Bovine Neutrophil β-Defensin-5 Provides Protection against Multidrug-Resistant Klebsiella pneumoniae via Regulating Pulmonary Inflammatory Response and Metabolic Response
Abstract
:1. Introduction
2. Results
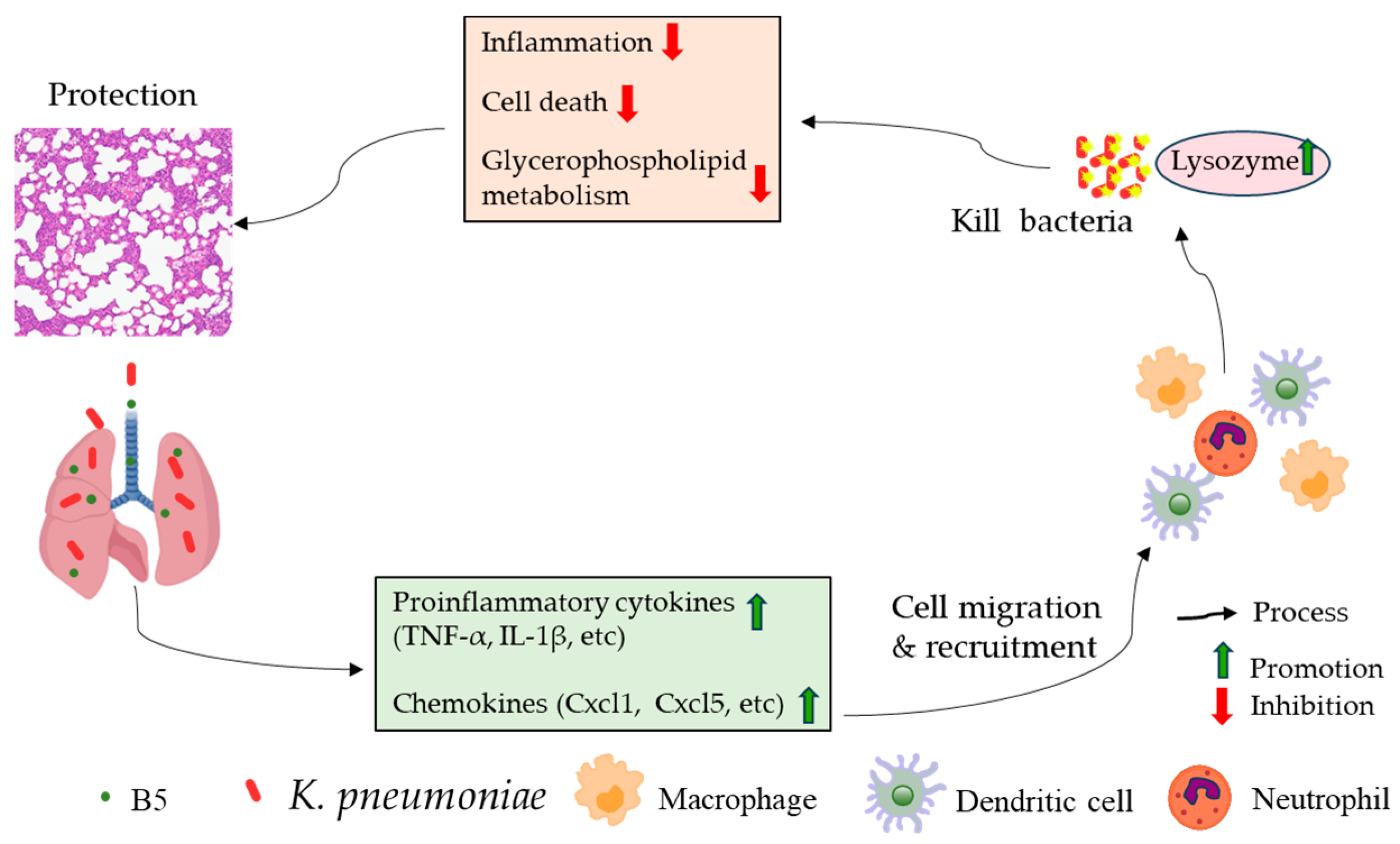
2.1. B5 Provides Protection against K. pneumoniae
2.2. B5 Up-Regulates Pulmonary mRNA Expressions of Cytokines and Chemokines in Early Infection Phase
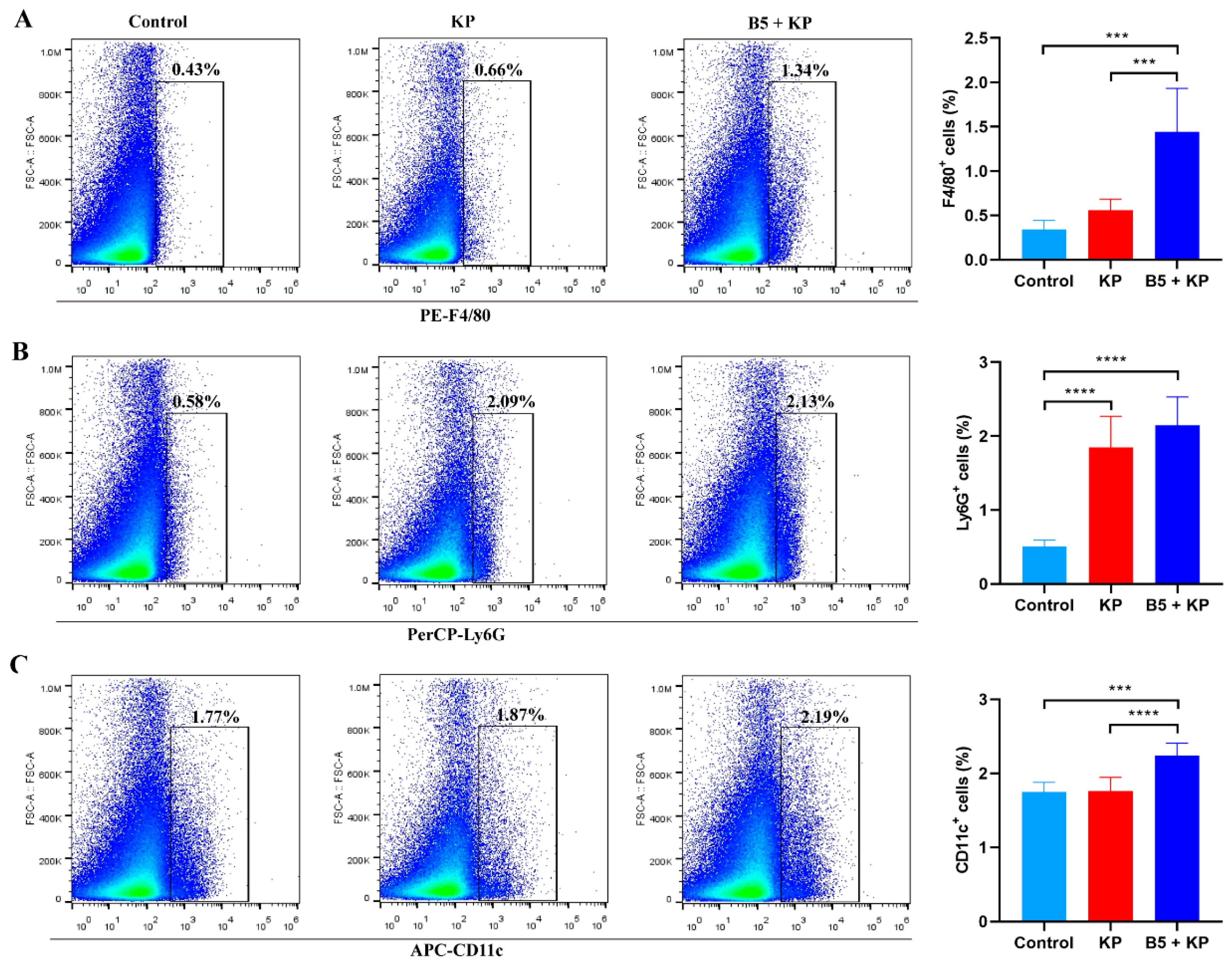
2.3. B5 Increases Pulmonary Macrophages and Dendritic Cells in Early Infection Phase
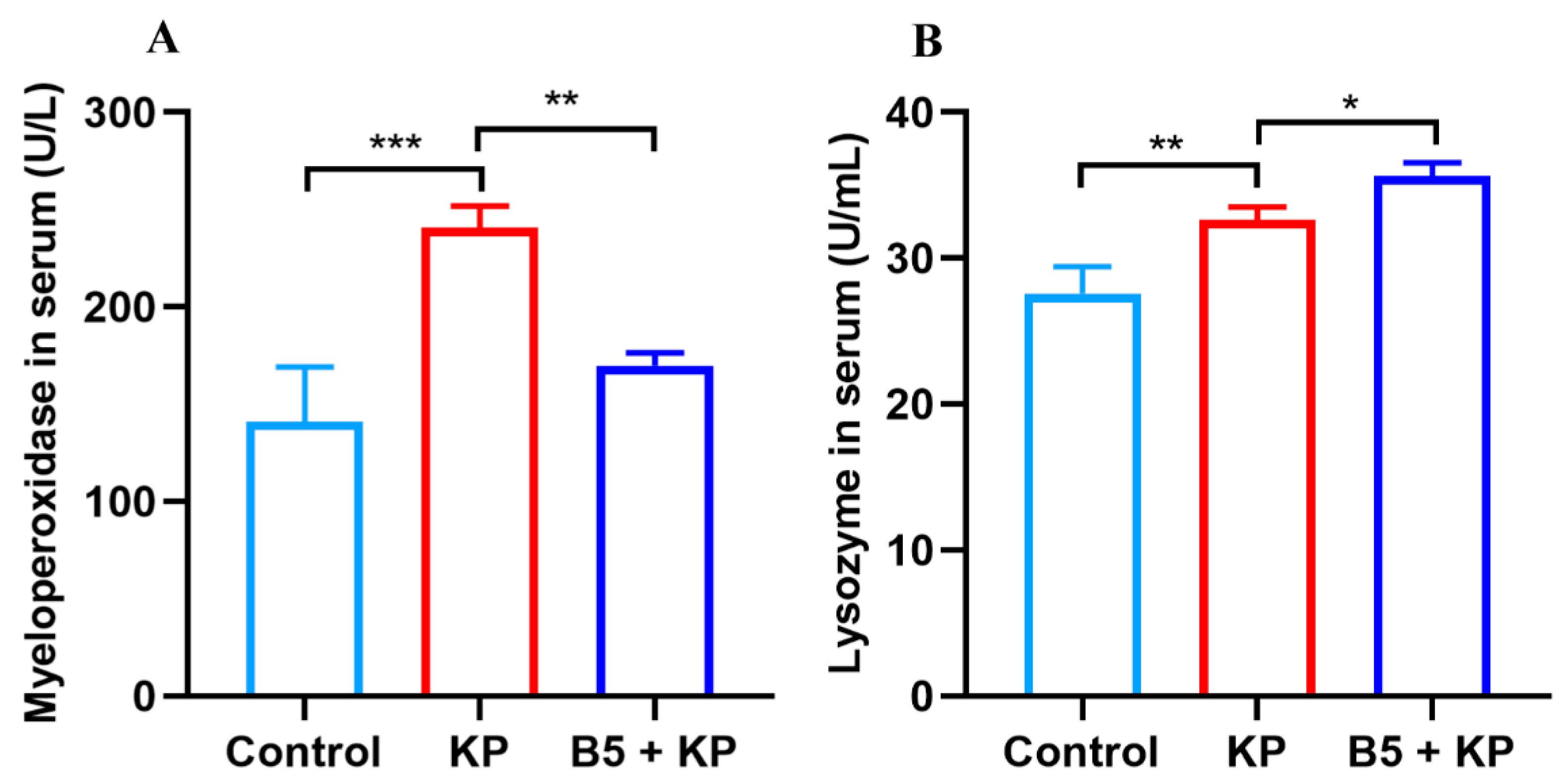
2.4. B5 Regulates Levels of Myeloperoxidase and Lysozyme in Serum in Later Infection Phase
2.5. B5 Ameliorates K. pneumoniae-Induced Pathological Damage in Lungs in Later Infection Phase
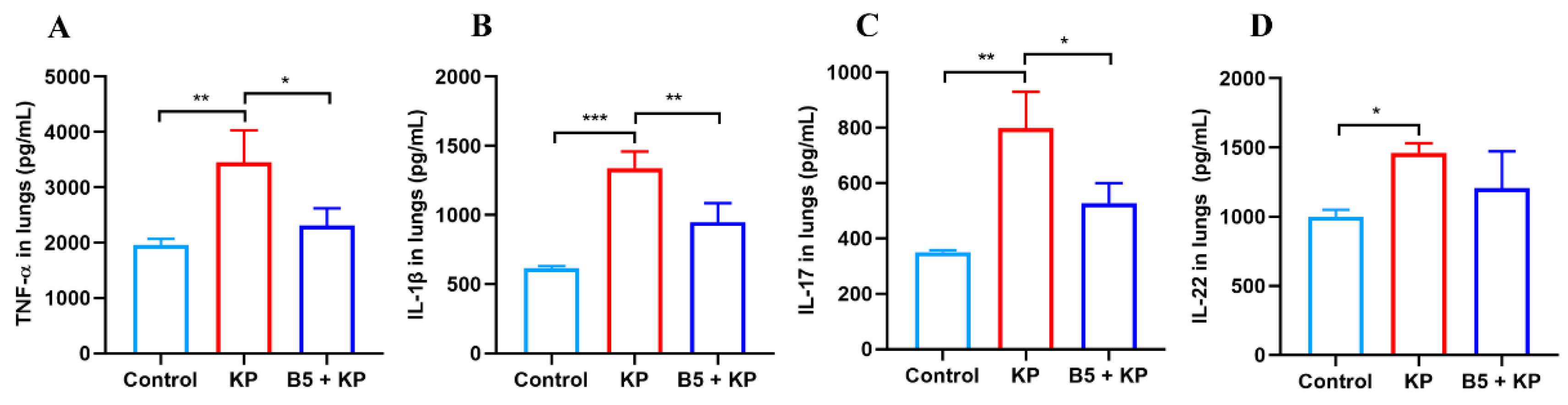
2.6. B5 Down-Regulates Pulmonary Cytokine Production in Later Infection Phase
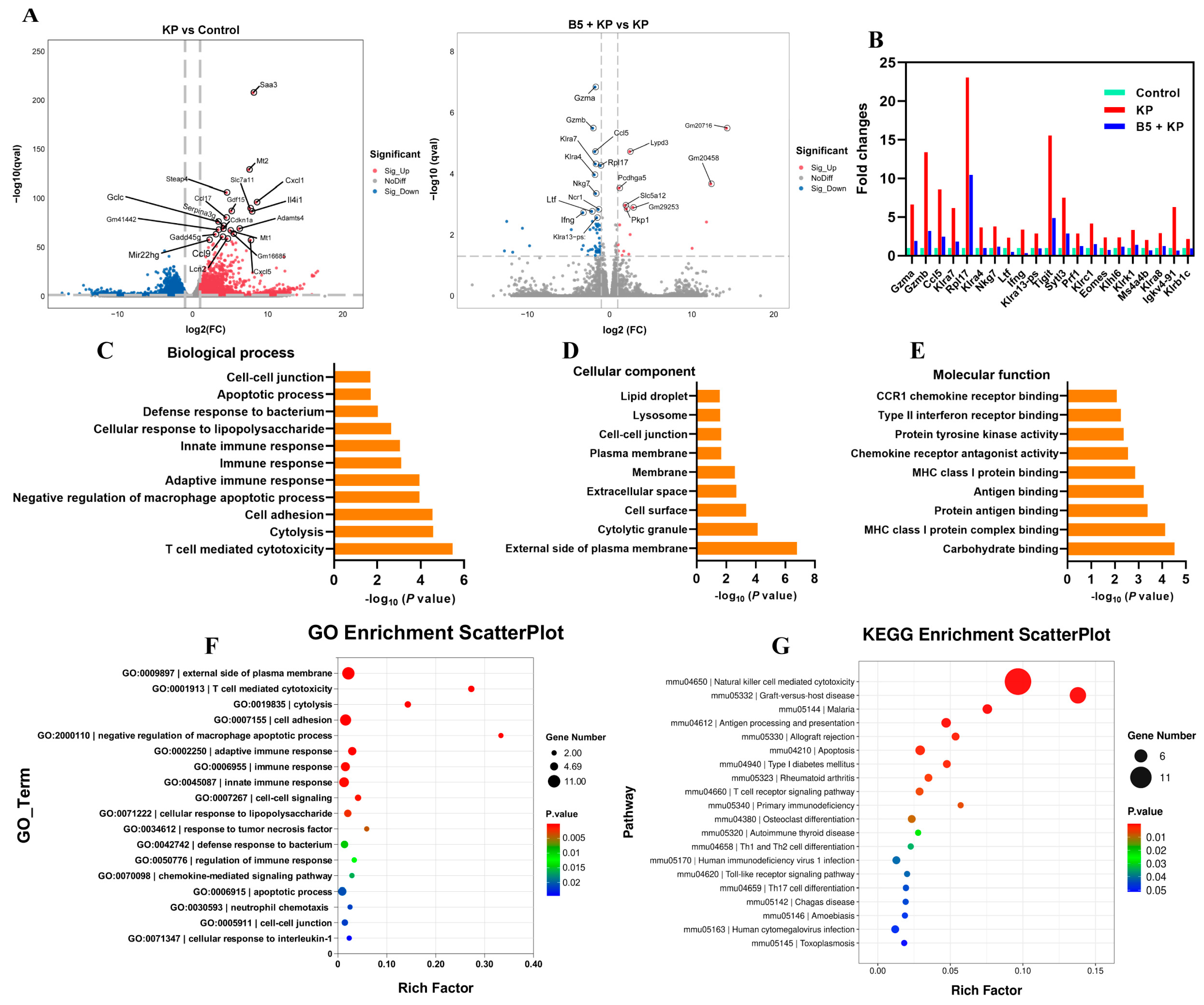
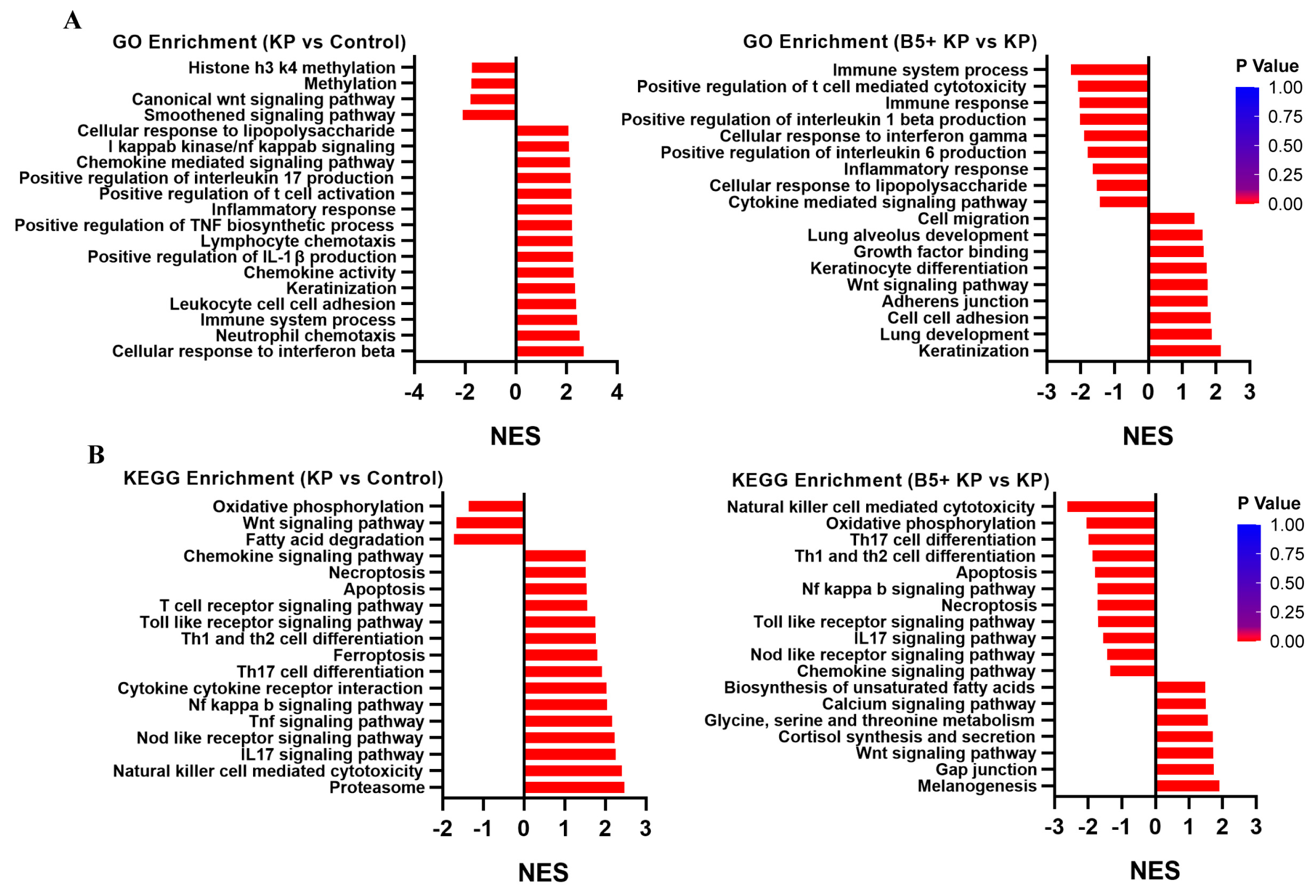
2.7. B5 Regulates Gene Expression Profile in Lungs
2.8. B5 Regulates Metabolic Response in Lungs
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Reagents
4.3. Bacterial Culture
4.4. B5 Pretreatment and K. pneumoniae-Induced Pneumonia in Mice
4.5. Bacterial Load Assay
4.6. Histopathological Assessments
4.7. ELISA
4.8. RT-qPCR
4.9. Flow Cytometry
4.10. RNA-Seq
4.11. Metabolomic Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Li, W.; Yang, Y.; Liu, Q.; Li, S.; Zheng, P.; Zheng, X.; Zhang, Y.; He, J.; Chen, Y.; et al. Inappropriate use of antibiotics exacerbates inflammation through OMV-induced pyroptosis in MDR Klebsiella pneumoniae infection. Cell Rep. 2021, 36, 109750. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Di Domenico, M.; Montagnani, M.; Jirillo, E. Antibiotic Resistance and Microbiota Response. Curr. Pharm. Des. 2023, 29, 356–364. [Google Scholar] [CrossRef] [PubMed]
- O’neill, J. Review on Antimicrobial Resistance. Tackling a Global Health Crisis: Rapid Diagnostics: Stopping Unnecessary Use of Antibiotics. Indep. Rev. AMR 2015, 1–36. [Google Scholar]
- Karami-Zarandi, M.; Rahdar, H.A.; Esmaeili, H.; Ranjbar, R. Klebsiella pneumoniae: An update on antibiotic resistance mechanisms. Future Microbiol. 2023, 18, 65–81. [Google Scholar] [CrossRef]
- Wyres, K.L.; Lam MM, C.; Holt, K.E. Population genomics of Klebsiella pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.S.; Price, L.B. Recent Research Examining Links Among Klebsiella pneumoniae from Food, Food Animals, and Human Extraintestinal Infections. Curr. Env. Health Rep. 2016, 3, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ubaldo, A.L.; Rivero-Perez, N.; Valladares-Carranza, B.; Velázquez-Ordoñez, V.; Delgadillo-Ruiz, L.; Zaragoza-Bastida, A. Bovine mastitis, a worldwide impact disease: Prevalence, antimicrobial resistance, and viable alternative approaches. Vet. Anim. Sci. 2023, 21, 100306. [Google Scholar] [CrossRef] [PubMed]
- Le Guern, R.; Grandjean, T.; Stabler, S.; Bauduin, M.; Gosset, P.; Kipnis, É.; Dessein, R. Gut colonisation with multidrug-resistant Klebsiella pneumoniae worsens Pseudomonas aeruginosa lung infection. Nat. Commun. 2023, 14, 78. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug. Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef] [PubMed]
- Kovach, M.A.; Ballinger, M.N.; Newstead, M.W.; Zeng, X.; Bhan, U.; Yu, F.S.; Moore, B.B.; Gallo, R.L.; Standiford, T.J. Cathelicidin-related antimicrobial peptide is required for effective lung mucosal immunity in Gram-negative bacterial pneumonia. J. Immunol. 2012, 189, 304–311. [Google Scholar] [CrossRef]
- Hitt, S.J.; Bishop, B.M.; Van Hoek, M.L. Komodo-dragon cathelicidin-inspired peptides are antibacterial against carbapenem-resistant Klebsiella pneumoniae. J. Med. Microbiol. 2020, 69, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, S.; Yao, Z.; Zheng, X.; Li, W.; Zhang, Y.; Wang, L.; Cao, J.; Zhou, T. Antimicrobial peptide WAM-1: A promising antibacterial and anti-inflammatory drug against carbapenem-resistant Klebsiella pneumoniae. J. Antimicrob. Chemother. 2022, 77, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Du, X.; Wang, Y.; Kong, J.; Zhang, X.; Wang, W.; Sun, Y.; Zhou, C.; Zhou, T.; Ye, J. Antimicrobial peptide A20L: In vitro and in vivo antibacterial and antibiofilm activity against carbapenem-resistant Klebsiella pneumoniae. Microbiol. Spectr. 2024, 12, e0397923. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Chen, X.C.; Yan, Z.H.; Tu, F.; He, T.; Gopinath SC, B.; Rui, X.H.; Cao, F.T. Human neutrophil peptide 1 promotes immune sterilization in vivo by reducing the virulence of multidrug-resistant Klebsiella pneumoniae and increasing the ability of macrophages. Biotechnol. Appl. Biochem. 2022, 69, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- Morici, P.; Rizzato, C.; Ghelardi, E.; Rossolini, G.M.; Lupetti, A. Sensitization of KPC and NDM Klebsiella pneumoniae To Rifampicin by the Human Lactoferrin-Derived Peptide hLF1-11. Microbiol. Spectr. 2023, 11, e0276722. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zong, X.; Jin, M.; Min, J.; Wang, F.; Wang, Y. Mechanisms and regulation of defensins in host defense. Signal Transduct. Target. Ther. 2023, 8, 300. [Google Scholar] [CrossRef]
- Ryan, L.K.; Wu, J.; Schwartz, K.; Yim, S.; Diamond, G. β-Defensins Coordinate In Vivo to Inhibit Bacterial Infections of the Trachea. Vaccines 2018, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Limjunyawong, N.; Sypek, E.I.; Wang, G.; Ortines, R.V.; Youn, C.; Alphonse, M.P.; Dikeman, D.; Wang, Y.; Lay, M.; et al. Keratinocyte-derived defensins activate neutrophil-specific receptors Mrgpra2a/b to prevent skin dysbiosis and bacterial infection. Immunity 2022, 55, 1645–1662.e1647. [Google Scholar] [CrossRef]
- Bolatchiev, A. Antimicrobial Peptides Epinecidin-1 and Beta-Defesin-3 Are Effective against a Broad Spectrum of Antibiotic-Resistant Bacterial Isolates and Increase Survival Rate in Experimental Sepsis. Antibiotics 2022, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Selsted, M.E.; Tang, Y.Q.; Morris, W.L.; Mcguire, P.A.; Novotny, M.J.; Smith, W.; Henschen, A.H.; Cullor, J.S. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J. Biol. Chem. 1993, 268, 6641–6648. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.K.; Rhodes, J.; Bhat, M.; Diamond, G. Expression of beta-defensin genes in bovine alveolar macrophages. Infect. Immun. 1998, 66, 878–881. [Google Scholar] [CrossRef]
- Goldammer, T.; Zerbe, H.; Molenaar, A.; Schuberth, H.J.; Brunner, R.M.; Kata, S.R.; Seyfert, H.M. Mastitis increases mammary mRNA abundance of beta-defensin 5, toll-like-receptor 2 (TLR2), and TLR4 but not TLR9 in cattle. Clin. Diagn. Lab. Immunol. 2004, 11, 174–185. [Google Scholar] [PubMed]
- Liang, Z.; Li, H.; Qu, M.; Liu, Y.; Wang, Y.; Wang, H.; Dong, Y.; Chen, Y.; Ge, X.; Zhou, X. Intranasal bovine β-defensin-5 enhances antituberculosis immunity in a mouse model by a novel protein-based respiratory mucosal vaccine. Virulence 2022, 13, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liu, Y.; Sun, X.; Lin, J.; Yao, J.; Song, Y.; Li, M.; Liu, T.; Zhou, X. Immunoregulatory and Antimicrobial Activity of Bovine Neutrophil β-Defensin-5-Loaded PLGA Nanoparticles against Mycobacterium bovis. Pharmaceutics 2020, 12, 1172. [Google Scholar] [CrossRef]
- Huang, J.; Kang, W.; Yi, D.; Zhu, S.; Xiang, Y.; Liu, C.; Li, H.; Dai, D.; Su, J.; He, J.; et al. Intranasal B5 promotes mucosal defence against Actinobacillus pleuropneumoniae via ameliorating early immunosuppression. Virulence 2024, 15, 2316459. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Z.; Yuan, Y.; Jing, H.; Zou, J.; Zhang, X.; Zeng, X.; Zhang, W.; Zou, Q.; Zhang, J. Innate Immune Effectors Play Essential Roles in Acute Respiratory Infection Caused by Klebsiella pneumoniae. J. Immunol. Res. 2020, 2020, 5291714. [Google Scholar] [CrossRef]
- Van Der Geest, R.; Peñaloza, H.F.; Xiong, Z.; Gonzalez-Ferrer, S.; An, X.; Li, H.; Fan, H.; Tabary, M.; Nouraie, S.M.; Zhao, Y.; et al. BATF2 enhances proinflammatory cytokine responses in macrophages and improves early host defense against pulmonary Klebsiella pneumoniae infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L604–L616. [Google Scholar] [CrossRef]
- Van Der Geest, R.; Fan, H.; Peñaloza, H.F.; Bain, W.G.; Xiong, Z.; Kohli, N.; Larson, E.; Sullivan ML, G.; Franks, J.M.; Stolz, D.B.; et al. Phagocytosis is a primary determinant of pulmonary clearance of clinical Klebsiella pneumoniae isolates. Front. Cell. Infect. Microbiol. 2023, 13, 1150658. [Google Scholar] [CrossRef]
- Iwanaga, N.; Chen, K.; Yang, H.; Lu, S.; Hoffmann, J.P.; Wanek, A.; Mccombs, J.E.; Song, K.; Rangel-Moreno, J.; Norton, E.B.; et al. Vaccine-driven lung TRM cells provide immunity against Klebsiella via fibroblast IL-17R signaling. Sci. Immunol. 2021, 6, eabf1198. [Google Scholar] [CrossRef] [PubMed]
- Dentice Maidana, S.; Imamura, Y.; Elean, M.; Albarracín, L.; Nishiyama, K.; Suda, Y.; Kurata, S.; Jure, M.; Kitazawa, H.; Villena, J. Oral Administration of Lacticaseibacillus rhamnosus CRL1505 Modulates Lung Innate Immune Response against Klebsiella pneumoniae ST25. Microorganisms 2023, 11, 1148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Jiang, J.; He, W.; Dong, G.; Xu, N.; Meng, L.; Zhao, Y.; Wang, M.; Tan, S.; Shi, Y.; et al. Chrysophanol alleviates acute lung injury caused by Klebsiella pneumoniae infection by inhibiting pro-inflammatory cytokine production. Phytother. Res. 2023, 37, 2965–2978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, G.; Wu, X.; Xu, Y.; Xu, L.; Zou, Y.; Yang, X.; Pan, L.; Lei, B.; Mu, J.; et al. Fei-Yan-Qing-Hua decoction decreases hyperinflammation by inhibiting HMGB1/RAGE signaling and promotes bacterial phagocytosis in the treatment of sepsis. J. Ethnopharmacol. 2024, 321, 117553. [Google Scholar] [CrossRef] [PubMed]
- Aujla, S.J.; Chan, Y.R.; Zheng, M.; Fei, M.; Askew, D.J.; Pociask, D.A.; Reinhart, T.A.; Mcallister, F.; Edeal, J.; Gaus, K.; et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008, 14, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Shen, S.; Chen, J.; Tian, Z.; Shi, Q.; Han, R.; Guo, Y.; Hu, F. Klebsiella pneumoniae carbapenemase variants: The new threat to global public health. Clin. Microbiol. Rev. 2023, 36, e0000823. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Y.; Tian, Y.Q.; Qin, Y.Y.; Jiao, X.; Pan, Z.M. Detection of cfr in Klebsiella pneumoniae from pig feed in China. J. Antimicrob. Chemother. 2023, 78, 2774–2776. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xin, L.; Peng, C.; Liu, C.; Wang, P.; Yu, L.; Liu, M.; Wang, F. Prevalence and antimicrobial susceptibility profiles of ESBL-producing Klebsiella Pneumoniae from broiler chicken farms in Shandong Province, China. Poult. Sci. 2022, 101, 102002. [Google Scholar] [CrossRef]
- Van Der Weide, H.; Vermeulen-De Jongh DM, C.; Van Der Meijden, A.; Boers, S.A.; Kreft, D.; Ten Kate, M.T.; Falciani, C.; Pini, A.; Strandh, M.; Bakker-Woudenberg, I.; et al. Antimicrobial activity of two novel antimicrobial peptides AA139 and SET-M33 against clinically and genotypically diverse Klebsiella pneumoniae isolates with differing antibiotic resistance profiles. Int. J. Antimicrob. Agents 2019, 54, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ormondes De Farias, J.; Resende Ferreira, A.C.; Cardoso Kostopoulos, A.G.; Berto Rezende, T.M.; Dias, S.C. Synergistic activity and immunomodulatory potential of levofloxacin and Synoeca-MP peptide against multi-resistant strains of Klebsiella pneumoniae. Microb. Pathog. 2022, 163, 105403. [Google Scholar] [CrossRef]
- Chatupheeraphat, C.; Peamchai, J.; Luk-In, S.; Eiamphungporn, W. Synergistic effect and antibiofilm activity of the antimicrobial peptide K11 with conventional antibiotics against multidrug-resistant and extensively drug-resistant Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2023, 13, 1153868. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Santiago, B.; Castañeda-Delgado, J.E.; Rivas Santiago, C.E.; Waldbrook, M.; González-Curiel, I.; León-Contreras, J.C.; Enciso-Moreno, J.A.; Del Villar, V.; Mendez-Ramos, J.; Hancock, R.E.; et al. Ability of innate defence regulator peptides IDR-1002, IDR-HH2 and IDR-1018 to protect against Mycobacterium tuberculosis infections in animal models. PLoS ONE 2013, 8, e59119. [Google Scholar] [CrossRef] [PubMed]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Eddens, T.; Trevejo-Nunez, G.; Way, E.E.; Elsegeiny, W.; Ricks, D.M.; Garg, A.V.; Erb, C.J.; Bo, M.; Wang, T.; et al. IL-17 Receptor Signaling in the Lung Epithelium Is Required for Mucosal Chemokine Gradients and Pulmonary Host Defense against K. pneumoniae. Cell Host Microbe 2016, 20, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, L.; Yang, Y.; Hou, Y.; Xu, Y.; Wang, Z.; Su, H.; Han, F.; Han, J.; Liu, P.; et al. LL-37 transports immunoreactive cGAMP to activate STING signaling and enhance interferon-mediated host antiviral immunity. Cell Rep. 2022, 39, 110880. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Zhang, N.; Yang, J.; Meng, X.; Yang, R.; Li, J.; Sun, T. Antimicrobial peptide LL-37 and IDR-1 ameliorate MRSA pneumonia in vivo. Cell. Physiol. Biochem. 2013, 32, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Int. J. Mol. Sci. 2020, 21, 5973. [Google Scholar] [CrossRef] [PubMed]
- Hirche, T.O.; Gaut, J.P.; Heinecke, J.W.; Belaaouaj, A. Myeloperoxidase plays critical roles in killing Klebsiella pneumoniae and inactivating neutrophil elastase: Effects on host defense. J. Immunol. 2005, 174, 1557–1565. [Google Scholar] [CrossRef]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, Y.; Lai, Y.; Zhang, J.; Yin, L.; Yu, X.; Zhou, Y.; Li, X.; Song, Y. Host defense against the infection of Klebsiella pneumoniae: New strategy to kill the bacterium in the era of antibiotics? Front. Cell. Infect. Microbiol. 2022, 12, 1050396. [Google Scholar] [CrossRef] [PubMed]
- García-Laorden, M.I.; Stroo, I.; Blok, D.C.; Florquin, S.; Medema, J.P.; De Vos, A.F.; Van Der Poll, T. Granzymes A and B Regulate the Local Inflammatory Response during Klebsiella pneumoniae Pneumonia. J. Innate Immun. 2016, 8, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Juang, S.W.; Kane, K.P. NK cells exacerbate the pathology of influenza virus infection in mice. Eur. J. Immunol. 2013, 43, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Wong Fok Lung, T.; Charytonowicz, D.; Beaumont, K.G.; Shah, S.S.; Sridhar, S.H.; Gorrie, C.L.; Mu, A.; Hofstaedter, C.E.; Varisco, D.; Mcconville, T.H.; et al. Klebsiella pneumoniae induces host metabolic stress that promotes tolerance to pulmonary infection. Cell Metab. 2022, 34, 761–774.e769. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yang, L.; Zhang, X.; Ji, P.; Hua, Y.; Wei, Y. Mechanism of Huang-lian-Jie-du decoction and its effective fraction in alleviating acute ulcerative colitis in mice: Regulating arachidonic acid metabolism and glycerophospholipid metabolism. J. Ethnopharmacol. 2020, 259, 112872. [Google Scholar] [CrossRef]
- Wang, S.; Tan, K.S.; Beng, H.; Liu, F.; Huang, J.; Kuai, Y.; Zhang, R.; Tan, W. Protective effect of isosteviol sodium against LPS-induced multiple organ injury by regulating of glycerophospholipid metabolism and reducing macrophage-driven inflammation. Pharmacol. Res. 2021, 172, 105781. [Google Scholar] [CrossRef]





| Targets | Forward | Reverse |
|---|---|---|
| TNF-α | CTCATGCACCACCATCAAGG | ACCTGACCACTCTCCCTTTG |
| IL-1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG |
| IL-17 | TTTAACTCCCTTGGCGCAAAA | CTTTCCCTCCGCATTGACAC |
| IL-22 | ATGAGTTTTTCCCTTATGGGGAC | GCTGGAAGTTGGACACCTCAA |
| Cxcl1 | CTGGGATTCACCTCAAGAACATC | CAGGGTCAAGGCAAGCCTC |
| Cxcl5 | GTTCCATCTCGCCATTCATGC | GCGGCTATGACTGAGGAAGG |
| Ccl17 | TACCATGAGGTCACTTCAGATGC | GCACTCTCGGCCTACATTGG |
| Ccl22 | CTTGCTGTGGCAATTCAGACC | ACTAAACGTGATGGCAGAGGG |
| GAPDH | GGTGAAGGTCGGTGTGAACGGA | TGTTAGTGGGGTCTCGCTCCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Dai, D.; Li, H.; Huang, J.; Kang, W.; Yang, Y.; Zhong, Y.; Xiang, Y.; Liu, C.; He, J.; et al. Bovine Neutrophil β-Defensin-5 Provides Protection against Multidrug-Resistant Klebsiella pneumoniae via Regulating Pulmonary Inflammatory Response and Metabolic Response. Int. J. Mol. Sci. 2024, 25, 10506. https://doi.org/10.3390/ijms251910506
Zhu S, Dai D, Li H, Huang J, Kang W, Yang Y, Zhong Y, Xiang Y, Liu C, He J, et al. Bovine Neutrophil β-Defensin-5 Provides Protection against Multidrug-Resistant Klebsiella pneumoniae via Regulating Pulmonary Inflammatory Response and Metabolic Response. International Journal of Molecular Sciences. 2024; 25(19):10506. https://doi.org/10.3390/ijms251910506
Chicago/Turabian StyleZhu, Shuxin, Dejia Dai, Han Li, Jingsheng Huang, Weichao Kang, Yunmei Yang, Yawen Zhong, Yifei Xiang, Chengzhi Liu, Jiakang He, and et al. 2024. "Bovine Neutrophil β-Defensin-5 Provides Protection against Multidrug-Resistant Klebsiella pneumoniae via Regulating Pulmonary Inflammatory Response and Metabolic Response" International Journal of Molecular Sciences 25, no. 19: 10506. https://doi.org/10.3390/ijms251910506






