Influence of Eu3+ Doping on Physiochemical Properties and Neuroprotective Potential of Polyacrylic Acid Functionalized Cerium Oxide Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Size Distribution and Stability
2.2. Absorption and Emission Characteristics
2.3. Visualization of the Red Fluorescence of Eu-CeONPs
2.4. X-ray Photoelectron Spectroscopy (XPS) Analysis
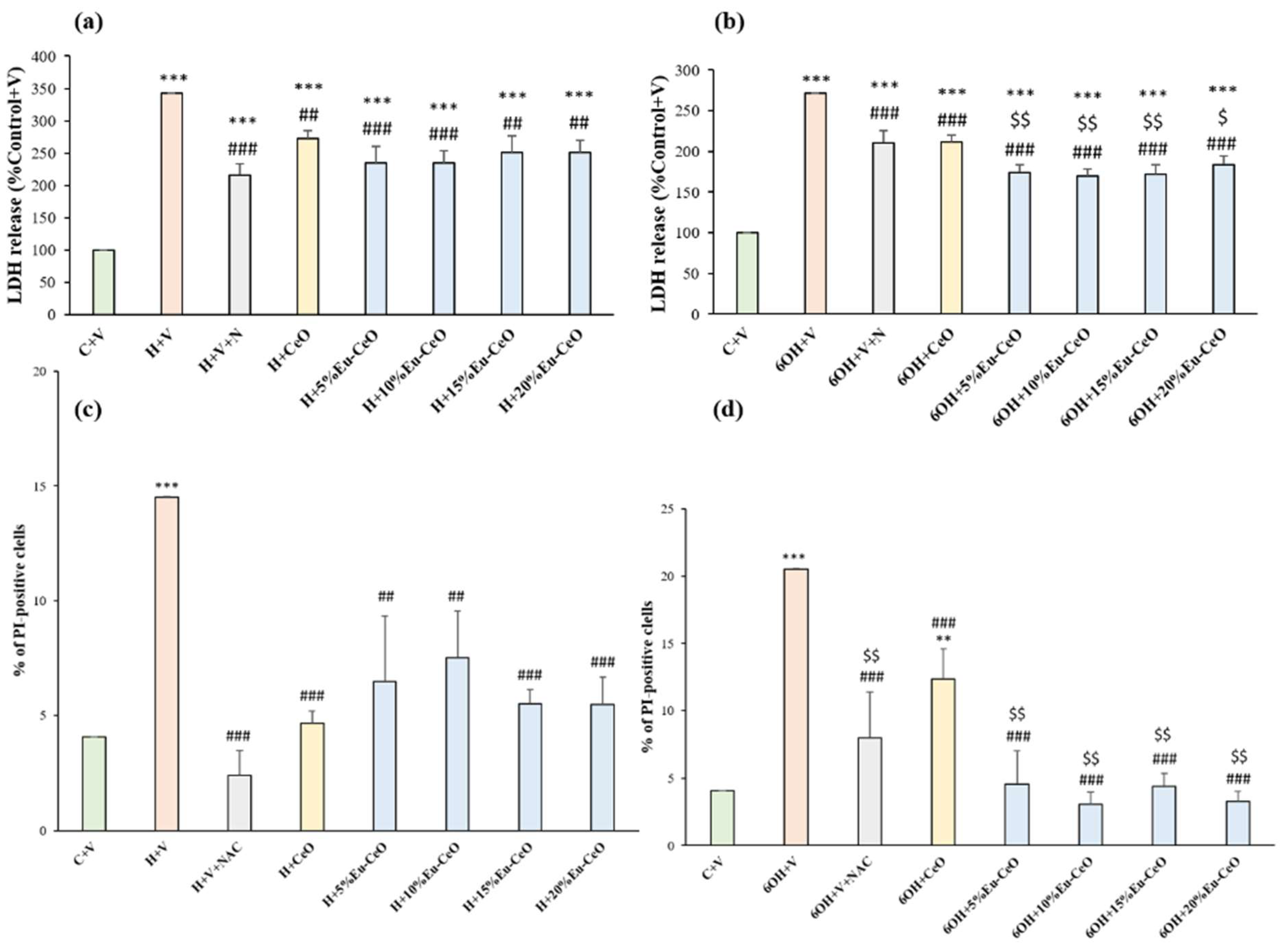
2.5. Biosafety Evaluation of Eu-CeO Nanoparticles
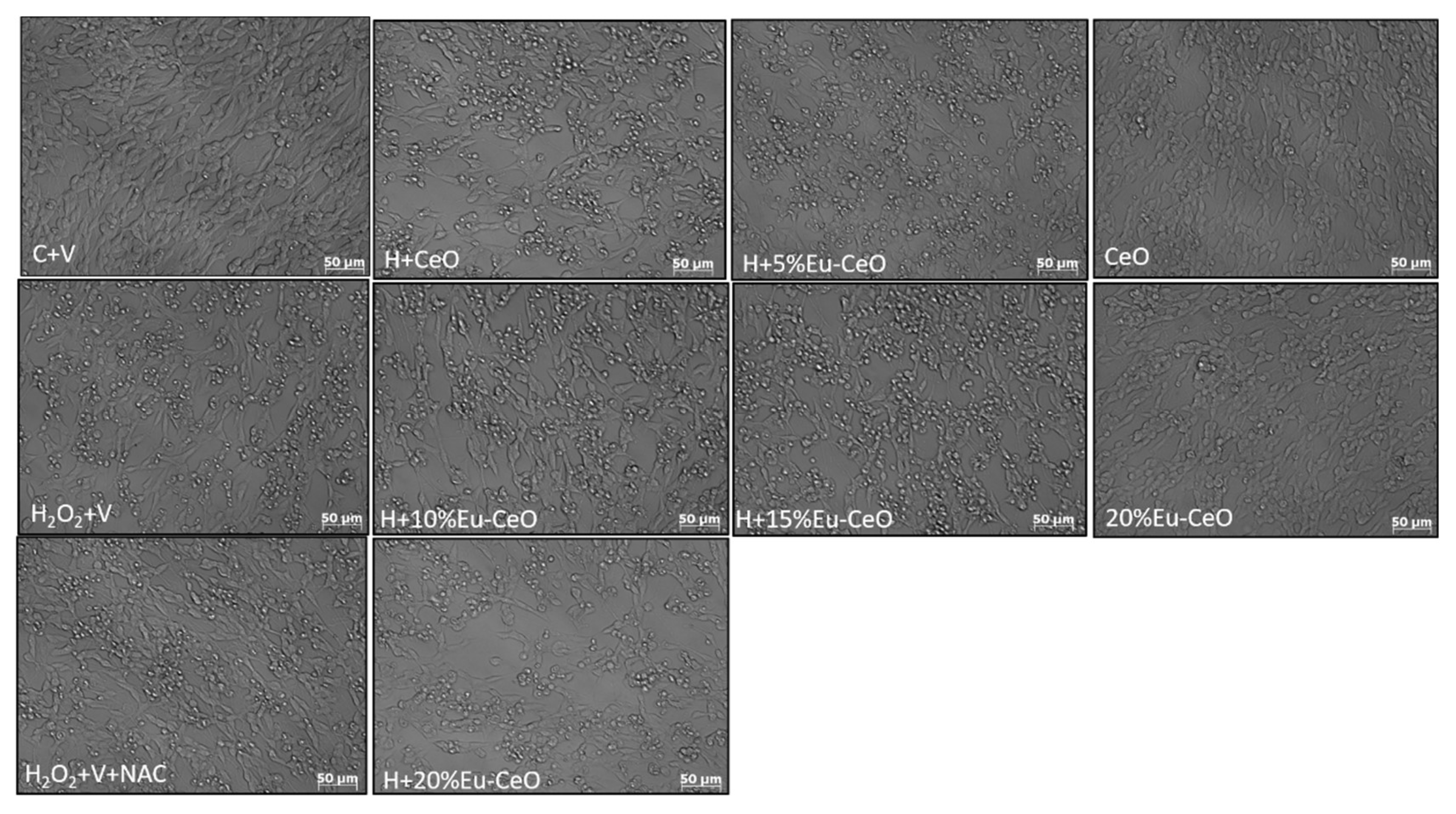
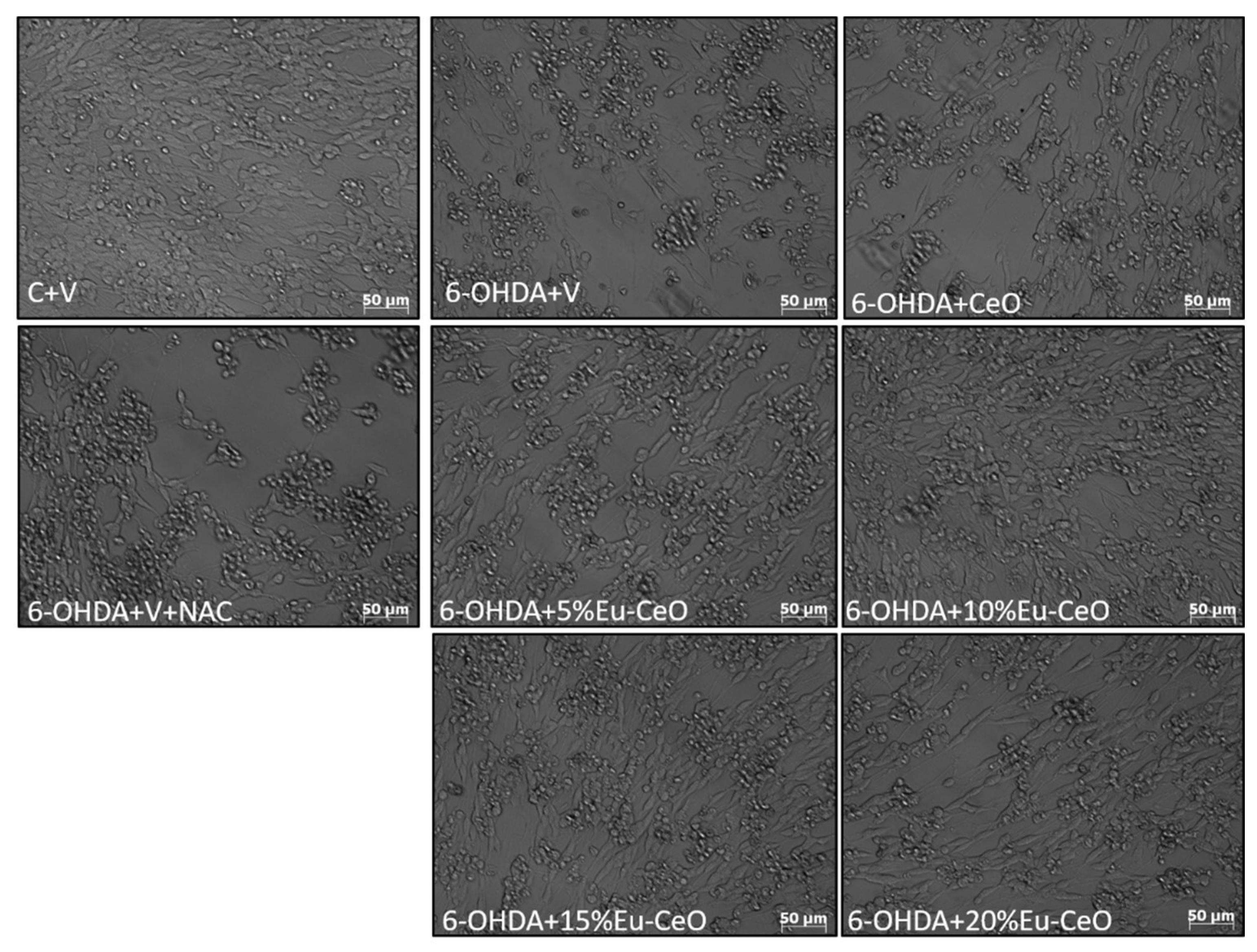
2.6. Neuroprotective Effects of Eu-CeO Nanoparticles against the H2O2 and 6-OHDA-Induced Cell Damage
2.7. Effect of Eu-CeO Nanoparticles on the H2O2-Induced Increase in Intracellular Reactive Oxygen Species (ROS) Level
2.8. Effect of Eu-CeONPs on Caspase-3 Activity
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Characterization of Eu-CeO Nanoparticles
3.3. Cell Culture
3.4. Cell Treatment
3.5. Cytotoxicity Assay
3.6. Microscopic Assessment of Morphological Changes
3.7. Propidium Iodide Staining and Flow Cytometry
3.8. Measurement of Intracellular Reactive Oxygen Species (ROS)
3.9. Caspase-3 Activity Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, F.; Ehlerding, E.B.; Cai, W. Theranostic Nanoparticles. J. Nucl. Med. 2014, 55, 1919–1922. [Google Scholar] [CrossRef]
- Indoria, S.; Singh, V.; Hsieh, M.F. Recent Advances in Theranostic Polymeric Nanoparticles for Cancer Treatment: A Review. Int. J. Pharm. 2020, 582, 119314. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Kazmi, S.T.B.; Zia, M. CeO2 Nanoparticles Synthesized through Green Chemistry Are Biocompatible: In Vitro and In Vivo Assessment. J. Biochem. Mol. Toxicol. 2019, 33, e22291. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, A.; Mohammadi-Aghdam, S.; Mortazavi-Derazkola, S. Novel CoFe2O4@ZnO-CeO2 Ternary Nanocomposite: Sonochemical Green Synthesis Using Crataegus Microphylla Extract, Characterization and Their Application in Catalytic and Antibacterial Activities. Bioorg. Chem. 2020, 103, 104194. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium Oxide Nanoparticles in Neuroprotection and Considerations for Efficacy and Safety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef] [PubMed]
- Zavvari, F.; Nahavandi, A.; Shahbazi, A. Neuroprotective Effects of Cerium Oxide Nanoparticles on Experimental Stress-Induced Depression in Male Rats. J. Chem. Neuroanat. 2020, 106, 101799. [Google Scholar] [CrossRef] [PubMed]
- Elshony, N.; Nassar, A.M.K.; El-Sayed, Y.S.; Samak, D.; Noreldin, A.; Wasef, L.; Saleh, H.; Elewa, Y.H.A.; Tawfeek, S.E.; Saati, A.A.; et al. Ameliorative Role of Cerium Oxide Nanoparticles Against Fipronil Impact on Brain Function, Oxidative Stress, and Apoptotic Cascades in Albino Rats. Front. Neurosci. 2021, 15, 525. [Google Scholar] [CrossRef] [PubMed]
- Meenambal, R.; Kruk, T.; Gurgul, J.; Warszyński, P.; Jantas, D. Neuroprotective Effects of Polyacrylic Acid (PAA) Conjugated Cerium Oxide against Hydrogen Peroxide- and 6-OHDA-Induced SH-SY5Y Cell Damage. Sci. Rep. 2023, 13, 18534. [Google Scholar] [CrossRef]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Monteiro-Riviere, N.A.; Aggarwal, R.; Davis, J.P.; Narayan, R.J.; Seif, W.T.; McGinnis, J.; Seal, S. Nanoceria as Antioxidant: Synthesis and Biomedical Applications. JOM 2008, 60, 33–37. [Google Scholar] [CrossRef]
- Lee, K.; Knoblauch, N.; Agrafiotis, C.; Pein, M.; Roeb, M.; Schmücker, M.; Sattler, C. Strategic Co-Doping of Ceria for Improved Oxidation Kinetics in Solar Thermochemical Fuel Production. Mater. Today Energy 2023, 35, 101321. [Google Scholar] [CrossRef]
- Arifin, D.; Ambrosini, A.; Wilson, S.A.; Mandal, B.; Muhich, C.L.; Weimer, A.W. Investigation of Zr, Gd/Zr, and Pr/Zr—Doped Ceria for the Redox Splitting of Water. Int. J. Hydrogen Energy 2020, 45, 160–174. [Google Scholar] [CrossRef]
- Fernandez-Garcia, S.; Jiang, L.; Tinoco, M.; Hungria, A.B.; Han, J.; Blanco, G.; Calvino, J.J.; Chen, X. Enhanced Hydroxyl Radical Scavenging Activity by Doping Lanthanum in Ceria Nanocubes. J. Phys. Chem. C 2016, 120, 1891–1901. [Google Scholar] [CrossRef]
- Kavi Rasu, K.; Moorthy Babu, S. Impact of Eu3+ Concentration on the Fluorescence Properties of the LiGd(W0.5Mo0.5O4)2 Novel Red Phosphors. Solid State Sci. 2019, 98, 106028. [Google Scholar] [CrossRef]
- Othman, A.; Hayat, A.; Andreescu, S. Eu-Doped Ceria Nanocrystals as Nanoenzyme Fluorescent Probes for Biosensing. ACS Appl. Nano Mater. 2018, 1, 5722–5735. [Google Scholar] [CrossRef]
- Hernández-Castillo, Y.; García-Hernández, M.; López-Marure, A.; Luna-Domínguez, J.H.; López-Camacho, P.Y.; Morales-Ramírez, Á.d.J. Antioxidant Activity of Cerium Oxide as a Function of Europium Doped Content. Ceram. Int. 2019, 45, 2303–2308. [Google Scholar] [CrossRef]
- Kumar, A.; Babu, S.; Karakoti, A.S.; Schulte, A.; Seal, S. Luminescence Properties of Europium-Doped Cerium Oxide Nanoparticles: Role of Vacancy and Oxidation States. Langmuir 2009, 25, 10998–11007. [Google Scholar] [CrossRef] [PubMed]
- MacHhi, J.; Yeapuri, P.; Markovic, M.; Patel, M.; Yan, W.; Lu, Y.; Cohen, J.D.; Hasan, M.; Abdelmoaty, M.M.; Zhou, Y.; et al. Europium-Doped Cerium Oxide Nanoparticles for Microglial Amyloid Beta Clearance and Homeostasis. ACS Chem. Neurosci. 2022, 13, 1232–1244. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Li, X.; Qiao, S.; Huang, G.; Hermann, D.M.; Doeppner, T.R.; Zeng, M.; Liu, W.; Xu, G.; et al. A Co-Doped Fe3O4Nanozyme Shows Enhanced Reactive Oxygen and Nitrogen Species Scavenging Activity and Ameliorates the Deleterious Effects of Ischemic Stroke. ACS Appl. Mater. Interfaces 2021, 13, 46213–46224. [Google Scholar] [CrossRef]
- Kumar Singh, R.; Som, S.; Lu, C.H. Spectroscopic Investigation of Red Eu3+ Doped Ceria Nanophosphors and Promising Color Rendition for Warm White LEDs. J. Alloys Compd. 2020, 816, 152653. [Google Scholar] [CrossRef]
- Dubey, V.; Kaur, J.; Agrawal, S. Effect of Europium Doping Levels on Photoluminescence and Thermoluminescence of Strontium Yttrium Oxide Phosphor. Mater. Sci. Semicond. Process. 2015, 31, 27–37. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, H.; Qian, J.; Li, Z.; Hu, X.; Guo, Y.; Fu, Y.; Zhu, H.; Nai, W.; Yang, Z.; et al. Rare Earth Ion Doped Luminescent Materials: A Review of Up/Down Conversion Luminescent Mechanism, Synthesis, and Anti-Counterfeiting Application. Photonics 2023, 10, 1014. [Google Scholar] [CrossRef]
- Sahu, I.P.; Bisen, D.P.; Brahme, N.; Tamrakar, R.K. Photoluminescence Properties of Europium Doped Di-Strontium Magnesium Di-Silicate Phosphor by Solid State Reaction Method. J. Radiat. Res. Appl. Sci. 2015, 8, 104–109. [Google Scholar] [CrossRef]
- Verma, N.; Michalska-Domańska, M.; Ram, T.; Kaur, J.; Misra, A.K.; Dubey, V.; Dubey, N.; Tiwari, K.; Rao, M.C. Optimizing the Luminescence Efficiency of an Europium (Eu3+) Doped SrY2O4 Phosphor for Flexible Display and Lighting Applications. RSC Adv. 2023, 13, 20217–20228. [Google Scholar] [CrossRef]
- Bezkrovnyi, O.S.; Blaumeiser, D.; Vorokhta, M.; Kraszkiewicz, P.; Pawlyta, M.; Bauer, T.; Libuda, J.; Kepinski, L. NAP-XPS and in Situ DRIFTS of the Interaction of CO with Au Nanoparticles Supported by Ce1-XEuxO2 Nanocubes. J. Phys. Chem. C 2020, 124, 5647–5656. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, R.; Choudhary, R.J.; Phase, D.M. Structural, XPS and Magnetic Studies of Pulsed Laser Deposited Fe Doped Eu2O3 Thin Film. Mater. Res. Bull. 2015, 70, 392–396. [Google Scholar] [CrossRef]
- Mercier, F.; Alliot, C.; Bion, L.; Thromat, N.; Toulhoat, P. XPS Study of Eu(III) Coordination Compounds: Core Levels Binding Energies in Solid Mixed-Oxo-Compounds EumXxOy. J. Electron Spectros. Relat. Phenomena 2006, 150, 21–26. [Google Scholar] [CrossRef]
- Kang, J.G.; Jung, Y.; Min, B.K.; Sohn, Y. Full Characterization of Eu(OH)3 and Eu2O3 Nanorods. Appl. Surf. Sci. 2014, 314, 158–165. [Google Scholar] [CrossRef]
- Shi, S.; Hossu, M.; Hall, R.; Chen, W. Solution Combustion Synthesis, Photoluminescence and X-ray Luminescence of Eu-Doped Nanoceria CeO2:Eu. J. Mater. Chem. 2012, 22, 23461–23467. [Google Scholar] [CrossRef]
- Mullins, D.R.; Overbury, S.H.; Huntley, D.R. Electron Spectroscopy of Single Crystal and Polycrystalline Cerium Oxide Surfaces. Surf. Sci. 1998, 409, 307–319. [Google Scholar] [CrossRef]
- Maslakov, K.I.; Teterin, Y.A.; Popel, A.J.; Teterin, A.Y.; Ivanov, K.E.; Kalmykov, S.N.; Petrov, V.G.; Petrov, P.K.; Farnan, I. XPS Study of Ion Irradiated and Unirradiated CeO2 Bulk and Thin Film Samples. Appl. Surf. Sci. 2018, 448, 154–162. [Google Scholar] [CrossRef]
- Paparazzo, E. Use and Mis-Use of X-ray Photoemission Spectroscopy Ce3d Spectra of Ce2O3 and CeO2. J. Phys. Condens. Matter 2018, 30, 343003. [Google Scholar] [CrossRef]
- Leel, N.S.; Kiran, M.; Kumawat, M.K.; Alvi, P.A.; Vats, V.S.; Patidar, D.; Dalela, B.; Kumar, S.; Dalela, S. Oxygen Vacancy Driven Luminescence, Ferromagnetic and Electronic Structure Properties of Eu Doped CeO2 Nanoparticles. J. Lumin. 2023, 263, 119981. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A Review on C1s XPS-Spectra for Some Kinds of Carbon Materials. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Castle, J.E.; Chapman-Kpodo, H.; Proctor, A.; Salvi, A.M. Curve-Fitting in XPS Using Extrinsic and Intrinsic Background Structure. J. Electron Spectrosc. Relat. Phenom. 2000, 106, 65–80. [Google Scholar] [CrossRef]
- Jantas, D.; Malarz, J.; Le, T.N.; Stojakowska, A. Neuroprotective Properties of Kempferol Derivatives from Maesa Membranacea against Oxidative Stress-Induced Cell Damage: An Association with Cathepsin d Inhibition and Pi3k/Akt Activation. Int. J. Mol. Sci. 2021, 22, 10363. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Chwastek, J.; Malarz, J.; Stojakowska, A.; Lasoń, W. Neuroprotective Effects of Methyl Caffeate against Hydrogen Peroxide-Induced Cell Damage: Involvement of Caspase 3 and Cathepsin d Inhibition. Biomolecules 2020, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
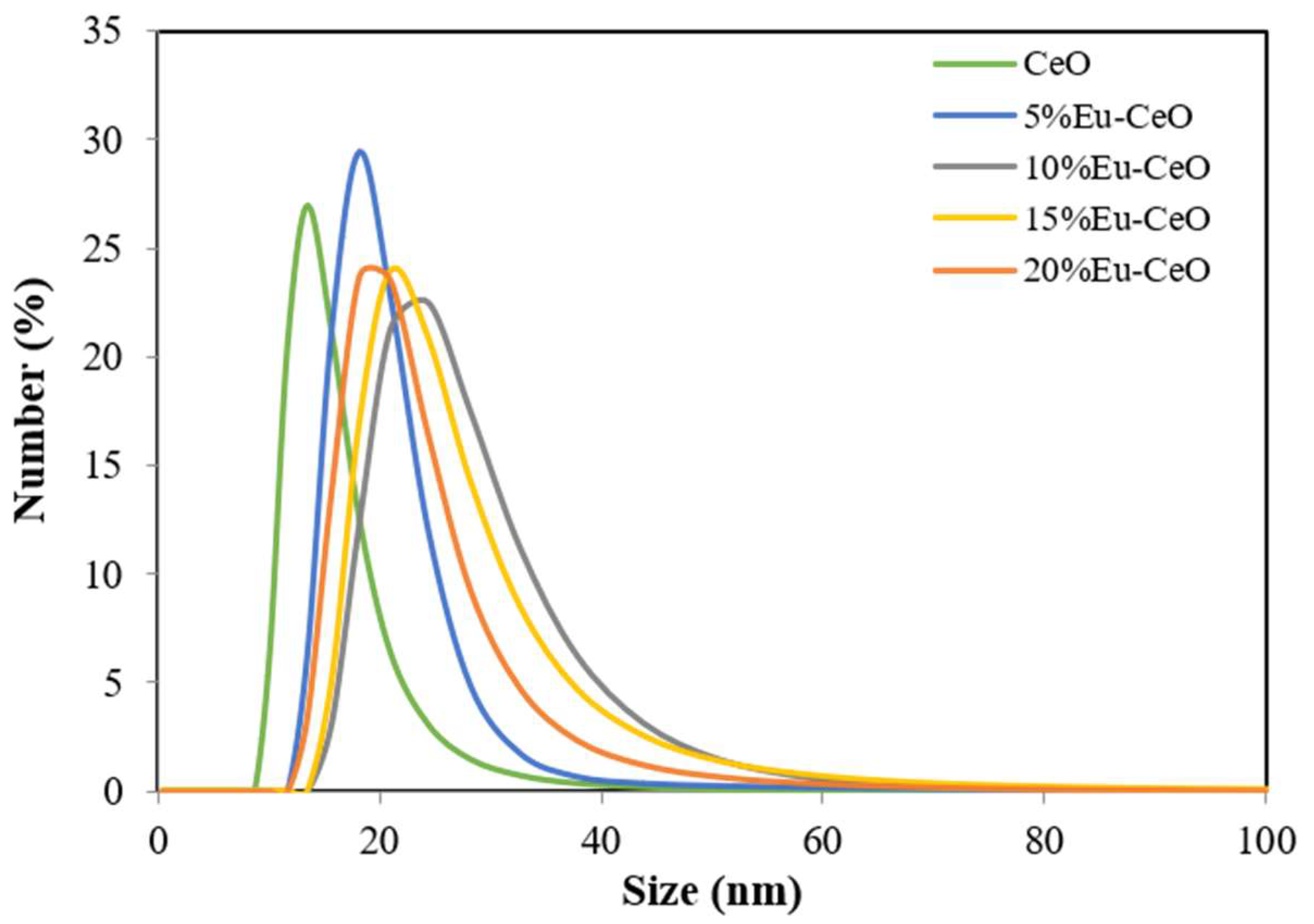
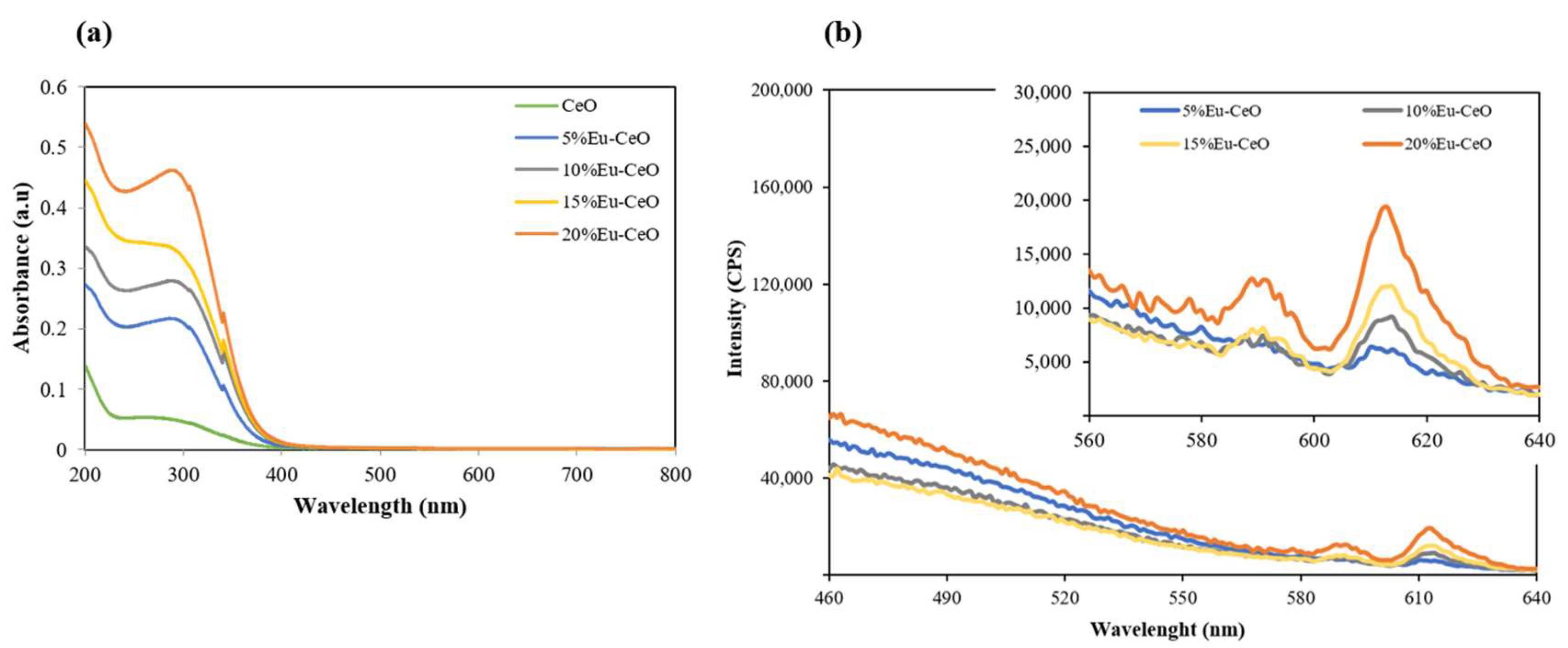

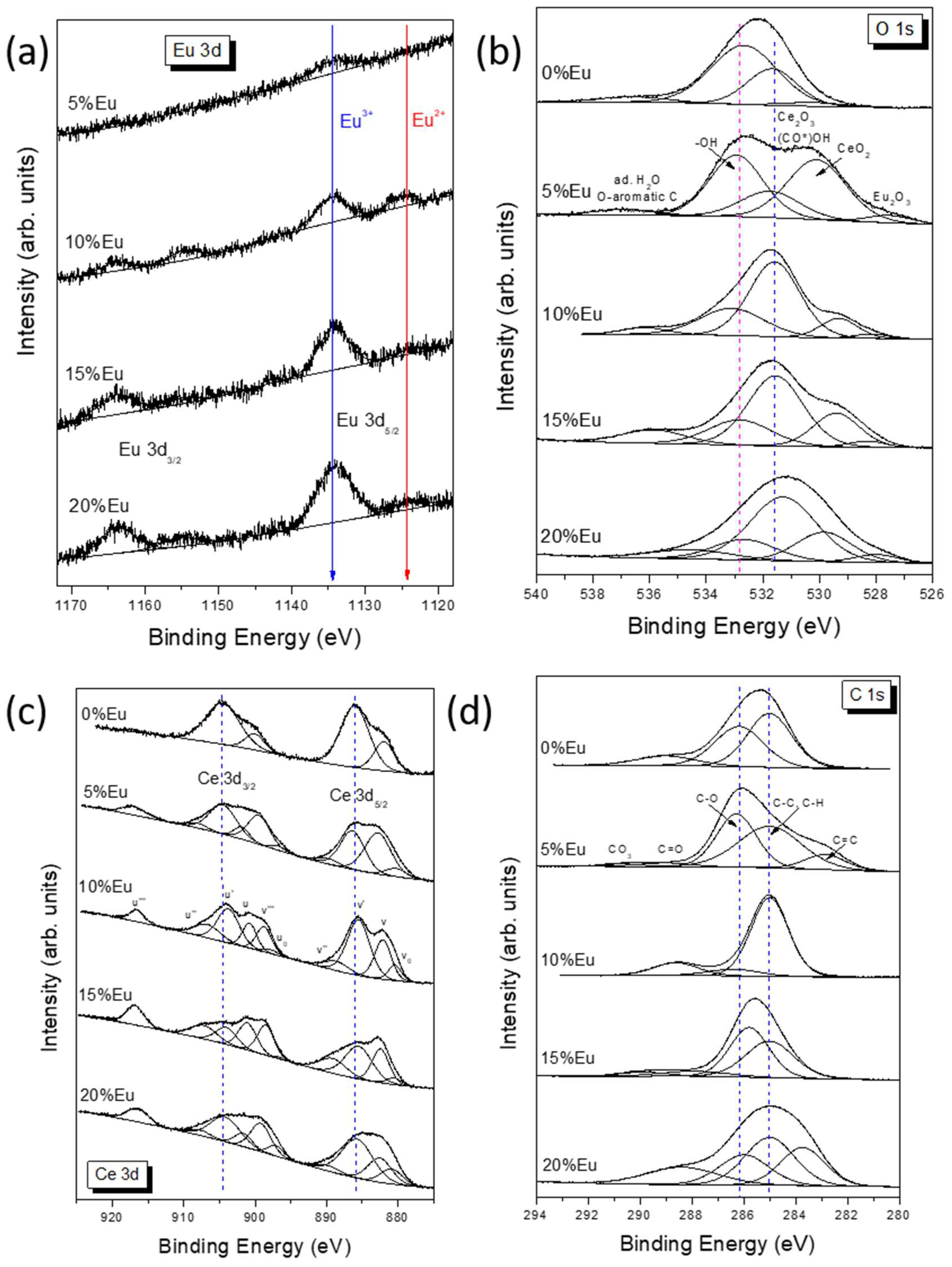

| Sample | Zeta Potential (mV) |
|---|---|
| CeO | −54 |
| 5%Eu-CeO | −58 |
| 10%Eu-CeO | −59 |
| 15%Eu-CeO | −59 |
| 20%Eu-CeO | −61 |
| Sample | Eu-O | CeO2 | Ce2O3, O-C=O | -OH | Ad. H2O O-Aromatic C |
|---|---|---|---|---|---|
| CeO | --- | 530.2 (3.5) | 531.7 (28.9) | 532.7 (62.0) | 536.5 (5.6) |
| 5%Eu-CeO | 527.5 (3.6) | 530.1 (39.0) | 531.7 (17.1) | 532.9 (36.3) | 536.9 (4.0) |
| 10%Eu-CeO | 528.1 (2.1) | 529.3 (10.8) | 531.6 (56.4) | 533.1 (26.2) | 536.2 (4.5) |
| 15%Eu-CeO | 528.3 (7.4) | 529.4 (18.3) | 531.5 (45.6) | 532.8 (18.6) | 535.9 (10.1) |
| 20%Eu-CeO | 528.0 (4.1) | 529.8 (19.8) | 531.3 (51.6) | 532.7 (15.6) | 534.5 (8.9) |
| Sample | Ce 3d5/2 (eV) | Ce 3d3/2 (eV) | Ce3+ (%) | Ce4+ (%) | Ce3+/Ce4+ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| v0 | v | v′ | v″ | v′′′ | u0 | u | u′ | u″ | u′′′ | ||||
| CeO | - | 881.9 | 886.0 | - | - | - | 900.1 | 904.5 | - | 917.1 | 77.2 | 22.8 | 3.39 |
| 5%Eu-CeO | 880.2 | 882.8 | 886.4 | 889.7 | 899.5 | 896.6 | 901.9 | 904.5 | 908.0 | 916.9 | 45.6 | 54.4 | 0.84 |
| 10%Eu-CeO | 880.4 | 882.1 | 885.5 | 888.6 | 898.8 | 897.9 | 900.8 | 903.8 | 906.7 | 916.6 | 51.1 | 48.9 | 1.04 |
| 15%Eu-CeO | 880.5 | 882.4 | 885.5 | 889.1 | 898.5 | 896.6 | 901.1 | 904.1 | 907.2 | 916.8 | 33.5 | 66.5 | 0.50 |
| 20%Eu-CeO | 880.9 | 882.5 | 885.7 | 889.7 | 899.2 | 897.1 | 901.6 | 904.2 | 907.7 | 916.5 | 56.2 | 43.8 | 1.28 |
| Sample | C=C | C-C | C-O | C=O | O-C=O | CO3 |
|---|---|---|---|---|---|---|
| 5%Eu-CeO | 282.8 (10.9) | 285.0 (46.7) | 286.3 (37.5) | --- | 289.0 (3.3) | 290.7 (1.6) |
| 10%Eu-CeO | 282.9 (0.6) | 285.0 (75.7) | 286.4 (8.9) | --- | 288.6 (14.8) | --- |
| 15%Eu-CeO | 282.3 (0.3) | 285.0 (41.4) | 285.8 (45.9) | 287.9 (7.8) | --- | 289.9 (4.6) |
| 20%Eu-CeO | 283.7 (24.8) | 285.0 (34.9) | 286.0 (22.0) | --- | 288.4 (18.3) | --- |
| Sample | CM-DCF Intensity (% Control + V) |
|---|---|
| Control + Vehicle | 100.00 ± 0.00 |
| H + V | 272.01 ± 3.74 ***, ### |
| H + CeO | 356.59 ± 11.35 ***, ### |
| H + 5%Eu-CeO | 368.61 ± 12.05 ***, ### |
| H + 10%Eu-CeO | 385.44 ± 14.16 ***, ### |
| H + 15%Eu-CeO | 364.25 ± 20.63 ***, ### |
| H + 20%Eu-CeO | 403.54 ± 24.49 ***, ### |
| CeO | 109.62 ± 5.62 |
| 5%Eu-CeO | 105.69 ± 2.27 |
| 10%Eu-CeO | 102.89 ± 5.44 |
| 15%Eu-CeO | 105.85 ± 8.03 |
| 20%Eu-CeO | 104.70 ± 1.16 |
| Sample | Caspase-3 Activity (%Control + V) | p-Value vs. 6OHDA from Duncan’s Post Hoc Test |
|---|---|---|
| Control + Vehicle | 100.00 ± 0.00 | |
| 6-OHDA + Vehicle | 499.23 ± 0.96 *** | |
| 6-OHDA + CeO | 428.56 ± 33.80 *** | 0.113611 |
| 6-OHDA + 5%Eu-CeO | 371.69 ± 32.54 ***, ## | 0.007474 |
| 6-OHDA + 10%Eu-CeO | 332.69 ± 42.39 ***, ## | 0.001045 |
| 6-OHDA + 15%Eu-CeO | 353.96 ± 36.46 ***, ## | 0.002326 |
| 6-OHDA + 20%Eu-CeO | 343.03 ± 37.43 ***, ## | 0.001793 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meenambal, R.; Kruk, T.; Jakubowska, K.; Gurgul, J.; Szczepanowicz, K.; Szczęch, M.; Szyk-Warszyńska, L.; Warszyński, P.; Jantas, D. Influence of Eu3+ Doping on Physiochemical Properties and Neuroprotective Potential of Polyacrylic Acid Functionalized Cerium Oxide Nanoparticles. Int. J. Mol. Sci. 2024, 25, 2501. https://doi.org/10.3390/ijms25052501
Meenambal R, Kruk T, Jakubowska K, Gurgul J, Szczepanowicz K, Szczęch M, Szyk-Warszyńska L, Warszyński P, Jantas D. Influence of Eu3+ Doping on Physiochemical Properties and Neuroprotective Potential of Polyacrylic Acid Functionalized Cerium Oxide Nanoparticles. International Journal of Molecular Sciences. 2024; 25(5):2501. https://doi.org/10.3390/ijms25052501
Chicago/Turabian StyleMeenambal, Rugmani, Tomasz Kruk, Klaudia Jakubowska, Jacek Gurgul, Krzysztof Szczepanowicz, Marta Szczęch, Lilianna Szyk-Warszyńska, Piotr Warszyński, and Danuta Jantas. 2024. "Influence of Eu3+ Doping on Physiochemical Properties and Neuroprotective Potential of Polyacrylic Acid Functionalized Cerium Oxide Nanoparticles" International Journal of Molecular Sciences 25, no. 5: 2501. https://doi.org/10.3390/ijms25052501








