The First Record of Marenzelleria neglecta and the Spread of Laonome xeprovala in the Danube Delta–Black Sea Ecosystem
Abstract
:1. Introduction
2. Materials and Methods
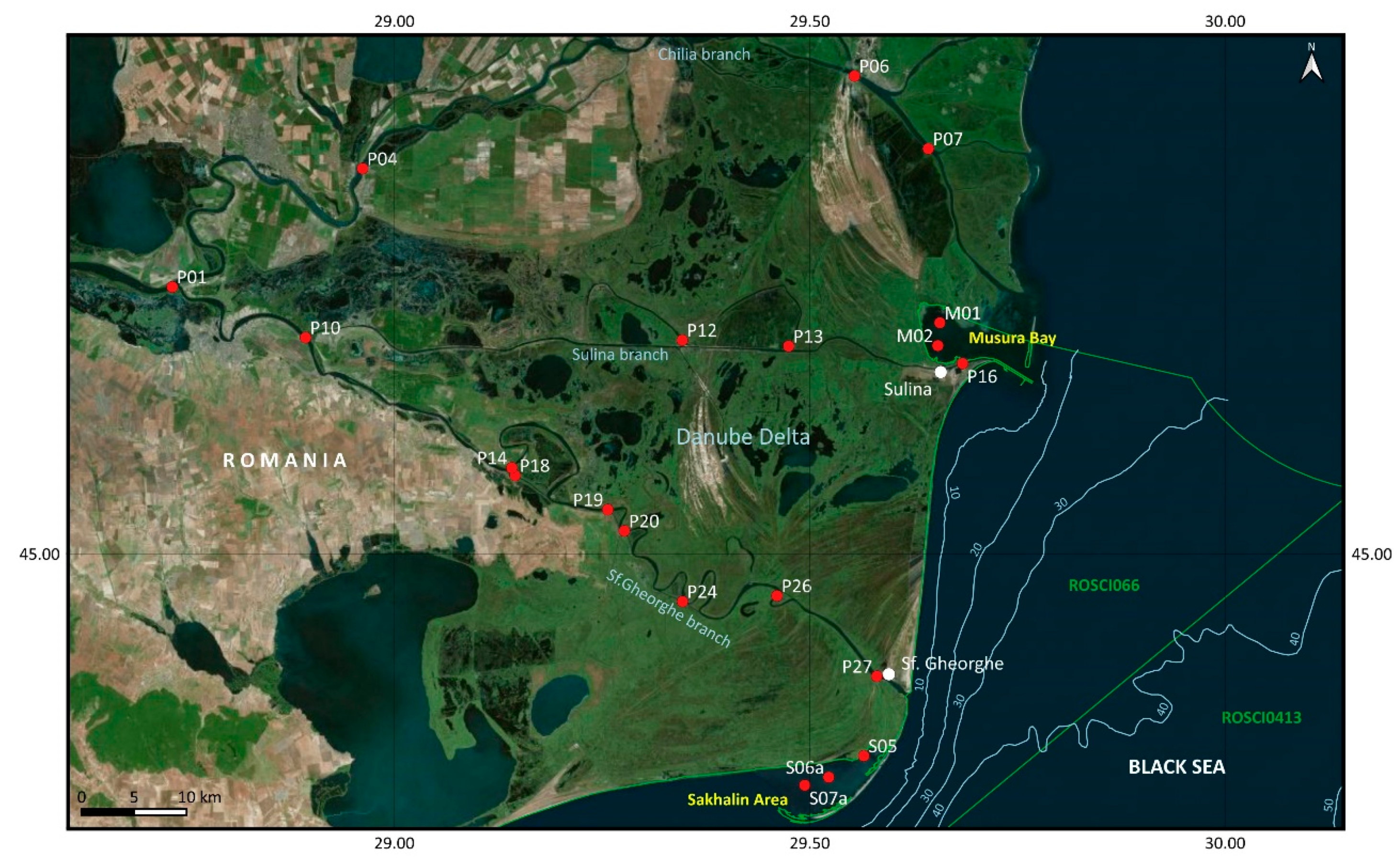
2.1. Area Description
2.2. Data Collection and Analysis
2.3. Morphological Examination
3. Results
3.1. Benthic Communities
3.2. Marenzelleria neglecta
3.3. Laonome xeprovala
4. Discussion
4.1. Marenzelleria neglecta Invasion
4.2. Laonome xeprovala Invasion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reise, K.; Olenin, S.; Thieltges, D.W. Are aliens threatening European aquatic coastal ecosystems? Helgol. Mar. Res. 2006, 60, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Grosholz, E. Ecological and evolutionary consequences of coastal invasions. Trends Ecol. 2002, 17, 22–27. [Google Scholar] [CrossRef]
- Williams, S.L.; Grosholz, E.D. The invasive species challenge in estuarine and coastal environments: Marrying management and science. Estuaries Coasts 2008, 31, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 2008, 6, 458–492. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; Agrosa, C.D.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A Global Map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [Green Version]
- Galil, B.S.; Marchini, A.; Occhipinti-Ambrogi, A.; Minchin, D.; Narščius, A.; Ojaveer, H.; Olenin, S. International arrivals: Widespread bioinvasions in European Seas. Ethol. Ecol. Evol. 2014, 26, 152–171. [Google Scholar] [CrossRef] [Green Version]
- Ojaveer, H.; Galil, B.S.; Gollasch, S.; Marchini, A.; Minchin, D.; Occhipinti-Ambrogi, A.; Olenin, S. Indentifying top issues of marine invasive alien species in Europe. Manag. Biol. Invasions 2014, 5, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Carlton, J.T.; Ruiz, G.M. Vector science and integrated vector management in bioinvasion ecology: Conceptual frameworks. In Invasive Alien Species: A New Synthesis; Mooney, H.A., Mack, R.N., McNeely, J.A., Schei, P.J., Waage, J.K., Neville, L.E., Eds.; Island Press: Washington, DC, USA, 2005; pp. 195–212. [Google Scholar]
- Teaca, A.; Begun, T.; Muresan, M. Annelid invaders in the Black Sea region: The distribution of Streblospio gynobranchiata and first occurrence of Laonome xeprovala. Glob. Ecol. Conserv. 2021, 32, e01920. [Google Scholar] [CrossRef]
- Regulation (EU). No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species. Off. J. Eur. Union 2014, 317, 35–55. [Google Scholar]
- DIRECTIVE, Strategy Framework. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Union 2008, 164, 19–40. [Google Scholar]
- EU Biodiversity Strategy to 2020–towards Implementation. 2014. Available online: http://ec.europa.eu/environment/nature/biodiversity/comm2006/2020.htm (accessed on 3 March 2022).
- Cardoso, A.C.; Free, G. Incorporating invasive alien species into ecological assessment in the context of the water framework directive. Aquat. Invasions 2008, 3, 361–366. [Google Scholar] [CrossRef]
- Carvalho, L.; Mackay, E.B.; Cardoso, A.C.; Baattrup-Pedersen, A.; Birk, S.; Blackstock, K.L.; Borics, G.; Borja, A.; Feld, C.K.; Ferreira, M.T.; et al. Protecting and restoring Europe’s waters: An analysis of the future development needs of the Water Framework Directive. Sci. Total Environ. 2019, 658, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Boon, P.J.; Clarke, S.A.; Copp, G.H. Alien species and the EU water framework directive: A comparative assessment of European approaches. Biol. Invasions 2020, 22, 1497–1512. [Google Scholar] [CrossRef] [Green Version]
- Magliozzi, C.; Tsiamis, K.; Vigiak, O.; Deriu, I.; Gervasini, E.; Cardoso, A.C. Assessing invasive alien species in European catchments: Distribution and impacts. Sci. Total Environ. 2020, 732, 138677. [Google Scholar] [CrossRef]
- Zaitsev, Y.; Öztürk, B. (Eds.) Exotic Species in the Aegean, Marmara, Black, Azov and Caspian Seas; Turkish Marine Research Foundation: Istanbul, Turkey, 2001; p. 267. [Google Scholar]
- Popa, L.O.; Popa, O.P.; Iorgu, E.I.; Krapal, A.M.; Pârvulescu, L.; Surugiu, V.; Petrescu, I.; Petrescu, A.M.; Ștefan, A.; Motoc, R.M.; et al. Raport Privind Lista Preliminară a Speciilor de Nevertebrate de apă Dulce Algogene din România în Format Tabelar. Raport Întocmit în Cadrul Proiectului POIM2014+120008-Managementul Adecvat al Speciilor Invazive din România, în Conformitate cu Regulamentul UE 1143/2014 Referitor la Prevenirea și Gestionarea Introducerii și Răspândirii Speciilor Alogene Invazive; Ministerul Mediului, Apelor şi Pădurilor & Universitatea din Bucureşti: Bucureşti, Romania, 2020; Available online: https://invazive.ccmesi.ro/wp-content/uploads/2020/10/POIM120008_Subact.-1.4.2-Lista-nevertebrate.pdf (accessed on 20 March 2022).
- Appeltans, W.; Ahyong, S.T.; Anderson, G.; Angel, M.V.; Artois, T.; Bailly, N.; Bamber, R.; Barber, A.; Bartsch, I.; Berta, A.; et al. The magnitude of global marine species diversity. Curr. Biol. 2012, 22, 2189–2202. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, P. Biodiversity and functioning of polychaetes in benthic sediments. Biodivers. Conserv. 1998, 7, 1133–1145. [Google Scholar] [CrossRef]
- Surugiu, V. Inventory of inshore polychaetes from the Romanian coast (Black Sea). Mediterr. Mar. Sci. 2005, 6, 51–73. [Google Scholar] [CrossRef] [Green Version]
- Begun, T.; Teaca, A.; Muresan, M.; Vasiliu, D.; Secrieru, D.; Pavel, B. Environmental assessment of two MPAs from the Romanian Black Sea Coast. J. Environ. Prot. Ecol. 2018, 19, 573–582. Available online: https://docs.google.com/a/jepe-journal.info/viewer?a=v&pid=sites&srcid=amVwZS1qb3VybmFsLmluZm98amVwZS1qb3VybmFsfGd4OmM2ODFlZGM4NWU2M2Q1NQ (accessed on 1 March 2022).
- Begun, T.; Teaca, A.; Muresan, M.; Quijon, P.A.; Menabit, S.; Surugiu, V. Habitat and macrozoobenthic diversity in marine protected areas of the Southern Romanian Black Sea Coast. Front. Mar. Sci. 2022, 9, 845507. [Google Scholar] [CrossRef]
- Teaca, A.; Muresan, M.; Begun, T.; Popa, A.; Ion, G. Marine benthic habitats within a physical disturbed site from the Romanian Coast of the Black Sea. J. Environ. Prot. Ecol. 2019, 20, 723–732. Available online: https://docs.google.com/a/jepe-journal.info/viewer?a=v&pid=sites&srcid=amVwZS1qb3VybmFsLmluZm98amVwZS1qb3VybmFsfGd4OjU4MTgzYWM2NGRhMWJhMzQ (accessed on 20 February 2022).
- Teaca, A.; Muresan, M.; Menabit, S.; Bucse, A.; Begun, T. Assessment of Romanian circalittoral soft bottom benthic habitats under Danube river influence. Reg. Stud. Mar. Sci. 2020, 40, 101523. [Google Scholar] [CrossRef]
- Muresan, M.; Teaca, A.; Popa, A.; Begun, T. Free-living marine nematodes community structural changes within a post-dredging site at the Romanian shelf. J. Environ. Prot. Ecol. 2019, 20, 753–760. [Google Scholar]
- Giangrande, A.; Licciano, M.; Musco, L. Polychaetes as environmental indicators revisited. Mar. Pollut. Bull. 2005, 50, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Pocklington, P.; Wells, P.G. Polychaetes: Key taxa for marine environmental quality monitoring. Mar. Pollut. Bull. 1992, 24, 593–598. [Google Scholar] [CrossRef]
- López, E.; Richter, A. Non-indigenous species (NIS) of polychaetes (Annelida: Polychaeta) from the Atlantic and Mediterranean coasts of the Iberian Peninsula: An annotated checklist. Helgol. Mar. Res. 2017, 71, 19. [Google Scholar] [CrossRef]
- Essink, K.; Dekker, R. General patterns in invasion ecology tested in the Dutch Wadden Sea: The case of a brackish-marine polychaetous worm. Biol. Invasions 2002, 4, 359–368. [Google Scholar] [CrossRef]
- Beesley, P.L.; Ross, G.J.B.; Glasby, C.J. Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula. In Polychaetes and Allies: The Southern Synthesis. Fauna of Australia; Australian Biological Resources Study/CSIRO Publishing: Melbourne, Australia, 2000; Volume 4A, p. 465. [Google Scholar]
- Çinar, M. Alien polychaete species worldwide: Current status and their impacts. J. Mar. Biol. Assoc. United Kingd. 2013, 93, 1257–1278. [Google Scholar] [CrossRef]
- Popescu, I. Directorate General for Internal Policies, Policy Department B: Structural and Cohesion Policies, Fisheries in the Black Sea Note, European Parliament. 2010. Available online: http://www.europarl.europa.eu/RegData/etudes/note/join/2010/438622/IPOL-PECH_NT(2010)438622_EN.pdf (accessed on 2 February 2022).
- Vignati, D.A.L.; Secrieru, D.; Bogatova, Y.I.; Dominik, J.; Céréghino, R.; Berlinsky, N.A.; Oaie, G.; Szobotka, S.; Stanica, A. Trace element contamination in the arms of the Danube Delta (Romania/Ukraine): Current state of knowledge and future needs. J. Environ. Manag. 2013, 125, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Panin, N.; Overmars, W. The Danube Delta evolution during the Holocene: Reconstruction attempt using geomorphological and geological data, and some of the existing cartographic documents. Geo-Eco-Marina 2012, 18, 75–104. [Google Scholar]
- Stanica, A.; Dan, S.; Ungureanu, V.G. Coastal changes at the Sulina mouth of the Danube River as a result of human activities. Mar. Pollut. Bull. 2007, 55, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Duţu, F.; Panin, N.; Ion, G.; Tiron Duţu, L. Multibeam bathymetric investigations of the morphology and associated bedforms, Sulina Channel, Danube Delta. Geosciences 2018, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Tiron Duţu, L.; Duțu, F.; Secrieru, D.; Opreanu, G. Sediments grain size and geo-chemical interpretation of three successive cutoff meanders of the Danube Delta, Romania. Geochemistry 2019, 79, 399–407. [Google Scholar] [CrossRef]
- Sikorski, A.V.; Bick, A. Revision of Marenzelleria Mesnil, 1896 (Spionidae, Polychaeta). Sarsia 2004, 89, 253–275. [Google Scholar] [CrossRef]
- Bick, A. A new Spionidae (Polychaeta) from North Carolina, and a redescription of Marenzelleria wireni Augener, 1913, from Spitsbergen, with a key for all species of Marenzelleria. Helgol. Mar. Res. 2005, 59, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Bick, A.; Bastrop, R.; Kotta, J.; Meißner, K.; Meyer, M.; Syomin, V. Description of a new species of Sabellidae (Polychaeta, Annelida) from fresh and brackish waters in Europe, with some remarks on the branchial crown of Laonome. Zootaxa 2018, 4483, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Schlitzer, R. Ocean Data View 2018. Available online: https://odv.awi.de (accessed on 15 March 2022).
- WoRMS Editorial Board. World Register of Marine Species. 2021. Available online: http://www.marinespecies.orgatVLIZ (accessed on 15 January 2022). [CrossRef]
- Begun, T.; Teaca, A.; Muresan, M.; Pavel, A.B. Current state of the mollusc populations in the Razim-Sinoe Lagoon System. Sci. Papers. Ser. D. Anim. Sci. 2020, 63, 553–561. Available online: http://animalsciencejournal.usamv.ro/pdf/2020/issue_1/Art81.pdf (accessed on 10 February 2022).
- Blank, M.; Bastrop, R. Phylogeny of the mud worm genus Marenzelleria (Polychaeta, Spionidae) inferred from mitochondrial DNA sequences. Zool. Scr. 2009, 38, 313–321. [Google Scholar] [CrossRef]
- George, J.D. Reproduction and early development of the spionid polychaete, Scolecolepides viridis (Verrill). Biol. Bull. 1966, 130, 76–93. [Google Scholar] [CrossRef]
- Bick, A.; Burckhardt, R. Erstnachweis von Marenzelleria viridis (Polychaeta, Spionidae) für den Ostseeraum, mit einem Bestimmungsschlüssel der Spioniden der Ostsee. Zoosystematics Evol. 1989, 65, 237–247. [Google Scholar] [CrossRef]
- Norkko, A.; Bonsdorff, E.; Bostrom, C. Observations of the polychaete Marenzelleria viridis (Verrill) on a shallow sandy bottom on the south coast of Finland. Memo. Soc. Pro Fauna Flora Fenn. 1993, 69, 112–113. [Google Scholar]
- Zettler, M.L.; Daunys, D.; Kotta, J.; Bick, A. History and success of an invasion into the Baltic Sea: The polychaete Marenzelleria cf. viridis development and strategies. In Aquatic Invasive Species of Europe—Distribution, Impacts and Management; Leppäkoski, E., Gollasch, S., Olenin, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 66–75. [Google Scholar]
- Ezhova, E.; Zmudzinski, L.; Maciejewska, K. Long Term Trends in the Macrozoobenthos of the Vistula Lagoon, Southerstern Baltic Sea. Species Composition and Biomass Distribution. Bull. Sea Fish. Inst. 2005, 1, 55–73. [Google Scholar]
- Kotta, J.; Kotta, I.; Simm, M.; Lankov, A.; Lauringson, V.; Pollumae, A.; Ojaveer, H. Ecological consequences of biological invasions: Three invertebrate case studies in the north-eastern Baltic Sea. Helgol. Mar. Res. 2006, 60, 106–112. [Google Scholar] [CrossRef]
- Maximov, A.A. Changes in bottom communities of the eastern Gulf of Finland after introduction of the polychaete Marenzelleria neglecta. Russ. J. Biol. Invasions 2010, 1, 11–16. [Google Scholar] [CrossRef]
- Maximov, A.A. Large-scale invasion of Marenzelleria spp. (Polychaeta; Spionidae) in the eastern Gulf of Finland, Baltic Sea. Russ. J. Biol. Invasions 2011, 2, 11–19. [Google Scholar] [CrossRef]
- Maximov, A.A. The long-term dynamics and current distribution of macrozoobenthos communities in the Eastern Gulf of Finland, Baltic Sea. Russ. J. Mar. Biol./Biol. Morya 2015, 41, 300–310. [Google Scholar] [CrossRef]
- Maximov, A.A.; Litvinchuk, L.F.; Eremina, T.R.; Lange, E.K.; Maximova, O.B. Regime shift in the ecosystem of the eastern Gulf of Finland caused by the invasion of the polychaete Marenzelleria arctia. Oceanology 2014, 54, 46–53. [Google Scholar] [CrossRef]
- Maximov, A.; Bonsdorff, E.; Eremina, T.; Kauppi, L.; Norkko, A.; Norkko, J. Context-dependent consequences of Marenzelleria spp. (Spionidae: Polychaeta) invasion for nutrient cycling in the Northern Baltic Sea. Oceanologia 2015, 57, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Syomin, V.L.; Sikorski, A.V.; Kovalenko, E.P.; Bulysheva, N.I. Penetration of genus Marenzelleria (Polychaeta: Spionidae) into the Don River estuary and the Taganrog Bay. Russ. J. Biol. Invasions 2016, 1, 109–120. [Google Scholar]
- Syomin, V.; Sikorski, A.; Bastrop, R.; Köhler, N.; Stradomsky, B.; Fomina, E.; Matishov, D. The invasion of the genus Marenzelleria (Polychaeta: Spionidae) into the Don River mouth and the Taganrog Bay: Morphological and genetic study. J. Mar. Biol. Assoc. UK 2017, 97, 975–984. [Google Scholar] [CrossRef]
- Boltachova, N.A.; Lisitskaya, E.V. Polychaetes of the Southwest of the Sea of Azov. Ekosistemy 2019, 19, 133–141. [Google Scholar]
- Boltachova, N.A.; Lisitskaya, E.V.; Podzorova, D.V. Distribution of alien polychaetes in biotopes of the northern part of the Black Sea. Russ. J. Biol. Invasions 2021, 12, 11–26. [Google Scholar] [CrossRef]
- Mikhailova, A.V.; Popova, E.V.; Shipulin, S.V.; Maximov, A.A.; Plotnikov, I.S.; Aladin, N.V. On the invasion of the genus Marenzelleria (Polychaeta, Spionidae) representatives into the Caspian Sea Basin. Russ. J. Biol. Invasions 2021, 12, 45–49. [Google Scholar] [CrossRef]
- Pavel, A.B.; Menabit, S.; Manzala, D.; Lupascu, N.; Pop, I.C.; Catianis, I. Benthic community structure characterization of the bed-sediment layer composition in the Musura Bay and Sakhalin area. Geo-Eco-Marina 2019, 25, 25–39. [Google Scholar]
- Pavel, A.B.; Dutu, L.; Patriche, N. The benthic fauna associations from the meanders area of Danube–Saint George Branch, in the period 2016–2017. Geo-Eco-Marina 2017, 23, 233–243. [Google Scholar]
- Boltacheva, N.A.; Lisitskaya, E.V.; Frolenko, L.N.; Kovalev, E.A.; Barabashin, T.O. The finding of Polychaete Laonome calida Capa, 2007 (Annelida: Sabellidae) in the Southeast Sea of Azov. Russ. J. Biol. Invasions 2017, 8, 303–306. [Google Scholar] [CrossRef]
- Capa, M.; Giangrande, A.; Nogueira, J.M.M.; Tovar-Hernández, M.A. Sabellidae Latreille, 1825. In Annelida; Westheide, G., Purschke, G., Eds.; Handbook of Zoology Online, 7-6; Walter de Gruyter: Berlin, Germany, 2014; p. 62. Available online: http://www.degruyter.com/view/Zoology/bp_02914 (accessed on 10 February 2022).
- Kotta, J.; Kotta, I.; Bick, A.; Bastrop, R.; Väinölä, R. Modelling habitat range and seasonality of a new, nonindigenous polychaete Laonome sp. (Sabellida, Sabellidae) in Pärnu Bay, the north-eastern Baltic Sea. Aquat. Invasions 2015, 10, 275–285. [Google Scholar] [CrossRef]
- Kocheshkova, O.V. Polychaetes of the Vistula Bay (Baltic Sea): Species composition and adaptation to the conditions of a eutrophic brackish lagoon. In Extended Abstract of Cand. Sci. (Biol.) Dissertation; Murmansk, Russia, 2017. [Google Scholar]
- Kocheshkova, O.V.; Ezhova, E.E. On alien polychaete species of the Russian part of South-Eastern Baltic. Mar. Biol. J. 2018, 3, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Tamulyonis, A.Y.; Gagaev, S.Y.; Stratanenko, E.A.; Zuyev, Y.A.; Potin, V.V. Invasion of the Polychaeta Laonome xeprovala Bick & Bastrop, 2018 (Sabellidae, Polychaeta) into the Estuary of the Luga and Khabolovka Rivers (Luga Bay, Gulf of Finland). Russ. J. Biol. Invasions 2020, 11, 148–154. [Google Scholar] [CrossRef]
- Rouse, G.; Fitzhugh, K. Broadcasting fables: Is external fertilization really primitive? Sex, size, and larvae in sabellid polychaetes. Zool. Scr. 1994, 23, 271–312. [Google Scholar] [CrossRef]


| No. Crt. | L [mm] | W [mm] | Chae | VHH | DHH | Br | NO | Br- VHH | BR- DHH | DHH- VHH |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8.07 | 0.69 | 41 * | 28 | 33 | 30 | m3 | 2 | −3 | 5 |
| 2 | 15.56 | 0.70 | 67 * | 28 | 33 | 30 | b3 | 2 | −3 | 5 |
| 3 | 8.01 | 0.72 | 38 * | 30 | 38 | 32 | b3 | 2 | −6 | 8 |
| 4 | 5.96 | 0.72 | 32 * | 29 | 31 | b2 | 2 | |||
| 5 | 12.97 | 0.73 | 61 * | 27 | 33 | 27 | b3 | 0 | −6 | 6 |
| 6 | 8.83 | 0.73 | 40 * | 27 | 36 | 34 | b3 | 7 | −2 | 9 |
| 7 | 4.81 | 0.74 | 24 * | m3 | ||||||
| 8 | 21.85 | 0.75 | 99 * | 29 | 40 | 31 | m3 | 2 | −9 | 11 |
| 9 | 27.75 | 0.75 | 133 | 34 | 42 | 38 | m3 | 4 | −4 | 8 |
| 10 | 7.94 | 0.75 | 36 * | 28 | 32 | m3 | 4 | |||
| 11 | 6.48 | 0.75 | 28 * | m3 | ||||||
| 12 | 6.72 | 0.76 | 35 * | 33 | 35 | b3 | 2 | |||
| 13 | 9.23 | 0.80 | 42 * | 31 | 37 | 34 | m3 | 3 | −3 | 6 |
| 14 | 6.60 | 0.80 | 29 * | b3 | ||||||
| 15 | 8.7 | 0.8 | 38 * | 29 | 31 | m3 | 2 | |||
| 16 | 6.86 | 0.81 | 29 * | b3 | ||||||
| 17 | 5.37 | 0.81 | 30 * | 28 | m3 | |||||
| 18 | 9.47 | 0.82 | 44 * | 30 | 36 | 32 | m3 | 2 | −4 | 6 |
| 19 | 8.21 | 0.82 | 37 * | 33 | 36 | m3 | 3 | |||
| 20 | 11.19 | 0.83 | 49 * | 30 | 36 | 35 | e3 | 5 | −1 | 6 |
| 21 | 14.72 | 0.85 | 57 * | 32 | 41 | 37 | m3 | 5 | −4 | 9 |
| 22 | 8.72 | 0.85 | 36 * | 31 | 34 | m3 | 3 | |||
| 23 | 22.83 | 0.86 | 97 * | 31 | 35 | 32 | m3 | 1 | −3 | 4 |
| 24 | 8.30 | 0.86 | 39 * | 33 | 34 | m3 | 1 | |||
| 25 | 14.93 | 0.87 | 62 * | 32 | 40 | 34 | m3 | 2 | −6 | 8 |
| 26 | 9.90 | 0.87 | 38 * | 31 | 33 | m3 | 2 | |||
| 27 | 10.73 | 0.89 | 47 * | 32 | 38 | 33 | e3 | 1 | −5 | 6 |
| 28 | 13.09 | 0.92 | 56 * | 32 | 39 | 32 | m3 | 0 | −7 | 7 |
| 29 | 9.48 | 0.93 | 41 * | 29 | 38 | 35 | m3 | 6 | −3 | 9 |
| 30 | 9.16 | 0.94 | 40 * | 29 | 38 | 30 | m3 | 1 | −8 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teacă, A.; Begun, T.; Menabit, S.; Mureșan, M. The First Record of Marenzelleria neglecta and the Spread of Laonome xeprovala in the Danube Delta–Black Sea Ecosystem. Diversity 2022, 14, 423. https://doi.org/10.3390/d14060423
Teacă A, Begun T, Menabit S, Mureșan M. The First Record of Marenzelleria neglecta and the Spread of Laonome xeprovala in the Danube Delta–Black Sea Ecosystem. Diversity. 2022; 14(6):423. https://doi.org/10.3390/d14060423
Chicago/Turabian StyleTeacă, Adrian, Tatiana Begun, Selma Menabit, and Mihaela Mureșan. 2022. "The First Record of Marenzelleria neglecta and the Spread of Laonome xeprovala in the Danube Delta–Black Sea Ecosystem" Diversity 14, no. 6: 423. https://doi.org/10.3390/d14060423
APA StyleTeacă, A., Begun, T., Menabit, S., & Mureșan, M. (2022). The First Record of Marenzelleria neglecta and the Spread of Laonome xeprovala in the Danube Delta–Black Sea Ecosystem. Diversity, 14(6), 423. https://doi.org/10.3390/d14060423






