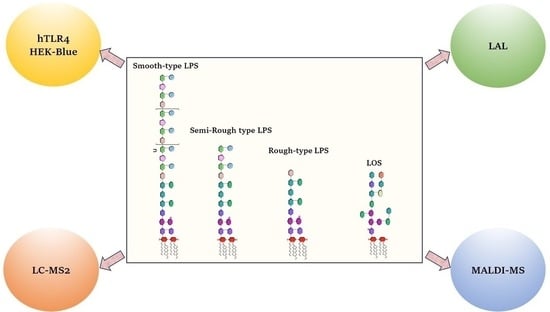
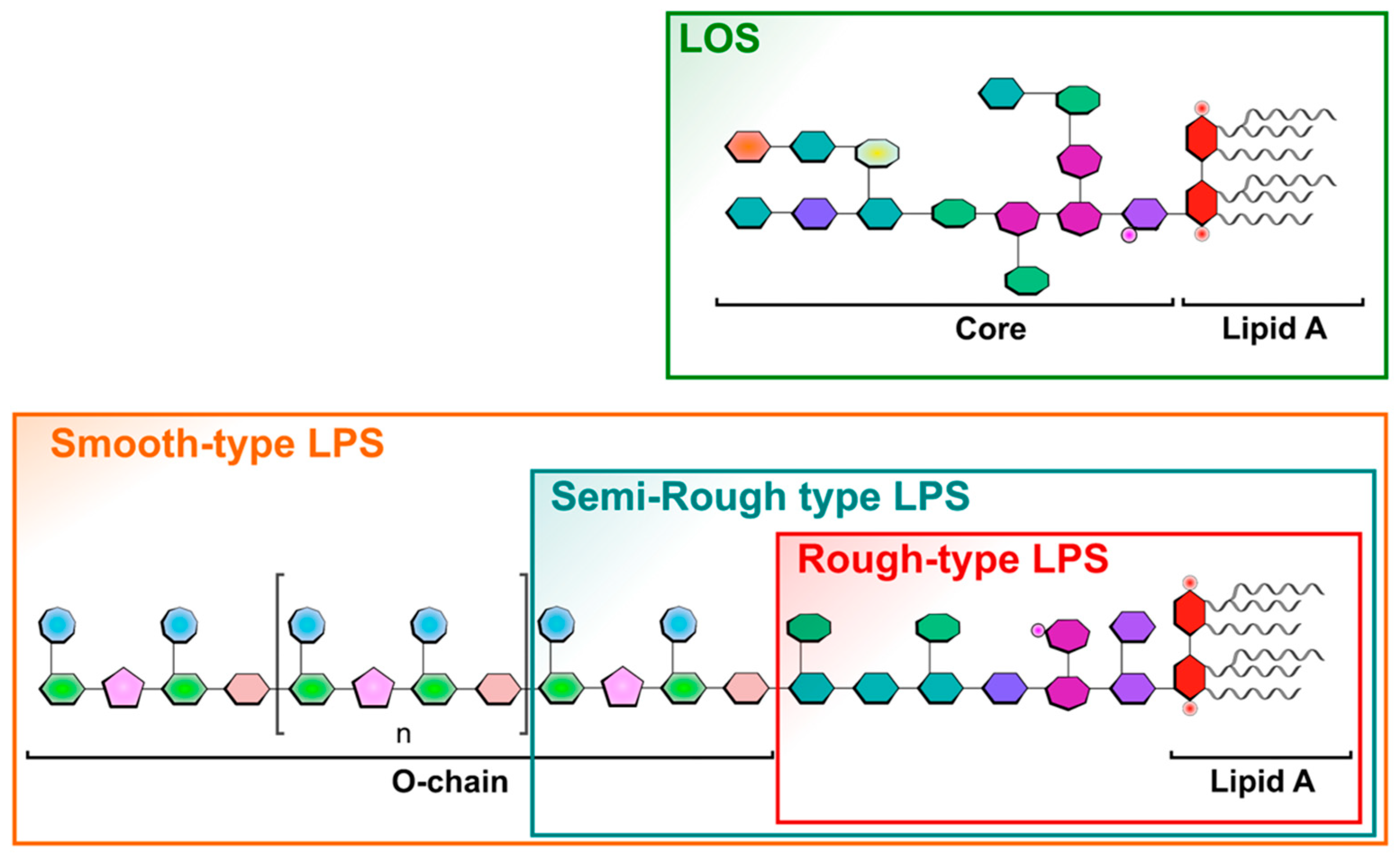
Diversity, Complexity, and Specificity of Bacterial Lipopolysaccharide (LPS) Structures Impacting Their Detection and Quantification †
Abstract
:1. Introduction
2. Results
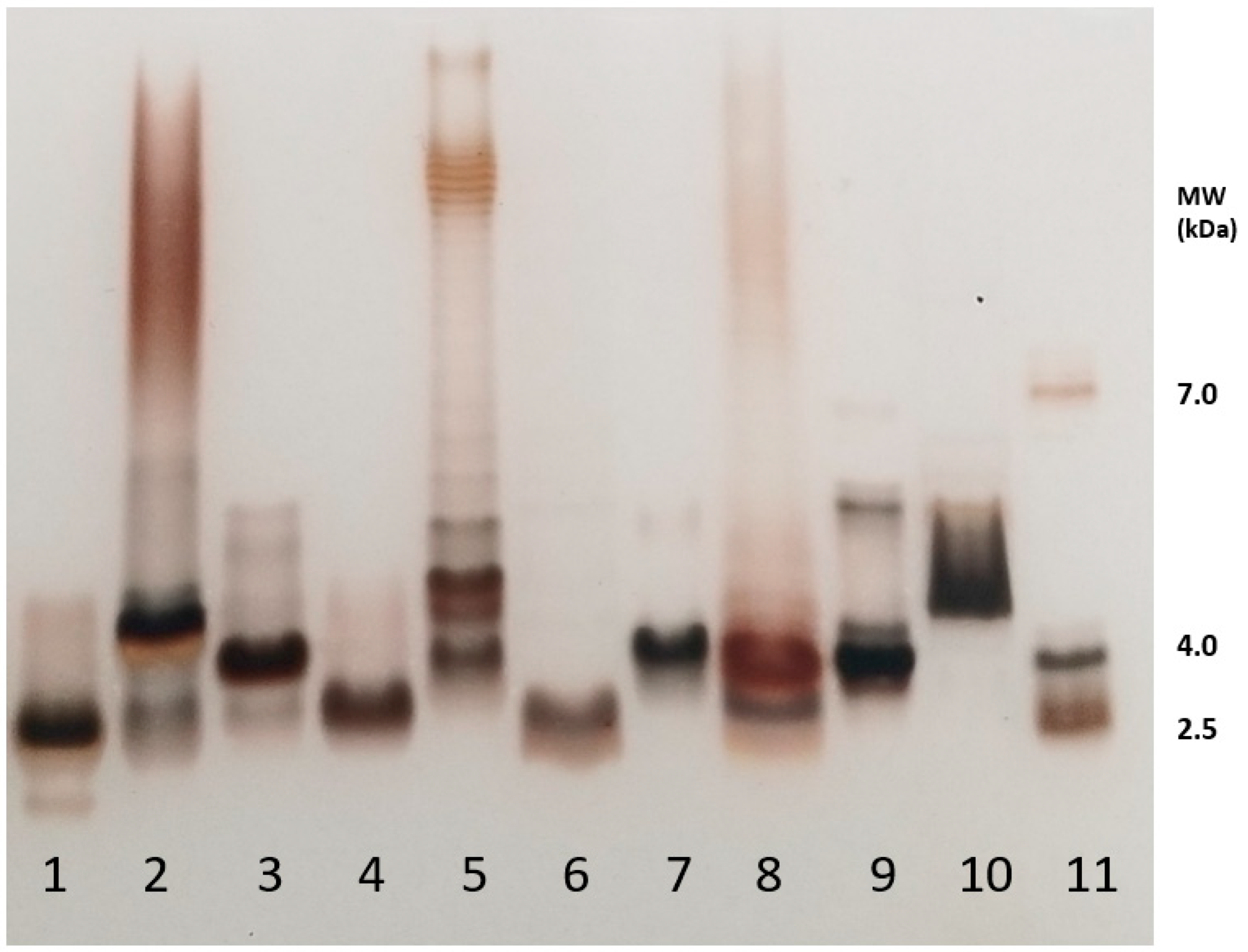
2.1. Illustration of LPSs’ Diversity by SDS-PAGE Electrophoresis of the Different LPS Samples Used in This Work
2.2. Impact of Growth Medium Temperature Conditions on Two Yersinia Species
2.2.1. Impact of Growth Temperature on Yersinia pestis LPSs
Impact on Y. pestis Lipid A Structures
Impact of Growth Temperatures on Y. pestis LPS Detection
2.2.2. Impact of Growth Temperatures on Yersinia enterocolitica LPSs
Impact on Y. enterocolitica Lipid A Structures
Impact on Y. enterocolitica LPS Detection
2.3. Impact of Lipid A Phosphate Group Decorations and Fatty Acid Number on LPS Biological Activities and Detection
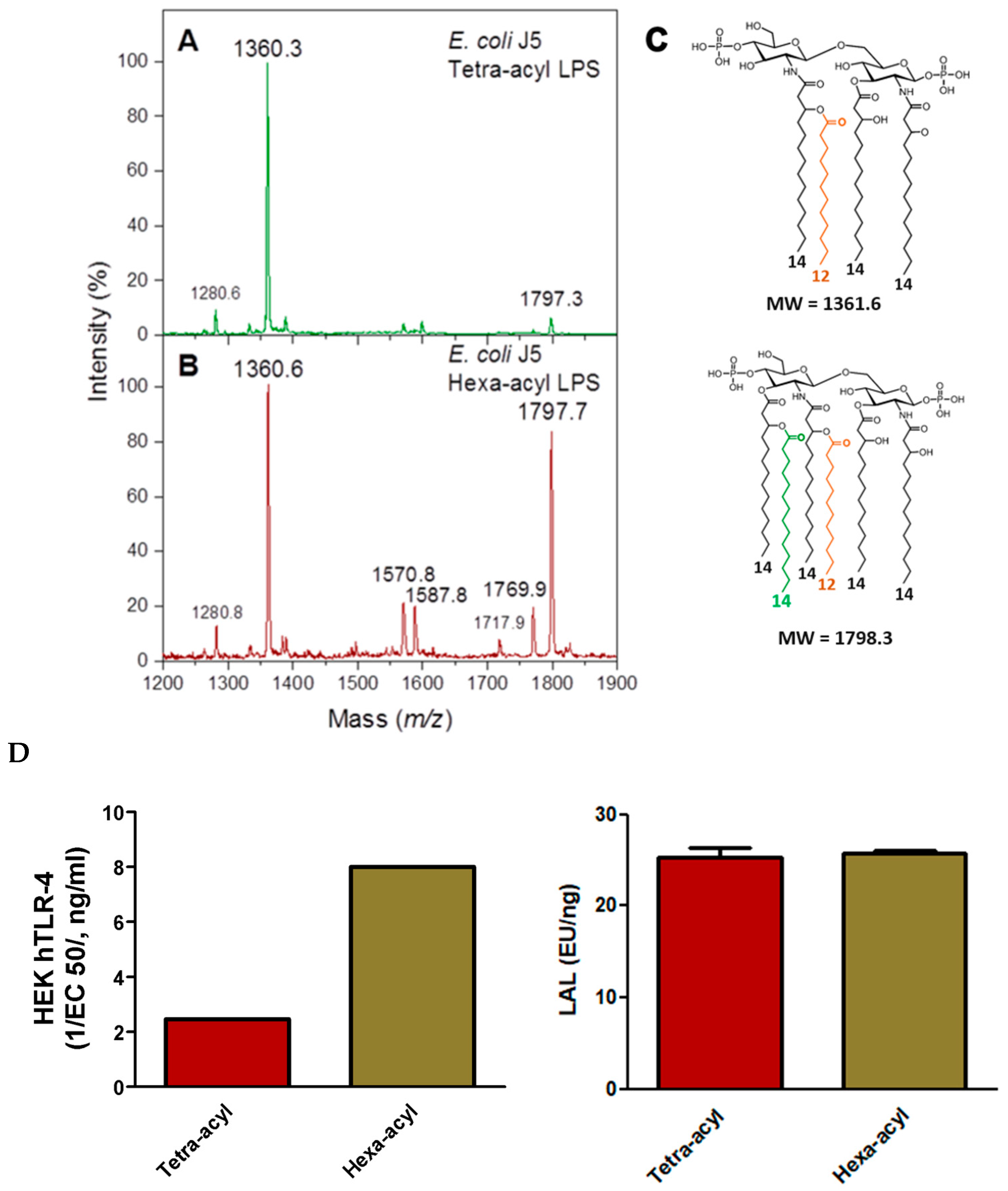
2.3.1. Comparison of TLR-4 Induction and LAL Detection with Tetra- and Hexa-Acylated Lipid A in E. coli J5 LPS
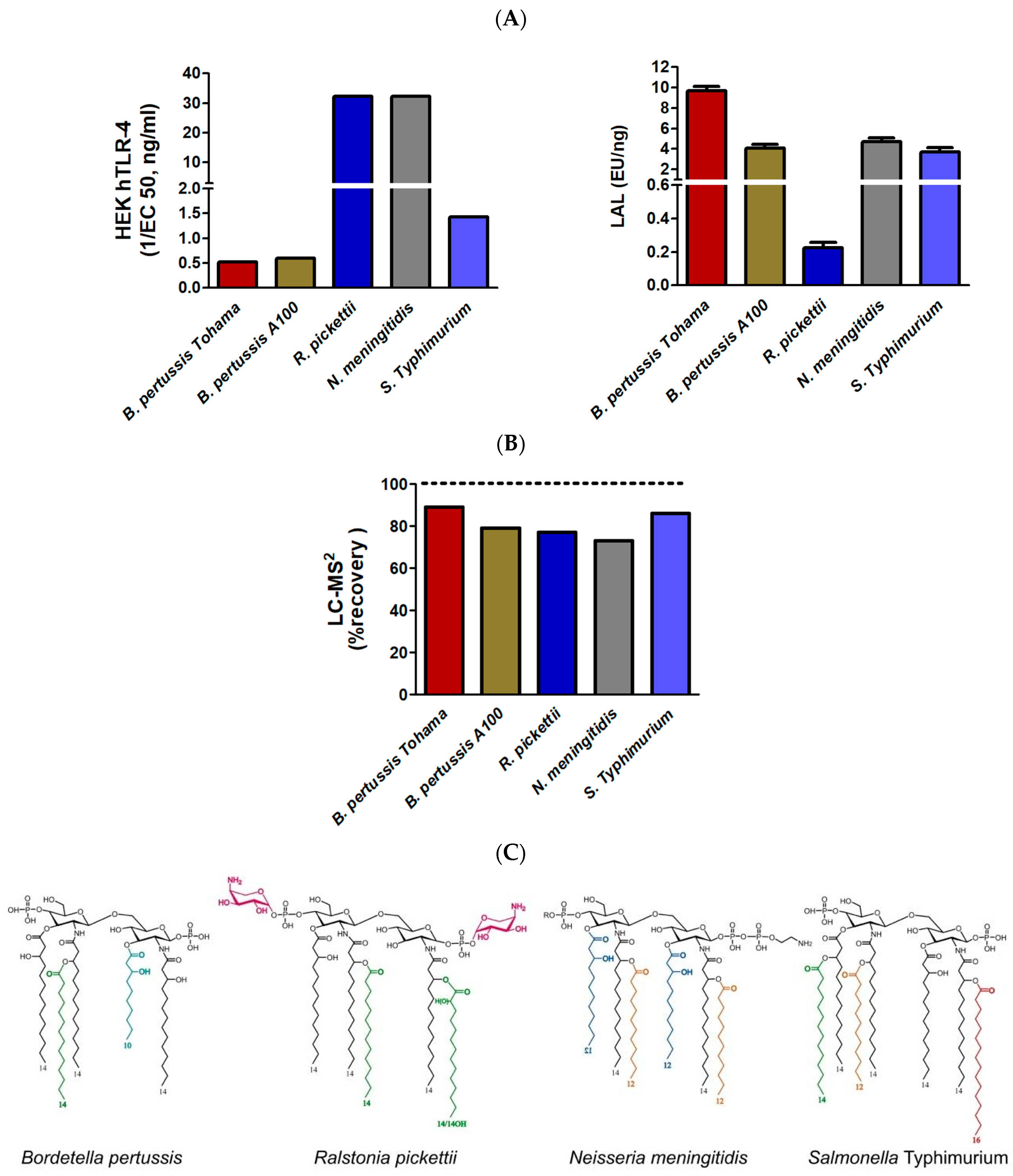
2.3.2. Comparison of TLR4, LAL, and LC-MS2 Analyses in LPSs from Different Genera with Various Lipid A Structures
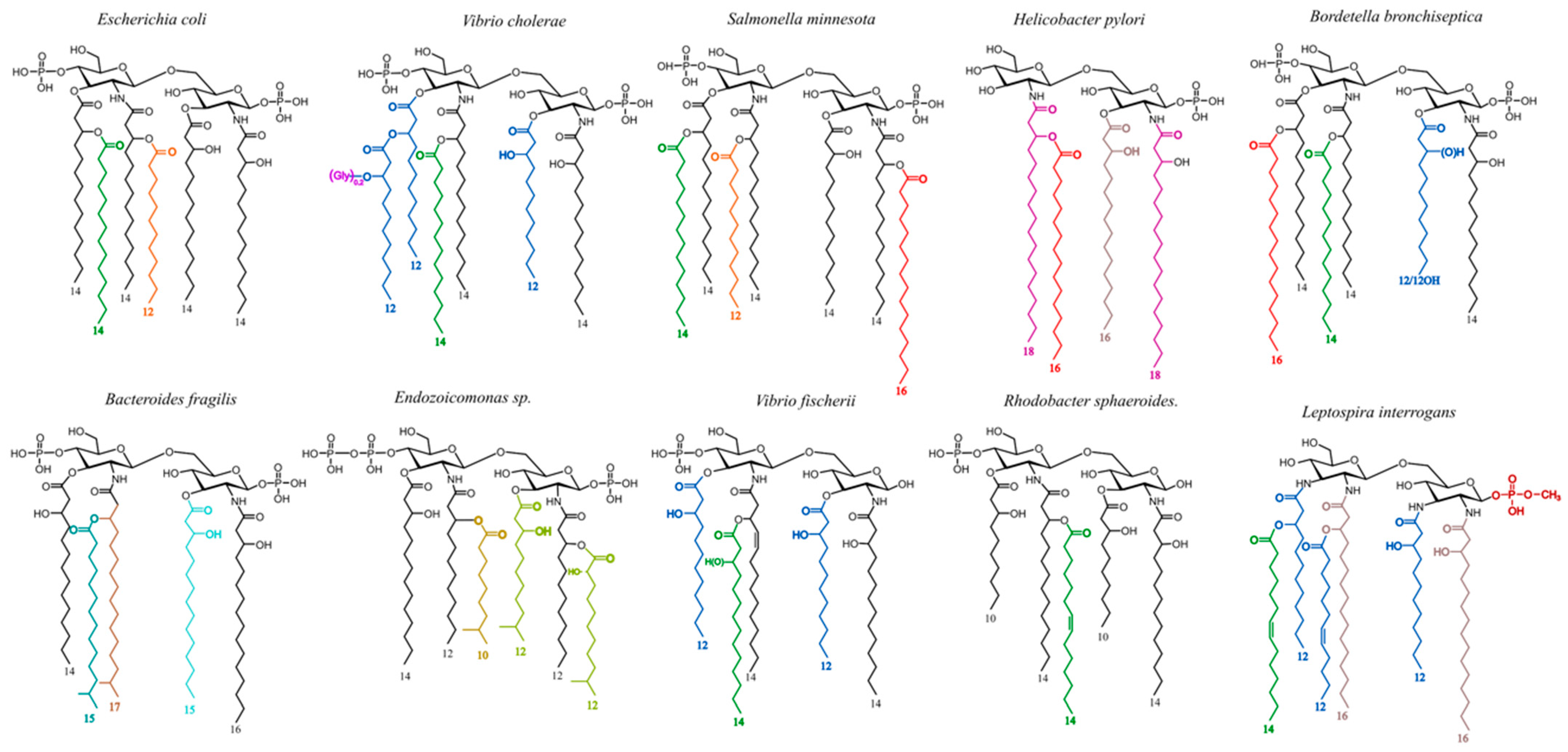
3. Discussion
4. Material and Methods
4.1. Bacteria
4.2. LPS and Lipid A Preparation
4.3. Sequential Ester-Linked Fatty Acids’ Release by Mild Alkali Treatments
4.4. LPS Quantification by 3-Hydroxy Fatty Acids’ Dosage
4.5. Chemical Analyses
4.6. SDS–Polyacrylamide Gel Analysis of LPS
4.7. MALDI Mass Spectrometry
4.8. LAL Detection Test
4.9. TLR-4 Reporter Assays
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DHB | 2,5-dihydroxybenzoic acid |
| EU | Endotoxin unit |
| FA | Fatty Acid |
| GlcN | Glucosamine |
| HEK | Human embryonic kidney |
| IL | Interleukin |
| LC-MS2 | Liquid Chromatography–Mass Spectrometry |
| LAL | Limulus amoebocyte Lysate |
| LOD | Limit of detection |
| LOS | Lipooligosaccharide |
| LPS | Lipopolysaccharide |
| MALDI | Matrix-assisted laser desorption ionization |
| MAT | Monocyte Activation Test |
| MD-2 | Myeloid Differentiation Protein-2 |
| OMV | Outer membrane vesicle |
| PBS | Phosphate Buffer Saline |
| PEA | Phosphoethanolamine |
| PS | Polysaccharide |
| rFC | Recombinant factor C |
| RPT | Rabbit pyrogen test |
| RSE | Reference Standard Endotoxin |
| TLR | Toll Like Receptor |
References
- Fivenson, E.M.; Rohs, P.D.A.; Vettiger, A.; Sardis, M.F.; Torres, G.; Forchoh, A.; Bernhardt, T.G. A Role for the Gram-Negative Outer Membrane in Bacterial Shape Determination. Proc. Natl. Acad. Sci. USA 2023, 120, e2301987120. [Google Scholar] [CrossRef]
- Valvano, M.A. Remodelling of the Gram-Negative Bacterial Kdo2-Lipid A and Its Functional Implications. Microbiology 2022, 168, 1–14. [Google Scholar] [CrossRef]
- Liu, B.; Furevi, A.; Perepelov, A.V.; Guo, X.; Cao, H.; Wang, Q.; Reeves, P.R.; Knirel, Y.A.; Wang, L.; Widmalm, G. Structure and Genetics of Escherichia Coli O Antigens. FEMS Microbiol. Rev. 2020, 44, 655–683. [Google Scholar] [CrossRef]
- Hong, Y.; Hu, D.; Verderosa, A.D.; Qin, J.; Totsika, M.; Reeves, P.R. Repeat-Unit Elongations To Produce Bacterial Complex Long Polysaccharide Chains, an O-Antigen Perspective. EcoSal Plus 2023, 11, eesp00202022. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide Endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.; Lindner, B.; Brade, H.; Raina, S. Molecular Basis of Lipopolysaccharide Heterogeneity in Escherichia Coli: Envelope Stress-Responsive Regulators Control the Incorporation of Glycoforms with a Third 3-Deoxy-α-D-Manno-Oct-2-Ulosonic Acid and Rhamnose. J. Biol. Chem. 2011, 286, 42787–42807. [Google Scholar] [CrossRef]
- Heinrichs, D.E.; Yethon, J.A.; Whitfield, C. Molecular Basis for Structural Diversity in the Core Regions of the Lipopolysaccharides of Escherichia Coli and Salmonella Enterica. Mol. Microbiol. 1998, 30, 221–232. [Google Scholar] [CrossRef]
- Fux, A.C.; Casonato Melo, C.; Michelini, S.; Swartzwelter, B.J.; Neusch, A.; Italiani, P.; Himly, M. Heterogeneity of Lipopolysaccharide as Source of Variability in Bioassays and LPS-Binding Proteins as Remedy. Int. J. Mol. Sci. 2023, 24, 8395. [Google Scholar] [CrossRef] [PubMed]
- Tuanyok, A.; Stone, J.K.; Mayo, M.; Kaestli, M.; Gruendike, J.; Georgia, S.; Warrington, S.; Mullins, T.; Allender, C.J.; Wagner, D.M.; et al. The Genetic and Molecular Basis of O-Antigenic Diversity in Burkholderia Pseudomallei Lipopolysaccharide. PLoS Negl. Trop. Dis. 2012, 6. [Google Scholar] [CrossRef]
- Wong, D.K.-H.; Morris, C.; Lam, T.L.; Wong, W.K.R.; Hackett, J. Identification of O-Antigen Polymerase Transcription and Translation Start Signals and Visualization of the Protein in Salmonella Enterica Serovar Typhimurium. Microbiology 1999, 145 Pt 9, 2443–2451. [Google Scholar] [CrossRef]
- Whitfield, C.; Trent, M.S. Biosynthesis and Export of Bacterial Lipopolysaccharides. Annu. Rev. Biochem. 2014, 83, 99–128. [Google Scholar] [CrossRef]
- Abeyrathne, P.D.; Daniels, C.; Poon, K.K.H.; Matewish, M.J.; Lam, J.S. Functional Characterization of WaaL, a Ligase Associated with Linking O-Antigen Polysaccharide to the Core of Pseudomonas Aeruginosa Lipopolysaccharide. J. Bacteriol. 2005, 187, 3002–3012. [Google Scholar] [CrossRef]
- Idosa, B.A.; Kelly, A.; Jacobsson, S.; Demirel, I.; Fredlund, H.; Särndahl, E.; Persson, A. Neisseria Meningitidis-Induced Caspase-1 Activation in Human Innate Immune Cells Is Los-Dependent. J. Immunol. Res. 2019, 2019, 6193186. [Google Scholar] [CrossRef]
- Gulati, S.; Shaughnessy, J.; Ram, S.; Rice, P.A. Targetting Lipooligosaccharide (LOS) for a Gonococcal Vaccine. Front. Immunol. 2019, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Caroff, M.; Brisson, J.; Martin, A.; Karibian, D. Structure of the Bordetella Pertussis 1414 Endotoxin. FEBS Lett. 2000, 477, 8–14. [Google Scholar] [CrossRef]
- Gaultier, G.N.; Colledanchise, K.N.; Alhazmi, A.; Ulanova, M. The Immunostimulatory Capacity of Nontypeable Haemophilus Influenzae Lipooligosaccharide. Pathog. Immun. 2017, 2, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Woodacre, A.; Machado, L.R.; Marsden, G.L. An Updated Classification System and Review of the Lipooligosaccharide Biosynthesis Gene Locus in Campylobacter jejuni. Front. Microbiol. 2020, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Campagnari, A.A.; Spinola, S.M.; Lesse, A.J.; Kwaik, Y.A.; Mandrell, R.E.; Apicella, M.A. Lipooligosaccharide Epitopes Shared among Gram-Negative Non-Enteric Mucosal Pathogens. Microb. Pathog. 1990, 8, 353–362. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Paracini, N.; Schneck, E.; Imberty, A.; Micciulla, S. Lipopolysaccharides at Solid and Liquid Interfaces: Models for Biophysical Studies of the Gram-Negative Bacterial Outer Membrane. Adv. Colloid Interface Sci. 2022, 301, 102603. [Google Scholar] [CrossRef]
- Romeyer Dherbey, J.; Parab, L.; Gallie, J.; Bertels, F. Stepwise Evolution of E. Coli C and ΦX174 Reveals Unexpected Lipopolysaccharide (LPS) Diversity. Mol. Biol. Evol. 2023, 40, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bonnington, K.E.; Kuehn, M.J. Outer Membrane Vesicle Production Facilitates LPS Remodeling and Outer Membrane Maintenance in Salmonella during Environmental Transitions. MBio 2016, 7, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-Negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 254572. [Google Scholar] [CrossRef]
- Kocijancic, D.; Leschner, S.; Felgner, S.; Komoll, R.-M.; Frahm, M.; Pawar, V.; Weiss, S. Therapeutic Benefit of Salmonella Attributed to LPS and TNF-α Is Exhaustible and Dictated by Tumor Susceptibility. Oncotarget 2017, 8, 36492–36508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Chen, B.; Fan, X.-Y.; Wu, S.-S.; Zhang, S.-Q.; Wu, H.-M. LPS Cooperates with Poly-L-Arginine to Promote IL-6 and IL-8 Release via the JNK Signaling Pathway in NCI-H292 Cells. J. Immunol. Res. 2016, 2016, 3421060. [Google Scholar] [CrossRef] [PubMed]
- van der Slikke, E.C.; Star, B.S.; van Meurs, M.; Henning, R.H.; Moser, J.; Bouma, H.R. Sepsis Is Associated with Mitochondrial DNA Damage and a Reduced Mitochondrial Mass in the Kidney of Patients with Sepsis-AKI. Crit. Care 2021, 25, 36. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.; Franchimont, D.; Piron, A.; Gevaert, Y.; Schaaf-Lafontaine, N.; Roland, S.; Mahieu, P.; Malaise, M.; De Groote, D.; Louis, R.; et al. Tumour Necrosis Factor (TNF) Gene Polymorphism Influences TNF-α Production in Lipopolysaccharide (LPS)-Stimulated Whole Blood Cell Culture in Healthy Humans. Clin. Exp. Immunol. 1998, 113, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, C.M.; Herrera, C.M.; Williams, P.E.; Ricci, D.P.; Swem, L.R.; Trent, M.S.; Brodbelt, J.S. Mapping Phosphate Modifications of Substituted Lipid A via a Targeted MS3 CID/UVPD Strategy. Analyst 2018, 143, 3091–3099. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.M. Dynamic Lipopolysaccharide Transfer Cascade to TLR4/MD2 Complex via LBP and CD14. BMB Rep. 2017, 50, 55–57. [Google Scholar] [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.-S.; Lee, H.; Lee, J.-O. The Structural Basis of Lipopolysaccharide Recognition by the TLR4-MD-2 Complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Cavaillon, J.M. Cytokines and Macrophages. Biomed. Pharmacother. 1994, 48, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Coats, S.R.; Berezow, A.B.; To, T.T.; Jain, S.; Bainbridge, B.W.; Banani, K.P.; Darveau, R.P. The Lipid A Phosphate Position Determines Differential Host Toll-like Receptor 4 Responses to Phylogenetically Related Symbiotic and Pathogenic Bacteria. Infect. Immun. 2011, 79, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.; Rooney, M.T.; Greenwood, A.I.; Cotten, M.L.; Wereszczynski, J. Lipopolysaccharide Simulations Are Sensitive to Phosphate Charge and Ion Parameterization. J. Chem. Theory Comput. 2020, 16, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Komazin, G.; Maybin, M.; Woodard, R.W.; Scior, T.; Schwudke, D.; Schombel, U.; Gisch, N.; Mamat, U.; Meredith, T.C. Substrate Structure-Activity Relationship Reveals a Limited Lipopolysaccharide Chemotype Range for Intestinal Alkaline Phosphatase. J. Biol. Chem. 2019, 294, 19405–19423. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ribeiro, A.A.; Lin, S.; Cotter, R.J.; Miller, S.I.; Raetz, C.R.H. Lipid A Modifications in Polymyxin-Resistant Salmonella Typhimurium: PmrA-Dependent 4-Amino-4-Deoxy-L-Arabinose, and Phosphoethanolamine Incorporation. J. Biol. Chem. 2001, 276, 43111–43121. [Google Scholar] [CrossRef] [PubMed]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Nagaoka, I.; Yamada, A.; Nagayama, M.; Miki, T. Effect of Hemoperfusion Using Polymyxin B-Immobilized Fibers on Acute Lung Injury in a Rat Sepsis Model. Int. J. Med. Sci. 2014, 11, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Laude-Sharp, M.; Caroff, M.; Simard, L.; Pusineri, C.; Kazatchkine, M.D.; Haeffner-Cavaillon, N. Induction of IL-1 during Hemodialysis: Transmembrane Passage of Intact Endotoxins (LPS). Kidney Int. 1990, 38, 1089–1094. [Google Scholar] [CrossRef]
- Conti, B.; Tabarean, I.; Andrei, C.; Bartfai, T. Cytokines and Fever. Front. Biosci. 2004, 9, 1433–1449. [Google Scholar] [CrossRef]
- Laude-Sharp, M.; Haeffner-Cavaillon, N.; Caroff, M.; Lantreibecq, F.; Pusineri, C.; Kazatchkine, M.D. Dissociation between the Interleukin 1-Inducing Capacity and Limulus Reactivity of Lipopolysaccharides from Gram-Negative Bacteria. Cytokine 1990, 2, 253–258. [Google Scholar] [CrossRef]
- Sweadner, K.J.; Forte, M.; Nelsen, L.L. Filtration Removal of Endotoxin (Pyrogens) in Solution in Different States of Aggregation. Appl. Environ. Microbiol. 1977, 34, 382–385. [Google Scholar] [CrossRef]
- Mueller, M.; Lindner, B.; Kusumoto, S.; Fukase, K.; Schromm, A.B.; Seydel, U. Aggregates Are the Biologically Active Units of Endotoxin. J. Biol. Chem. 2004, 279, 26307–26313. [Google Scholar] [CrossRef] [PubMed]
- Caroff, M.; Deprun, C.; Karibian, D.; Szabó, L. Analysis of Unmodified Endotoxin Preparations by 252Cf Plasma Desorption Mass Spectrometry. Determination of Molecular Masses of the Constituent Native Lipopolysaccharides. J. Biol. Chem. 1991, 266, 18543–18549. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.L.; Ho, B. A New Area in Pyrogen Testing. Trends Biotechnol. 2001, 19, 277–281. [Google Scholar] [CrossRef]
- Franco, E.; Garcia-Recio, V.; Jiménez, P.; Garrosa, M.; Girbés, T.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Endotoxins from a Pharmacopoeial Point of View. Toxins 2018, 10, 331. [Google Scholar] [CrossRef]
- Williams, K.L. Practical Limulus-Based Testing. In Endotoxin Detection and Control in Pharma, Limulus, and Mammalian Systems, 1st ed.; Springer: Cham, Switzerland, 2019; pp. 203–268. ISBN 978-3-030-17148-3. [Google Scholar]
- Cirefice, G.; Schütte, K.; Spreitzer, I.; Charton, E.; Shaid, S.; Viviani, L.; Rubbrecht, M.; Manou, I. The Future of Pyrogenicity Testing: Phasing out the Rabbit Pyrogen Test. A Meeting Report. Biologicals 2023, 84, 101702. [Google Scholar] [CrossRef]
- 2.6.30, 21. Monocyte Activation Test. In European Pharmacopeia 11.3; Council of Europe: Strasbourg, France, 2023.
- Spreitzer, I. Evolution and Characteristics of the Monocyte Activation Test (MAT). In Endotoxin Detection and Control in Pharma, Limulus, and Mammalian Systems; Springer: Cham, Switzerland, 2019; pp. 523–535. [Google Scholar]
- Rudbach, J.A.; Akiya, F.I.; Elin, R.J.; Hochstein, H.D.; Luoma, M.K.; Milner, E.C.; Milner, K.C.; Thomas, K.R. Preparation and Properties of a National Reference Endotoxin. J. Clin. Microbiol. 1976, 3, 21–25. [Google Scholar] [CrossRef]
- Findlay, L.; Desai, T.; Heath, A.; Poole, S.; Crivellone, M.; Hauck, W.; Ambrose, M.; Morris, T.; Daas, A.; Rautmann, G.; et al. Collaborative Study for the Establishment of the WHO 3(Rd) International Standard for Endotoxin, the Ph. Eur. Endotoxin Biological Reference Preparation Batch 5 and the USP Reference Standard for Endotoxin Lot H0K354. Pharmeur. Bio Sci. Notes 2015, 2015, 73–98. [Google Scholar] [PubMed]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like Receptor-4 Mediates Lipopolysaccharide-Induced Signal Transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef]
- Muller-Loennies, S.; Brade, L.; MacKenzie, C.R.; Di Padova, F.E.; Brade, H. Identification of a Cross-Reactive Epitope Widely Present in Lipopolysaccharide from Enterobacteria and Recognized by the Cross-Protective Monoclonal Antibody WN1 222-5. J. Biol. Chem. 2003, 278, 25618–25627. [Google Scholar] [CrossRef]
- Hillenkamp, F.; Karas, M. [12] Mass Spectrometry of Peptides and Proteins by Matrix-Assisted Ultraviolet Laser Desorption/Ionization. Methods Enzymol. 1990, 193, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.M.; Frasch, C.E. A Sensitive Silver Stain for Detecting Lipopolysaccharides in Polyacrylamide Gels. Anal. Biochem. 1982, 119, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.E.; Anderson, A.N.; Perry, M.B. Differences between the LPS Cores in Adherent and Non-Adherent Strains of Enteropathogenic Escherichia Coli 0119. FEMS Microbiol. Lett. 1991, 64, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.; Burrows, L.L.; Lam, J.S. Functional Analysis of Genes Responsible for the Synthesis of the B-Band O Antigen of Pseudomonas Aeruginosa Serotype O6 Lipopolysaccharide. Microbiology 1999, 145, 3505–3521. [Google Scholar] [CrossRef]
- Lam, J.S.; Taylor, V.L.; Islam, S.T.; Hao, Y.; Kocíncová, D. Genetic and Functional Diversity of Pseudomonas Aeruginosa Lipopolysaccharide. Front. Microbiol. 2011, 2, 118. [Google Scholar] [CrossRef] [PubMed]
- Vaneechoutte, M.; Kämpfer, P.; De Baere, T.; Falsen, E.; Verschraegen, G. Wautersia Gen. Nov., a Novel Genus Accomodating the Phylogenetic Lineage Including Ralstonia Eutropha and Related Species, and Proposal of Ralstonia [Pseudomonas] syzygii (Roberts et Al. 1990) Comb. Nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang-Sun, W.; Tercé, F.; Burcelin, R.; Novikov, A.; Serino, M.; Caroff, M. Structure Function Relationships in Three Lipids A from the Ralstonia Genus Rising in Obese Patients. Biochimie 2019, 159, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Aussel, L.; Thérisod, H.; Karibian, D.; Perry, M.B.; Bruneteau, M.; Caroff, M. Novel Variation of Lipid A Structures in Strains of Different Yersinia Species. FEBS Lett. 2000, 465, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lim, K.B.; Poduje, C.M.; Daniel, M.; Gunn, J.S.; Hackett, M.; Miller, S.I. Lipid A Acylation and Bacterial Resistance against Vertebrate Antimicrobial Peptides. Cell 1998, 95, 189–198. [Google Scholar] [CrossRef]
- Kawahara, K.; Tsukano, H.; Watanabe, H.; Lindner, B.; Matsuura, M. Modification of the Structure and Activity of Lipid A in Yersinia pestis Lipopolysaccharide by Growth Temperature. Infect. Immun. 2002, 70, 4092–4098. [Google Scholar] [CrossRef]
- Marr, N.; Hajjar, A.M.; Shah, N.R.; Novikov, A.; Yam, C.S.; Caroff, M.; Fernandez, R.C. Substitution of the Bordetella Pertussis Lipid A Phosphate Groups with Glucosamine Is Required for Robust NF-KappaB Activation and Release of Proinflammatory Cytokines in Cells Expressing Human but Not Murine Toll-like Receptor 4-MD-2-CD14. Infect. Immun. 2010, 78, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Knirel, Y.A.; Dentovskaya, S.V.; Senchenkova, S.N.; Shaikhutdinova, R.Z.; Kocharova, N.A.; Anisimov, A.P. Structural Features and Structural Variability of the Lipopolysaccharide of Yersinia Pestis, the Cause of Plague. J. Endotoxin Res. 2006, 12, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zähringer, U.; Salvetzki, R.; Wagner, F.; Lindner, B.; Ulmer, A.J. Structural and Biological Characterisation of a Novel Tetra-Acyl Lipid A from Escherichia Coli F515 Lipopolysaccharide Acting as Endotoxin Antagonist in Human Monocytes. J. Endotoxin Res. 2001, 7, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, S.; Fukase, K.; Fukase, Y.; Kataoka, M.; Yoshizaki, H.; Sato, K.; Oikawa, M.; Suda, Y. Structural Basis for Endotoxic and Antagonistic Activities: Investigation with Novel Synthetic Lipid A Analogs. J. Endotoxin Res. 2003, 9, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Carfrae, L.A.; Rachwalski, K.; French, S.; Gordzevich, R.; Seidel, L.; Tsai, C.N.; Tu, M.M.; MacNair, C.R.; Ovchinnikova, O.G.; Clarke, B.R.; et al. Inhibiting Fatty Acid Synthesis Overcomes Colistin Resistance. Nat. Microbiol. 2023, 8, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Caroff, M.; Novikov, A. Lipopolysaccharides: Structure, Function and Bacterial Identification. OCL 2020, 27, 31. [Google Scholar] [CrossRef]
- Stenutz, R.; Weintraub, A.; Widmalm, G. The Structures of Escherichia Coli O-Polysaccharide Antigens. FEMS Microbiol. Rev. 2006, 30, 382–403. [Google Scholar] [CrossRef]
- Whitfield, C.; Williams, D.M.; Kelly, S.D. Lipopolysaccharide O-Antigens-Bacterial Glycans Made to Measure. J. Biol. Chem. 2020, 295, 10593–10609. [Google Scholar] [CrossRef]
- Novikov, A.; Shah, N.R.; AlBitar-Nehme, S.; Basheer, S.M.; Trento, I.; Tirsoaga, A.; Moksa, M.; Hirst, M.; Perry, M.B.; El Hamidi, A.; et al. Complete Bordetella Avium, Bordetella Hinzii and Bordetella Trematum Lipid A Structures and Genomic Sequence Analyses of the Loci Involved in Their Modifications. Innate Immun. 2014, 20, 659–672. [Google Scholar] [CrossRef]
- Saha, S.; Pupo, E.; Zariri, A.; Van Der Ley, P. Lipid A Heterogeneity and Its Role in the Host Interactions with Pathogenic and Commensal Bacteria. MicroLife 2022, 3, uqac011. [Google Scholar] [CrossRef]
- Velasco, J.; Moll, H.; Knirel, Y.A.; Sinnwell, V.; Moriyón, I.; Zähringer, U. Structural Studies on the Lipopolysaccharide from a Rough Strain of Ochrobactrum Anthropi Containing a 2,3-Diamino-2,3-Dideoxy-d-Glucose Disaccharide Lipid A Backbone. Carbohydr. Res. 1998, 306, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, D.V.; Kannenberg, E.L.; Sherrier, D.J.; Buhr, R.J.; Carlson, R.W. The Lipopolysaccharide Lipid a Long-Chain Fatty Acid Is Important for Rhizobium Leguminosarum Growth and Stress Adaptation in Free-Living and Nodule Environments. Mol. Plant-Microbe Interact. 2017, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Kirikae, T.; Ulrich Schade, F.; Kirikae, F.; Qureshi, N.; Takayama, K.; Rietschel, E.T. Diphosphoryl Lipid A Derived from the Lipopolysaccharide (LPS) of Rhodobacter Sphaeroides ATCC 17023 Is a Potent Competitive LPS Inhibitor in Murine Macrophage-like J774.1 Cells. FEMS Immunol. Med. Microbiol. 1994, 9, 231–236. [Google Scholar] [CrossRef]
- Cullen, T.W.; Giles, D.K.; Wolf, L.N.; Ecobichon, C.; Boneca, I.G.; Trent, M.S. Helicobacter pylori versus the Host: Remodeling of the Bacterial Outer Membrane Is Required for Survival in the Gastric Mucosa. PLoS Pathog. 2011, 7, e1002454. [Google Scholar] [CrossRef]
- Lepper, P.M.; Triantafilou, M.; Schumann, C.; Schneider, E.M.; Triantafilou, K. Lipopolysaccharides from Helicobacter pylori Can Act as Antagonists for Toll-like Receptor 4. Cell. Microbiol. 2005, 7, 519–528. [Google Scholar] [CrossRef]
- Moran, A.P. Lipopolysaccharide in Bacterial Chronic Infection: Insights from Helicobacter pylori Lipopolysaccharide and Lipid A. Int. J. Med. Microbiol. 2007, 297, 307–319. [Google Scholar] [CrossRef]
- Ogawa, T.; Asai, Y.; Sakai, Y.; Oikawa, M.; Fukase, K.; Suda, Y.; Kusumoto, S.; Tamura, T. Endotoxic and Immunobiological Activities of a Chemically Synthesized Lipid A of Helicobacter pylori Strain 206-1. FEMS Immunol. Med. Microbiol. 2003, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.P.; Rietschel, E.T.; Kosunen, T.U.; Zahringer, U. Chemical Characterization of Campylobacter jejuni Lipopolysaccharides Containing N-Acetylneuraminic Acid and 2,3-Diamino-2,3-Dideoxy-D-Glucose. J. Bacteriol. 1991, 173, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Edwards, N.J.; Monteiro, M.A.; Faller, G.; Walsh, E.J.; Moran, A.P.; Roberts, I.S.; High, N.J. Lewis X Structures in the O Antigen Side-Chain Promote Adhesion of Helicobacter pylori to the Gastric Epithelium. Mol. Microbiol. 2000, 35, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Chmiela, M.; Miszczyk, E.; Rudnicka, K. Structural Modifications of Helicobacter pylori Lipopolysaccharide: An Idea for How to Live in Peace. World J. Gastroenterol. 2014, 20, 9882–9897. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Wang, J.; Chen, S.; Wang, Z.; Zhao, L.; Wang, X. Colanic Acid Biosynthesis in Escherichia Coli Is Dependent on Lipopolysaccharide Structure and Glucose Availability. Microbiol. Res. 2020, 239, 126527. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Trent, M.S. Fortifying the Barrier: The Impact of Lipid A Remodelling on Bacterial Pathogenesis. Nat. Rev. Microbiol. 2013, 11, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Troudi, A.; Pagès, J.M.; Brunel, J.M. Chemical Highlights Supporting the Role of Lipid A in Efficient Biological Adaptation of Gram-Negative Bacteria to External Stresses. J. Med. Chem. 2021, 64, 1816–1834. [Google Scholar] [CrossRef] [PubMed]
- Dalebroux, Z.D.; Matamouros, S.; Whittington, D.; Bishop, R.E.; Miller, S.I. PhoPQ Regulates Acidic Glycerophospholipid Content of the Salmonella Typhimurium Outer Membrane. Proc. Natl. Acad. Sci. USA 2014, 111, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Chalabaev, S.; Chauhan, A.; Novikov, A.; Iyer, P.; Szczesny, M.; Beloin, C.; Caroff, M.; Ghigo, J.M. Biofilms Formed by Gram-Negative Bacteria Undergo Increased Lipid a Palmitoylation, Enhancing In Vivo Survival. MBio 2014, 5, 10-1128. [Google Scholar] [CrossRef]
- Bishop, R.E. The Lipid A Palmitoyltransferase PagP: Molecular Mechanisms and Role in Bacterial Pathogenesis. Mol. Microbiol. 2005, 57, 900–912. [Google Scholar] [CrossRef]
- Janusch, H.; Brecker, L.; Lindner, B.; Alexander, C.; Gronow, S.; Heine, H.; Ulmer, A.J.; Rietschel, E.T.; Zähringer, U. Structural and Biological Characterization of Highly Purified Hepta-Acyl Lipid A Present in the Lipopolysaccharide of the Salmonella Enterica Sv. Minnesota Re Deep Rough Mutant Strain R595. J. Endotoxin Res. 2002, 8, 343–356. [Google Scholar] [CrossRef]
- Holst, O.; Müller-Loennies, S.; Lindner, B.; Brade, H. Chemical Structure of the Lipid A of Escherichia Coli J-5. Eur. J. Biochem. 1993, 214, 695–701. [Google Scholar] [CrossRef]
- Novem, V.; Shui, G.; Wang, D.; Bendt, A.K.; Sim, S.H.; Liu, Y.; Thong, T.W.; Sivalingam, S.P.; Ooi, E.E.; Wenk, M.R.; et al. Structural and Biological Diversity of Lipopolysaccharides from Burkholderia Pseudomallei and Burkholderia Thailandensis. Clin. Vaccine Immunol. 2009, 16, 1420–1428. [Google Scholar] [CrossRef]
- Teghanemt, A.; Zhang, D.; Levis, E.N.; Weiss, J.P.; Gioannini, T.L. Molecular Basis of Reduced Potency of Underacylated Endotoxins. J. Immunol. 2005, 175, 4669–4676. [Google Scholar] [CrossRef]
- Novikov, A.; Breton, A.; Caroff, M. Micromethods for Isolation and Structural Characterization of Lipid A, and Polysaccharide Regions of Bacterial Lipopolysaccharides. In Microbial Toxins: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017; pp. 167–186. [Google Scholar]
- Fujita, Y.; Nabetani, T. Iron Sulfate Inhibits Limulus Activity by Induction of Structural and Qualitative Changes in Lipid A. J. Appl. Microbiol. 2014, 116, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Baeva, L.F.; Sarkar Das, S.; Hitchins, V.M. Bacterial Endotoxin Detection in Hyaluronic Acid-Based Medical Devices. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2017, 105, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Nagase, T.; Matsumoto, T.; Suzuki, S.; Suzuki, M. Gelatin of Limulus Amoebocyte Lysate by Simple Polysaccharides. Microbiol. Immunol. 1982, 26, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Reich, J.; Nagaoka, I. Outstanding Contributions of LAL Technology to Pharmaceutical and Medical Science: Review of Methods, Progress, Challenges, and Future Perspectives in Early Detection and Management of Bacterial Infections and Invasive Fungal Diseases. Biomedicines 2021, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Harm, S.; Schildböck, C.; Cont, D.; Weber, V. Heparin Enables the Reliable Detection of Endotoxin in Human Serum Samples Using the Limulus Amebocyte Lysate Assay. Sci. Rep. 2024, 14, 2410. [Google Scholar] [CrossRef] [PubMed]
- Reich, J.; Lang, P.; Grallert, H.; Motschmann, H. Masking of Endotoxin in Surfactant Samples: Effects on Limulus-Based Detection Systems. Biologicals 2016, 44, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Gorman, A.; Golovanov, A.P. Lipopolysaccharide Structure and the Phenomenon of Low Endotoxin Recovery. Eur. J. Pharm. Biopharm. 2022, 180, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T. Pyrogen Testing Revisited on Occasion of the 25th Anniversary of the Whole Blood Monocyte Activation Test. Altex 2021, 38, 3–19. [Google Scholar] [CrossRef]
- Rietschel, E.T.; Gotter, H.; Lüderitz, O.; Westphal, O. Nature and Linkages of the Fatty Acids Present in the Lipid-A Component of Salmonella Lipopolysaccharides. Eur. J. Biochem. 1972, 28, 166–173. [Google Scholar] [CrossRef]
- Seid, R.C.; Schneider, H.; Bondarew, S.; Boykins, R.A. Quantitation of L-Glycero-d-Manno-Heptose and 3-Deoxy-d-Manno-Octulosonic Acid in Rough Core Lipopolysaccharides by Partition Chromatography. Anal. Biochem. 1982, 124, 320–326. [Google Scholar] [CrossRef]
- De Santana-Filho, A.P.; Noleto, G.R.; Gorin, P.A.J.; De Souza, L.M.; Iacomini, M.; Sassaki, G.L. GC-MS Detection and Quantification of Lipopolysaccharides in Polysaccharides through 3-O-Acetyl Fatty Acid Methyl Esters. Carbohydr. Polym. 2012, 87, 2730–2734. [Google Scholar] [CrossRef]
- Sonesson, A.; Bryn, K.; Jantzen, E.; Larsson, L. Gas Chromatographic Determination of (Phosphorylated) 2-Keto-3-Deoxyoctonic Acid, Heptoses and Glucosamine in Bacterial Lipopolysaccharides after Treatment with Hydrofluoric Acid, Methanolysis and Trifluoroacetylation. J. Chromatogr. B Biomed. Sci. Appl. 1989, 487, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pais de Barros, J.-P.; Gautier, T.; Sali, W.; Adrie, C.; Choubley, H.; Charron, E.; Lalande, C.; Le Guern, N.; Deckert, V.; Monchi, M.; et al. Quantitative Lipopolysaccharide Analysis Using HPLC/MS/MS and Its Combination with the Limulus Amebocyte Lysate Assay. J. Lipid Res. 2015, 56, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Caroff, M.; Lebbar, S.; Szabó, L. Do Endotoxins Devoid of 3-Deoxy-D-Manno-2-Octulosonic Acid Exist? Biochem. Biophys. Res. Commun. 1987, 143, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Jann, B.; Hofmann, P.; Jann, K. Structure of the 3-Deoxy-D-Manno-Octulosonic Acid-(KDO)-Containing Capsular Polysaccharide (K14 Antigen) from Escherichia Coli 06:K14:H31. Carbohydr. Res. 1983, 120, 131–141. [Google Scholar] [CrossRef]
- Huddleston, J.P.; Raushel, F.M. Biosynthesis of GDP-d-Glycero-α-d-Manno-Heptose for the Capsular Polysaccharide of Campylobacter jejuni. Biochemistry 2019, 58, 3893–3902. [Google Scholar] [CrossRef]
- Hu, G.; Varisco, D.J.; Das, S.; Middaugh, C.R.; Gardner, F.; Ernst, R.K.; Picking, W.L.; Picking, W.D. Physicochemical Characterization of Biological and Synthetic Forms of Two Lipid A-Based TLR4 Agonists. Heliyon 2023, 9, e18119. [Google Scholar] [CrossRef] [PubMed]
- Chettab, K.; Fitzsimmons, C.; Novikov, A.; Denis, M.; Phelip, C.; Mathé, D.; Choffour, P.A.; Beaumel, S.; Fourmaux, E.; Norca, P.; et al. A Systemically Administered Detoxified TLR4 Agonist Displays Potent Antitumor Activity and an Acceptable Tolerance Profile in Preclinical Models. Front. Immunol. 2023, 14, 1066402. [Google Scholar] [CrossRef] [PubMed]
- Caroff, M.; Bundle, D.R.; Perry, M.B. Structure of the O-Chain of the Phenol-Phase Soluble Cellular Lipopolysaccharide of Yersinia Enterocolitica Serotype O:9. Eur. J. Biochem. 1984, 139, 195–200. [Google Scholar] [CrossRef]
- Caroff, M. Novel Method for Isolating Endotoxins. WO2004062690A1, 29 July 2004. [Google Scholar]
- Volz, T.; Nega, M.; Buschmann, J.; Kaesler, S.; Guenova, E.; Peschel, A.; Röcken, M.; Götz, F.; Biedermann, T. Natural Staphylococcus Aureus-Derived Peptidoglycan Fragments Activate NOD2 and Act as Potent Costimulators of the Innate Immune System Exclusively in the Presence of TLR Signals. FASEB J. 2010, 24, 4089–4102. [Google Scholar] [CrossRef]
- El Hamidi, A.; Tirsoaga, A.; Novikov, A.; Hussein, A.; Caroff, M. Microextraction of Bacterial Lipid A: Easy and Rapid Method for Mass Spectrometric Characterization. J. Lipid Res. 2005, 46, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Karibian, D.; Deprun, C.; Caroff, M. Comparison of Lipids A of Several Salmonella and Escherichia Strains by 252Cf Plasma Desorption Mass Spectrometry. J. Bacteriol. 1993, 175, 2988–2993. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dardelle, F.; Phelip, C.; Darabi, M.; Kondakova, T.; Warnet, X.; Combret, E.; Juranville, E.; Novikov, A.; Kerzerho, J.; Caroff, M. Diversity, Complexity, and Specificity of Bacterial Lipopolysaccharide (LPS) Structures Impacting Their Detection and Quantification. Int. J. Mol. Sci. 2024, 25, 3927. https://doi.org/10.3390/ijms25073927
Dardelle F, Phelip C, Darabi M, Kondakova T, Warnet X, Combret E, Juranville E, Novikov A, Kerzerho J, Caroff M. Diversity, Complexity, and Specificity of Bacterial Lipopolysaccharide (LPS) Structures Impacting Their Detection and Quantification. International Journal of Molecular Sciences. 2024; 25(7):3927. https://doi.org/10.3390/ijms25073927
Chicago/Turabian StyleDardelle, Flavien, Capucine Phelip, Maryam Darabi, Tatiana Kondakova, Xavier Warnet, Edyta Combret, Eugenie Juranville, Alexey Novikov, Jerome Kerzerho, and Martine Caroff. 2024. "Diversity, Complexity, and Specificity of Bacterial Lipopolysaccharide (LPS) Structures Impacting Their Detection and Quantification" International Journal of Molecular Sciences 25, no. 7: 3927. https://doi.org/10.3390/ijms25073927
APA StyleDardelle, F., Phelip, C., Darabi, M., Kondakova, T., Warnet, X., Combret, E., Juranville, E., Novikov, A., Kerzerho, J., & Caroff, M. (2024). Diversity, Complexity, and Specificity of Bacterial Lipopolysaccharide (LPS) Structures Impacting Their Detection and Quantification. International Journal of Molecular Sciences, 25(7), 3927. https://doi.org/10.3390/ijms25073927