Q09151 | Glutelin type-A 3 | Highest Sequest HT score and most abundance |
| P14614 | Glutelin type-B 4 | Highest Sequest HT score and most abundance |
| P07728 | Glutelin type-A 1 | Highest Sequest HT score and most abundance |
| P07730 | Glutelin type-A 2 | Highest Sequest HT score and most abundance |
| A0A0P0W399 | Alpha-1,4 glucan phosphorylase (Fragment) | Highest Sequest HT score and most abundance |
| Q653V7 | Probable alpha-glucosidase | Highest Sequest HT score and most abundance |
| Q01401 | 1,4-alpha-glucan-branching enzyme, chloroplastic/amyloplastic | Highest Sequest HT score and most abundance |
| Most enriched pathway or terms | ||
| Q852L2 | Cupincin | Highest Sequest HT score and most abundance |
| seed vigor correlation | ||
| Q75GX9 | 63 kDa globulin-like protein | Highest Sequest HT score and most abundance |
| Q42456 | Aspartic proteinase oryzasin-1 | Highest Sequest HT score and most abundance |
| P37833 | Aspartate aminotransferase, cytoplasmic | Most enriched pathway or terms and |
| most abundance | ||
| P48494 | Triosephosphate isomerase, cytosolic | Most enriched pathway or terms and |
| Most abundance | ||
| Q53MW2 | Non-specific lipid-transfer protein | Most abundance |
| O82451 | Probable glutathione S-transferase GSTF2 | Most abundance |
| Q33AE4 | Phosphoglucomutase (alpha-D-glucose-1,6-bisphosphate-dependent) | Most enriched pathway or terms |
| Q9AUQ4 | Phosphoglucomutase (alpha-D-glucose-1,6-bisphosphate-dependent) | Most enriched pathway or terms |
| P17784 | Fructose-bisphosphate aldolase 1, cytoplasmic | Most enriched pathway or terms |
| Q5N725 | Fructose-bisphosphate aldolase 3, cytoplasmic | Most enriched pathway or terms |
| P42862 | Glucose-6-phosphate isomerase, cytosolic A | Most enriched pathway or terms |
| Q2QXR8 | Pyruvate kinase 2, cytosolic | Most enriched pathway or terms |
| Q0DCI1 | Pyrophosphate-fructose 6-phosphate 1-phosphotransferase subunit alpha | Most enriched pathway or terms |
| Q0DD75 | Pyrophosphate-fructose 6-phosphate 1-phosphotransferase subunit beta | Most enriched pathway or terms |
| Q2R1J8 | 30S ribosomal protein S4, chloroplastic | Most enriched pathway or terms |
| Q6K8B8 | 40S ribosomal protein S3a | Most enriched pathway or terms |
| Q75J18 | 60S ribosomal protein L13a-2, putative, expressed | Most enriched pathway or terms |
| Q2QNF3 | 60S ribosomal protein L2 | Most enriched pathway or terms |
| Q6K4T1 | 60S ribosomal protein L27 | Most enriched pathway or terms |
| Q6YY64 | 60S ribosomal protein L6 | Most enriched pathway or terms |
| P0CH34 | Ubiquitin-60S ribosomal protein L40-1 | Most enriched pathway or terms |
| Q948T6 | Lactoylglutathione lyase | Most enriched pathway or terms |
| Q7XUG1 | Malate synthase | Most enriched pathway or terms |
| Q5JKW5 | Malic enzyme | Most enriched pathway or terms |
| Q94JA2 | Malate dehydrogenase | Most enriched pathway or terms |
| Q7XDC8 | Malate dehydrogenase, cytoplasmic | Most enriched pathway or terms |
| Q6Z6M4 | Isocitrate lyase | Most enriched pathway or terms |
| Q7F280 | Isocitrate dehydrogenase [NADP] | Most enriched pathway or terms |
| Q0JBV4 | Isocitrate dehydrogenase [NADP] | Most enriched pathway or terms |
| Q9LST8 | Proteasome subunit beta | Most enriched pathway or terms |
| Q9LST4 | Proteasome subunit beta | Most enriched pathway or terms |
| Q0DS36 | Os03g0336100 protein | Highest Sequest HT score and most abundance |
| A0A0P0VUB4 | Os03g0197300 protein (Fragment) | Highest Sequest HT score and most abundance |
| A0A0P0V679 | Os01g0663400 protein (Fragment) | Highest Sequest HT score and most abundance |
| Q6ZK46 | Os08g0127900 protein | Highest Sequest HT score and most abundance |
| Q6Z7B0 | Heat shock 70 kDa protein BiP1 | Seed vigor correlation |
| Q53RJ5 | Heat shock 70 kDa protein BiP2 | Seed vigor correlation |
| Q69P84 | Aldehyde dehydrogenase (NAD(+)) | Seed vigor correlation |
| A0A0P0UZH3 | Phospholipase D (Fragment) | Seed vigor correlation |
| Q53LQ0 | Protein disulfide isomerase-like 1-1 | Seed vigor correlation |
| Q67IX6 | Protein disulfide isomerase-like 1-4 | Seed vigor correlation |
| Q942L2 | Protein disulfide isomerase-like 2-2 | Seed vigor correlation |
| Comparison Group | miRNA_ID | Rapdb | Description | Gene Count | Distinctiveness |
|---|---|---|---|---|---|
| H vs. L | osa-miR164e | Os03g0278000/Os06g0590800 | D-xylose metabolic process | 1 | p. adjust < 0.05 |
| UDP-D-xylose biosynthetic process | 1 | p. adjust < 0.05 | |||
| UDP-glucuronate decarboxylase activity | 1 | p. adjust < 0.05 | |||
| UDP-glycosyltransferase activity | 1 | ||||
| Intracellular membrane-bounded organelle | 1 | ||||
| NAD+ binding | 1 | ||||
| Lyase activity | 1 | ||||
| Carboxy-lyase activity | 1 | ||||
| Quercetin 7-O-glucosyltransferase activity | 1 | ||||
| Quercetin 3-O-glucosyltransferase activity | 1 | ||||
| Hexosyltransferase activity | 1 | ||||
| H vs. L, H vs. M | osa-miR159a.1 | Os11g0153400/Os03g0683866/Os02g0717400/Os03g0578900/Os12g0616400/Os05g0358700/Os01g0812000/Os05g0490600 | cell differentiation | 3 | p. adjust < 0.05 |
| Positive regulation of mitochondrial fusion | 1 | p. adjust < 0.05 | |||
| Intracellular distribution of mitochondria | 1 | p. adjust < 0.05 | |||
| Organelle localization | 1 | p. adjust < 0.05 | |||
| Organelle organization | 1 | p. adjust < 0.05 | |||
| Phospholipid catabolic process | 1 | p. adjust < 0.05 | |||
| Mitochondrion organization | 1 | ||||
| Protein polyubiquitination | 1 | ||||
| Regulation of catalytic activity | 1 | ||||
| Multicellular organism development | 1 | ||||
| Signal transduction | 1 | ||||
| Ubiquitin-dependent protein catabolic process | 1 | ||||
| Intracellular organelle | 1 | ||||
| Phospholipase D activity | 1 | ||||
| Ubiquitin conjugating enzyme activity | 1 | ||||
| GTPase activator activity | 1 | ||||
| Translation initiation factor activity | 1 | ||||
| Ligase activity | 1 | ||||
| Ubiquitin-protein transferase activity | 1 | ||||
| Ubiquitin protein ligase activity | 1 | ||||
| H vs. L, H vs. M | osa-miR159b | Os03g0578900/Os01g0812000/Os05g0490600 | Cell differentiation | 3 | p. adjust < 0.05 |
| Anther development | 1 | p. adjust < 0.05 | |||
| Pollen development | 1 | ||||
| Flower development | 1 | ||||
| mRNA binding | 1 | ||||
| H vs. L, L vs. M | osa-miR168a-5p | Os02g0831600/Os04g0566500/Os02g0672200/Os07g0529000 | Gene silencing by RNA | 3 | p. adjust < 0.05 |
| Post-transcriptional gene silencing by RNA | 2 | p. adjust < 0.05 | |||
| Glyoxylate cycle | 1 | p. adjust < 0.05 | |||
| Glyoxysome | 1 | p. adjust < 0.05 | |||
| Carboxylic acid metabolic process | 1 | p. adjust < 0.05 | |||
| Tricarboxylic acid cycle | 1 | p. adjust < 0.05 | |||
| Isocitrate lyase activity | 1 | p. adjust < 0.05 | |||
| Regulation of secondary shoot formation | 1 | p. adjust < 0.05 | |||
| Peroxisome | 1 | ||||
| Lyase activity | 1 | ||||
| H vs. L, L vs. M | osa-miR166a-3p/osa-miR166j-3p/osa-miR166b-3p/osa-miR166c-3p/osa-miR166d-3p/osa-miR166f | Os04g0571600/Os02g0676400/Os03g0640800/Os10g0480200/Os03g0109400/Os12g0612700 | Lipid binding | 4 | p. adjust < 0.05 |
| Xenobiotic detoxification by transmembrane export across the plasma membrane | 2 | p. adjust < 0.05 | |||
| Xenobiotic transmembrane transport | 2 | p. adjust < 0.05 | |||
| Xenobiotic transmembrane transporter activity | 2 | p. adjust < 0.05 | |||
| Antiporter activity | 2 | p. adjust < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Chen, B.; Jia, J.; Liu, J. Relationship between Protein, MicroRNA Expression in Extracellular Vesicles and Rice Seed Vigor. Int. J. Mol. Sci. 2024, 25, 10504. https://doi.org/10.3390/ijms251910504
Wu R, Chen B, Jia J, Liu J. Relationship between Protein, MicroRNA Expression in Extracellular Vesicles and Rice Seed Vigor. International Journal of Molecular Sciences. 2024; 25(19):10504. https://doi.org/10.3390/ijms251910504
Chicago/Turabian StyleWu, Rouxian, Bingxian Chen, Junting Jia, and Jun Liu. 2024. "Relationship between Protein, MicroRNA Expression in Extracellular Vesicles and Rice Seed Vigor" International Journal of Molecular Sciences 25, no. 19: 10504. https://doi.org/10.3390/ijms251910504






