Definition of Synovial Mesenchymal Stem Cells for Meniscus Regeneration by the Mechanism of Action and General Amp1200 Gene Expression
Abstract
:1. Introduction
2. Results
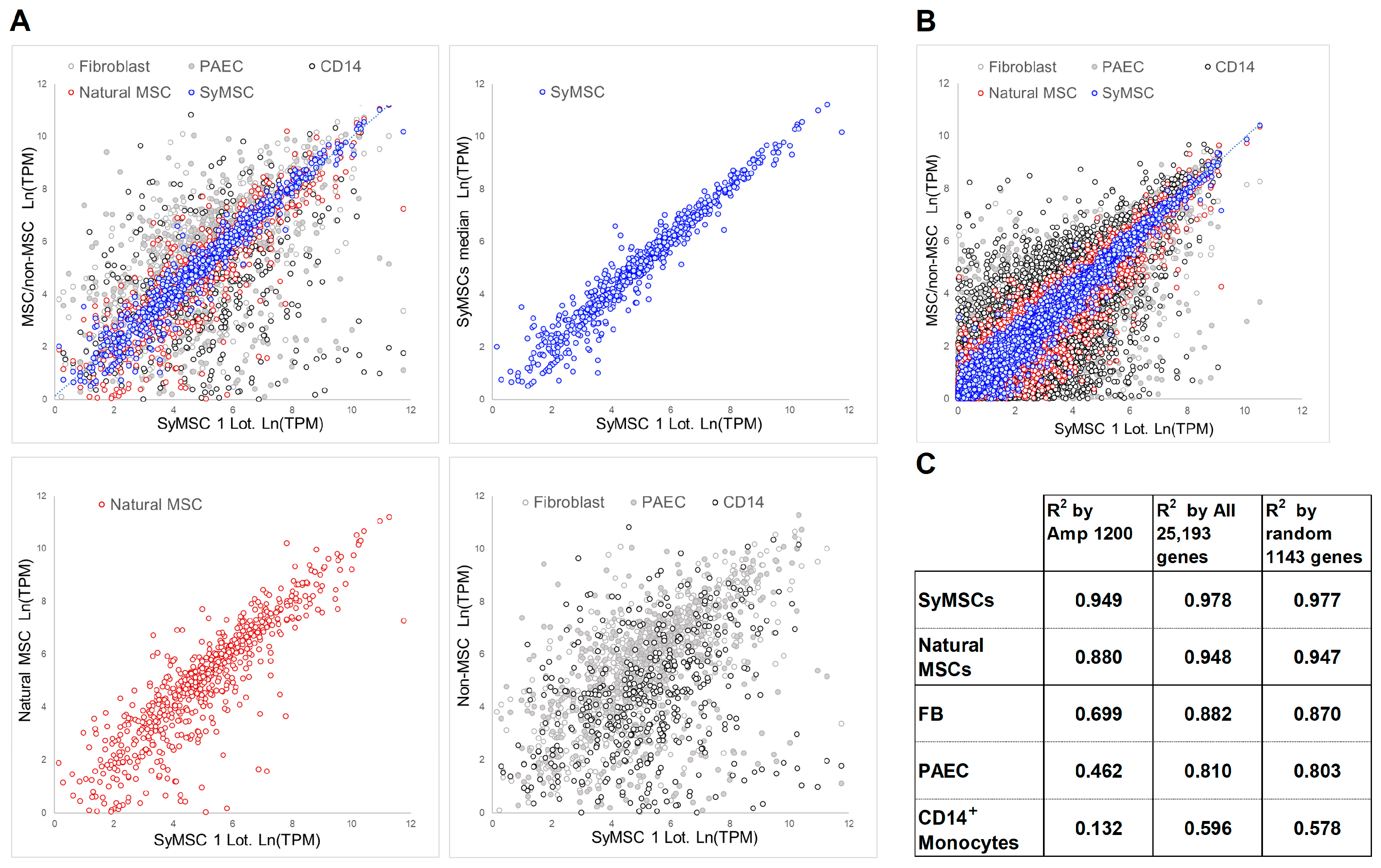
2.1. QC Correlation Method in SyMSCs, Amp1200 Construction of MSC Gene Groups
2.2. Expression of Amp1200-Selected Genes, All Human Genes, and 1143 Random Genes in SyMSCs
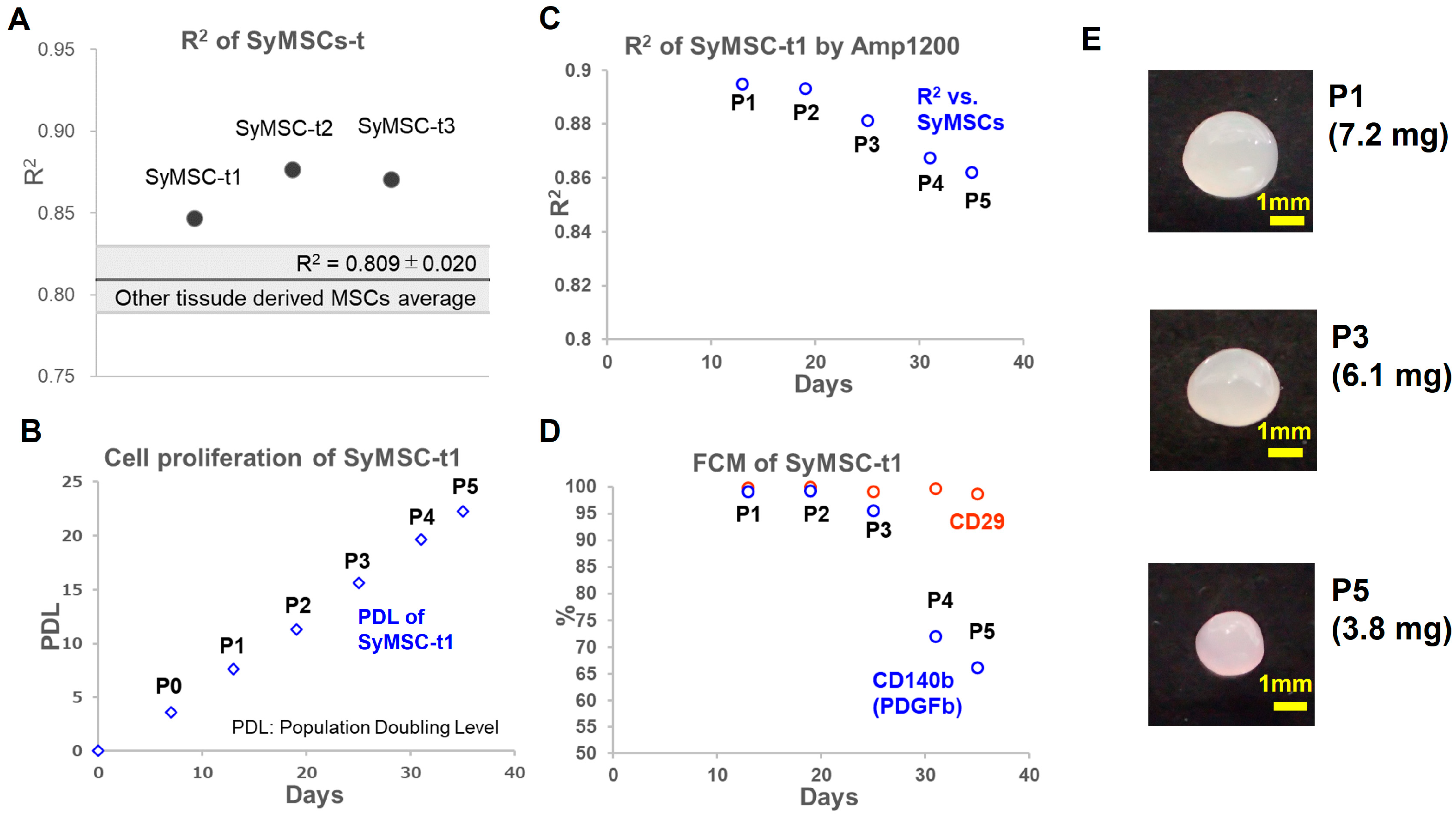
2.3. SyMSCs Isolated from Human Synovium over Time in Cultures
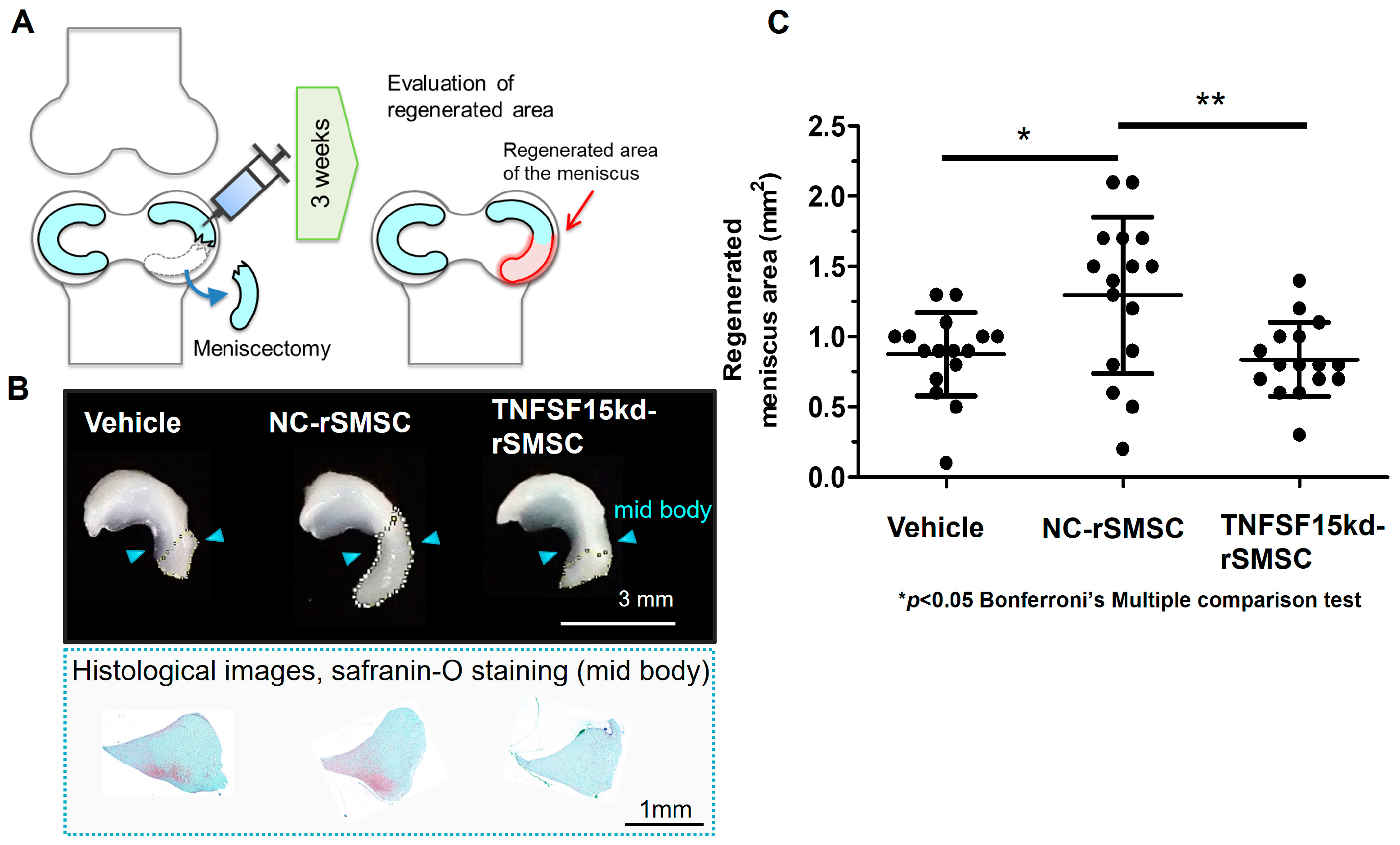
2.4. Contribution of the SyMSC-Characteristic Molecule TNFSF-15 to In Vivo Efficacy of Rat SyMSCs
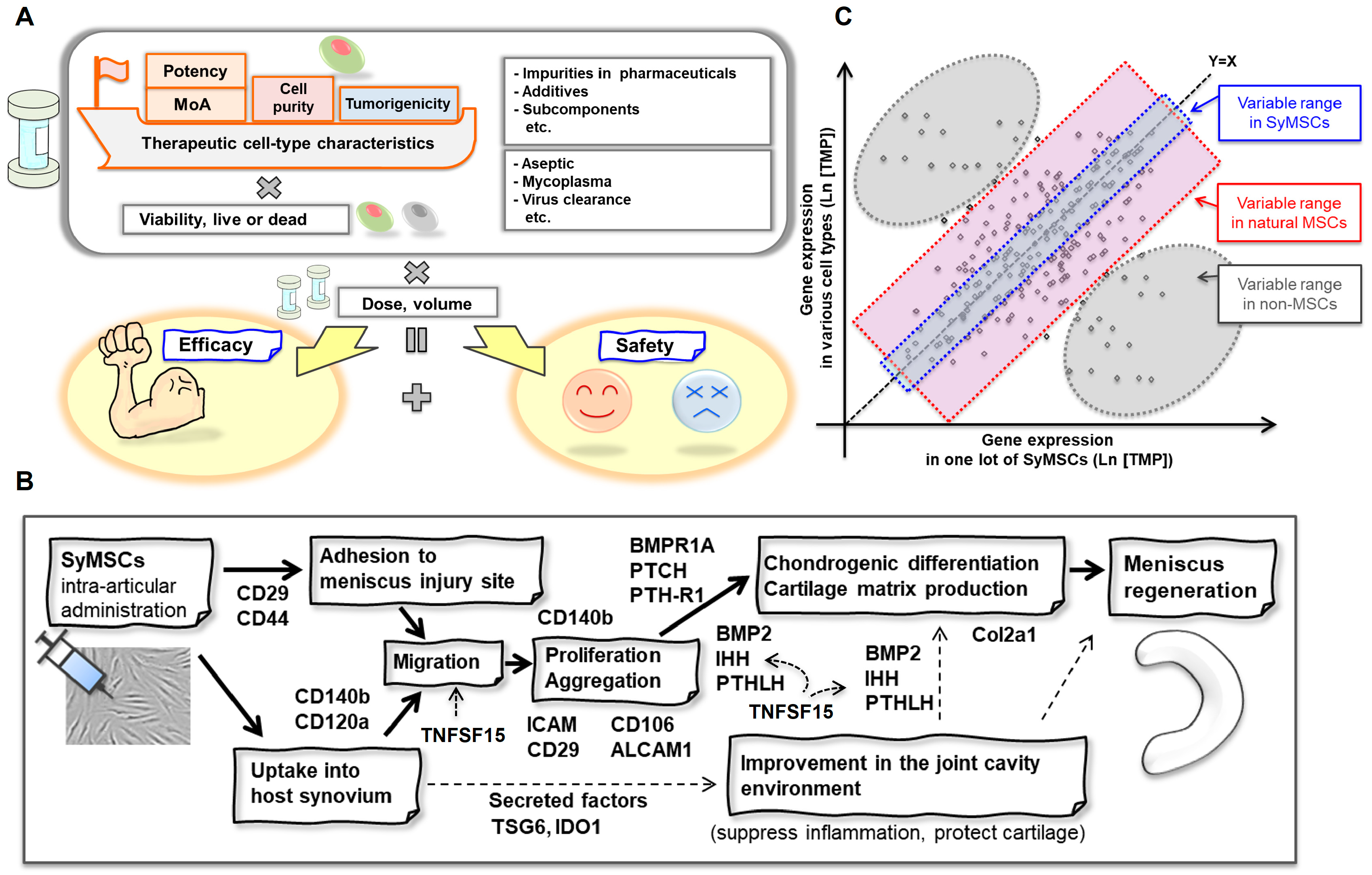
2.5. QC Concept for SyMSCs
3. Discussion
3.1. Cell-Type-Dependent Correlation QC Method
3.2. MoA-Dependent QC Method
4. Materials and Methods
4.1. Identification of MoA Molecules
4.2. Preparation of Amp1200 for MSCs
4.3. MSC Culture and RNA Preparation
4.4. Non-MSC Culture and RNA Preparation
4.5. Gene Library Construction, Quantitative PCR (qPCR), and Sequencing
4.6. Data Processing
4.7. Expression Analysis of All Human Genes
4.8. Human Synovial MSC Isolation and Culture, and Ethics Approval
4.9. Rat Experiments and Ethics Approval
4.10. TNFSF-15 Knockdown in rSMSCs
4.11. Meniscectomy and Transplantation of TNSF15-Knockdown Cells
4.12. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Why are MSCs therapeutic? New data: New insight. J. Pathol. 2009, 217, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.S.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef]
- Mishra, V.K.; Shih, H.H.; Parveen, F.; Lenzen, D.; Ito, E.; Chan, T.F.; Ke, L.Y. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells 2020, 9, 1145. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef]
- Hashmi, S.; Ahmed, M.; Murad, M.H.; Litzow, M.R.; Adams, R.H.; Ball, L.M.; Prasad, V.K.; Kebriaei, P.; Ringden, O. Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: Systematic review and meta-analysis. Lancet. Haematol. 2016, 3, e45–e52. [Google Scholar] [CrossRef]
- Murata, M.; Terakura, S.; Wake, A.; Miyao, K.; Ikegame, K.; Uchida, N.; Kataoka, K.; Miyamoto, T.; Onizuka, M.; Eto, T.; et al. Off-the-shelf bone marrow-derived mesenchymal stem cell treatment for acute graft-versus-host disease: Real-world evidence. Bone Marrow Transplant. 2021, 56, 2355–2366. [Google Scholar] [CrossRef]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C.Y. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013, 19, 35–42. [Google Scholar] [CrossRef]
- Rebelatto, C.L.K.; Boldrini-Leite, L.M.; Daga, D.R.; Marsaro, D.B.; Vaz, I.M.; Jamur, V.R.; de Aguiar, A.M.; Vieira, T.B.; Furman, B.P.; Aguiar, C.O.; et al. Quality Control Optimization for Minimizing Security Risks Associated with Mesenchymal Stromal Cell-Based Product Development. Int. J. Mol. Sci. 2023, 24, 12955. [Google Scholar] [CrossRef]
- Stroncek, D.F.; Jin, P.; McKenna, D.H.; Takanashi, M.; Fontaine, M.J.; Pati, S.; Schäfer, R.; Peterson, E.; Benedetti, E.; Reems, J.A. Human Mesenchymal Stromal Cell (MSC) Characteristics Vary Among Laboratories When Manufactured From the Same Source Material: A Report by the Cellular Therapy Team of the Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Front. Cell Dev. Biol. 2020, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Briefing Document. In Proceedings of the Oncologic Drugs Advisory Committee (ODAC) Meeting, Online, 13 August 2020; BLA 125706; Remestemcel-L: CMC. FDA: Silver Spring, MD, USA, 2020. [Google Scholar]
- Mabuchi, Y.; Okawara, C.; Mendez-Ferrer, S.; Akazawa, C. Cellular Heterogeneity of Mesenchymal Stem/Stromal Cells in the Bone Marrow. Front. Cell Dev. Biol. 2021, 9, 689366. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, U.; Peluso, G.; Di-Bernardo, G. Clinical Trials Based on Mesenchymal Stromal Cells are Exponentially Increasing: Where are We in Recent Years? Stem Cell Rev. Rep. 2022, 18, 23–36. [Google Scholar] [CrossRef]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De-Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Moll, G.; Hoogduijn, M.J.; Ankrum, J.A. Editorial: Safety, Efficacy and Mechanisms of Action of Mesenchymal Stem Cell Therapies. Front. Immunol. 2020, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, P.; Lim, R.; Ng, J.; Vemuri, M.C. Acceleration of Translational Mesenchymal Stromal Cell Therapy through Consistent Quality GMP Manufacturing. Front. Cell Dev. Biol. 2021, 9, 648472. [Google Scholar] [CrossRef]
- Grangeia, H.B.; Silva, C.; Simões, S.P.; Reis, M.S. Quality by design in pharmaceutical manufacturing: A systematic review of current status, challenges and future perspectives. Eur. J. Pharm. Biopharm. 2020, 147, 19–37. [Google Scholar] [CrossRef]
- Dominici, M.; Le, B.K.; Mueller, I.; Slaper, C.I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Viganò, M.; Budelli, S.; Lavazza, C.; Montemurro, T.; Montelatici, E.; de Cesare, S.; Lazzari, L.; Orlandi, A.R.; Lunghi, G.; Giordano, R. Tips and Tricks for Validation of Quality Control Analytical Methods in Good Manufacturing Practice Mesenchymal Stromal Cell Production. Stem Cells Int. 2018, 2018, 3038565. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Koga, H.; Otabe, K.; Nakagawa, Y.; Katano, H.; Ozeki, N.; Mizuno, M.; Horie, M.; Kohno, Y.; Katagiri, K.; et al. Additional Use of Synovial Mesenchymal Stem Cell Transplantation Following Surgical Repair of a Complex Degenerative Tear of the Medial Meniscus of the Knee: A Case Report. Cell Transpl. 2019, 28, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Katano, H.; Mizuno, M.; Koga, H.; Masumoto, J.; Tomita, M.; Ozeki, N. Alterations in cartilage quantification before and after injections of mesenchymal stem cells into osteoarthritic knees. Sci. Rep. 2021, 11, 13832. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Muneta, T.; Horie, M.; Koga, H. Arthroscopic Transplantation of Synovial Stem Cells Improves Clinical Outcomes in Knees With Cartilage Defects. Clin. Orthop. Relat. Res. 2015, 473, 2316–2326. [Google Scholar] [CrossRef]
- Kitahashi, T.; Kogawa, R.; Nakamura, K.; Sekiya, I. Integrin β1, PDGFRβ, and type II collagen are essential for meniscus regeneration by synovial mesenchymal stem cells in rats. Sci. Rep. 2022, 12, 14148. [Google Scholar] [CrossRef]
- Nakamura, K.; Yoshino, Y.; Hando, R. Method for Managin Quality of Specific Cells, and Method for Manufacturing Specific Cells. EP4372098A1, 19 January 2023. [Google Scholar]
- Bian, L.; Guvendiren, M.; Mauck, R.L.; Burdick, J.A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 10117–10122. [Google Scholar] [CrossRef]
- Endo, K.; Horiuchi, K.; Katano, H.; Ozeki, N.; Sakamaki, Y.; Koga, H.; Sekiya, I. Intra-articular Injection of PDGF-BB Explored in a Novel In Vitro Model Mobilizes Mesenchymal Stem Cells from the Synovium into Synovial Fluid in Rats. Stem Cell Rev. Rep. 2021, 17, 1768–1779. [Google Scholar] [CrossRef]
- Horie, M.; Choi, H.; Lee, R.H.; Reger, R.L.; Ylostalo, J.; Muneta, T.; Sekiya, I.; Prockop, D.J. Intra-articular injection of human mesenchymal stem cells (MSCs) promote rat meniscal regeneration by being activated to express Indian hedgehog that enhances expression of type II collagen. Osteoarthr. Cartil. 2012, 20, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Shimaya, M.; Muneta, T.; Nimura, A.; Morito, T.; Hayashi, M.; Suzuki, S.; Ju, Y.J.; Mochizuki, T.; Sekiya, I. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res. Ther. 2008, 10, R84. [Google Scholar] [CrossRef]
- Nimura, A.; Muneta, T.; Koga, H.; Mochizuki, T.; Suzuki, K.; Makino, H.; Umezawa, A.; Sekiya, I. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: Comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008, 58, 501–510. [Google Scholar] [CrossRef]
- Ozeki, N.; Muneta, T.; Koga, H.; Nakagawa, Y.; Mizuno, M.; Tsuji, K.; Mabuchi, Y.; Akazawa, C.; Kobayashi, E.; Matsumoto, K.; et al. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthr. Cartil. 2016, 24, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Shimaya, M.; Muneta, T.; Ichinose, S.; Tsuji, K.; Sekiya, I. Magnesium enhances adherence and cartilage formation of synovial mesenchymal stem cells through integrins. Osteoarthr. Cartil. 2010, 18, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Budeus, B.; Unger, K.; Hess, J.; Sentek, H.; Klein, D. Comparative computational analysis to distinguish mesenchymal stem cells from fibroblasts. Front. Immunol. 2023, 14, 1270493. [Google Scholar] [CrossRef] [PubMed]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, S.; Okada, H.; Hojo, H.; Ohba, S.; Iwata, J.; Komura, M.; Hikita, A.; Hoshi, K. Mesenchymal stromal cells in the bone marrow niche consist of multi-populations with distinct transcriptional and epigenetic properties. Sci. Rep. 2021, 11, 15811. [Google Scholar] [CrossRef]
- Yi, N.; Zeng, Q.; Zheng, C.; Li, S.; Lv, B.; Wang, C.; Li, C.; Jiang, W.; Liu, Y.; Yang, Y.; et al. Functional variation among mesenchymal stem cells derived from different tissue sources. PeerJ 2024, 12, e17616. [Google Scholar] [CrossRef]
- Yamatani, Y.; Nakai, K. Comprehensive comparison of gene expression diversity among a variety of human stem cells. NAR Genom. Bioinform. 2022, 4, lqac087. [Google Scholar] [CrossRef]
- Al-Azab, M.; Qaed, E.; Ouyang, X.; Elkhider, A.; Walana, W.; Li, H.; Li, W.; Tang, Y.; Adlat, S.; Wei, J.; et al. TL1A/TNFR2-mediated mitochondrial dysfunction of fibroblast-like synoviocytes increases inflammatory response in patients with rheumatoid arthritis via reactive oxygen species generation. FEBS J. 2020, 287, 3088–3104. [Google Scholar] [CrossRef]
- Xu, W.D.; Li, R.; Huang, A.F. Role of TL1A in Inflammatory Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 2022, 13, 891328. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Fahmi, H.; Wojcik, S.; Fikes, J.; Yu, Y.; Wu, J.; Luo, H. Role of TL1A in the pathogenesis of rheumatoid arthritis. J. Immunol. 2009, 183, 5350–5357. [Google Scholar] [CrossRef]
- Cassatella, M.A.; Pereira-da-Silva, G.; Tinazzi, I.; Facchetti, F.; Scapini, P.; Calzetti, F.; Tamassia, N.; Wei, P.; Nardelli, B.; Roschke, V.; et al. Soluble TNF-like cytokine (TL1A) production by immune complexes stimulated monocytes in rheumatoid arthritis. J. Immunol. 2007, 178, 7325–7333. [Google Scholar] [CrossRef] [PubMed]
- Al-Azab, M.; Wei, J.; Ouyang, X.; Elkhider, A.; Walana, W.; Sun, X.; Tang, Y.; Wang, B.; Li, X. TL1A mediates fibroblast-like synoviocytes migration and Indian Hedgehog signaling pathway via TNFR2 in patients with rheumatoid arthritis. Eur. Cytokine Netw. 2018, 29, 27–35. [Google Scholar] [CrossRef]
- Raghav, P.K.; Mann, Z.; Ahlawat, S.; Mohanty, S. Mesenchymal stem cell-based nanoparticles and scaffolds in regenerative medicine. Eur. J. Pharmacol. 2022, 918, 174657. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, T.; Feng, R.; Iwahashi, S.I.; Yamada, S.; Saito, Y.; Morine, Y.; Imura, S.; Matsuhisa, M.; Shimada, M. In vitro and in vivo effects of insulin-producing cells generated by xeno-antigen free 3D culture with RCP piece. Sci. Rep. 2019, 9, 10759. [Google Scholar] [CrossRef]
- Nakamura, K. Cell Structure for Brain Damage Treatment, Production Method Thereof, and Brain Damage Treatment Agent. U.S. Patent 10500311B2, 6 April 2017. [Google Scholar]
- Nakamura, K. CellSaic, A Cell Aggregate-Like Technology Using Recombinant Peptide Pieces for MSC Transplantation. Curr. Stem Cell Res. Ther. 2019, 14, 52–56. [Google Scholar] [CrossRef]
- Takamiya, S.; Kawabori, M.; Kitahashi, T.; Nakamura, K.; Mizuno, Y.; Yasui, H.; Kuge, Y.; Tanimori, A.; Takamatsu, Y.; Yuyama, K.; et al. Intracerebral Transplantation of Mesenchymal Stromal Cell Compounded with Recombinant Peptide Scaffold against Chronic Intracerebral Hemorrhage Model. Stem Cells Int. 2022, 2022, 8521922. [Google Scholar] [CrossRef]
- Tanimoto, T.; Endo, K.; Sakamaki, Y.; Ozeki, N.; Katano, H.; Mizuno, M.; Koga, H.; Sekiya, I. Human synovial mesenchymal stem cells show time-dependent morphological changes and increased adhesion to degenerated porcine cartilage. Sci. Rep. 2022, 12, 16619. [Google Scholar] [CrossRef]

| GO Functions | Number of Genes | Representative Genes Related to MSC Function a |
|---|---|---|
| Immunoregulation | 282 | IDO1, IDO2, PTGES, TGFB1, HGF |
| Anti-inflammatory | 303 | PTGS2, IDO1, NOS2, TGFB1, TNFAIP6 |
| Angiogenesis | 121 | VEGFA, TGFB1, CCL2, ANGPT1, FGF2 |
| Neurogenesis | 109 | BDNF, GDNF, VEGFA, NGF, NTF3 |
| Osteogenesis | 96 | CBFA1, ALPL, COL1A1, BGLAP, SPP1 |
| Adipogenesis | 101 | PPARG, FABP4, CEBPA, LPL, ADIPOQ |
| Chondrogenesis | 119 | COL2A1, ACAN, SOX9, COL10A1, COL1A1 |
| Fibrosis | 112 | HGF, VEGFA, IL10, ACTA2, PGE2 |
| Immunogenicity | 42 | CD274, CD40, CD80, CD86, TNFSF14 |
| Migration | 137 | CXCR4, CCR1, CCR2, MMP2, CXCR6 |
| Adhesion | 95 | ITGA4, ITGB1, VCAM1, ICAM1, ITGA5 |
| Senescence | 113 | CDKN2A, GLB1, CDKN1A, TRP53, HAS1 |
| MSC selection markers | 110 | EGF, CD19, ZFP42, NOTCH1, TGFB3 |
| Others | 346 | CCL2, IL6, VEGFA, CXCL12, IL8 |
| Total | 1199 | |
| Excluded genes | 18 | |
| 38 | Genes without readily available PCR primers | |
| Amp 1200 | 1143 | High-expression genes |
| Amp1200 Gene Set R2 | SyMSC CV | Random-Human Gene Set R2 | SyMSC CV | All-Human Gene Set R2 | SyMSC CV | Detection Power (Amp1200 vs. Random- Human) | Detection Power (Amp1200 vs. All-Human) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| MSCs | Natural MSCs | Secondary lot of SyMSCs | 0.886 | 7% | 0.951 | 3% | 0.947 | 3% | 2.47 | 2.07 |
| SyMSCs med | 0.949 | 0% | 0.977 | 0% | 0.978 | 0% | N/A | N/A | ||
| BMSCs, med | 0.821 | 14% | 0.931 | 5% | 0.928 | 5% | 2.88 | 2.62 | ||
| RECs, med | 0.786 | 17% | 0.913 | 7% | 0.912 | 7% | 2.63 | 2.56 | ||
| ADSCs, med | 0.821 | 13% | 0.917 | 6% | 0.919 | 6% | 2.19 | 2.24 | ||
| Artificial MSCs | iMSCs | 0.663 | 30% | 0.859 | 12% | 0.866 | 11% | 2.49 | 2.63 | |
| Non-MSCs | FBs | 0.699 | 26% | 0.870 | 11% | 0.882 | 10% | 2.40 | 2.67 | |
| WPs | 0.720 | 24% | 0.890 | 9% | 0.886 | 9% | 2.71 | 2.58 | ||
| PAECs | 0.462 | 51% | 0.803 | 18% | 0.810 | 17% | 2.88 | 2.99 | ||
| CD14+ monocytes | 0.132 | 86% | 0.578 | 41% | 0.596 | 39% | 2.11 | 2.20 | ||
| T cells | 0.100 | 89% | 0.541 | 45% | 0.592 | 39% | 2.00 | 2.27 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, K.; Kitahashi, T.; Kogawa, R.; Yoshino, Y.; Ogura, I. Definition of Synovial Mesenchymal Stem Cells for Meniscus Regeneration by the Mechanism of Action and General Amp1200 Gene Expression. Int. J. Mol. Sci. 2024, 25, 10510. https://doi.org/10.3390/ijms251910510
Nakamura K, Kitahashi T, Kogawa R, Yoshino Y, Ogura I. Definition of Synovial Mesenchymal Stem Cells for Meniscus Regeneration by the Mechanism of Action and General Amp1200 Gene Expression. International Journal of Molecular Sciences. 2024; 25(19):10510. https://doi.org/10.3390/ijms251910510
Chicago/Turabian StyleNakamura, Kentaro, Tsukasa Kitahashi, Ryo Kogawa, Yuichi Yoshino, and Izumi Ogura. 2024. "Definition of Synovial Mesenchymal Stem Cells for Meniscus Regeneration by the Mechanism of Action and General Amp1200 Gene Expression" International Journal of Molecular Sciences 25, no. 19: 10510. https://doi.org/10.3390/ijms251910510







