Plant Diversity and Conservation Role of Three Indigenous Agroforestry Systems of Southeastern Rift-Valley Landscapes, Ethiopia
Abstract
:1. Introduction
2. Materials and Methods
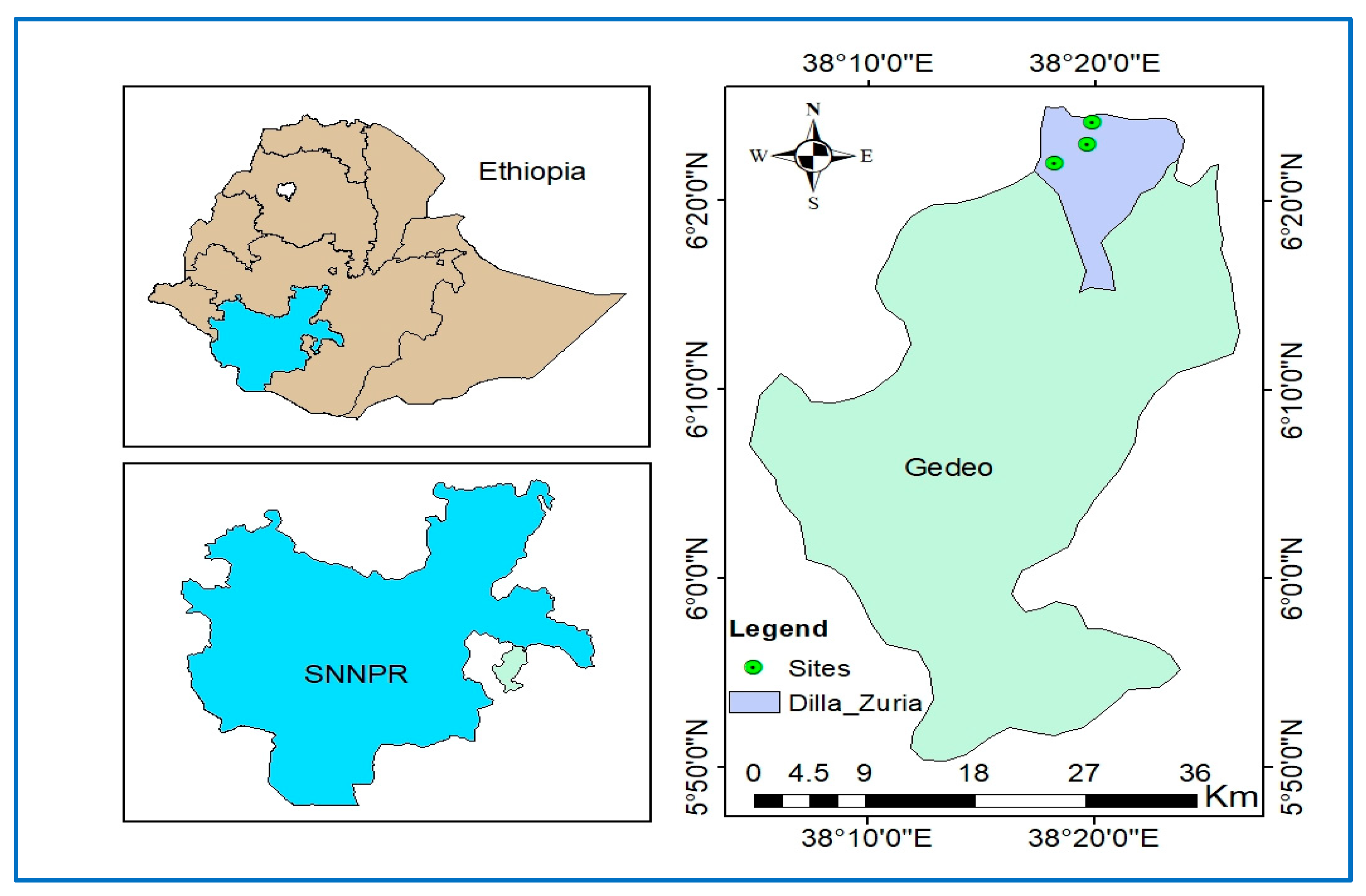
2.1. Study Area and Sites
2.1.1. Indigenous Agroforestry Systems as Focus of the Study
Enset Based Agroforestry
Coffee–Enset Based Agroforestry (C–E Based AF)
Coffee–Fruit Tree –Enset Based Agroforestry (C–Ft–E Based AF)
2.2. Methods
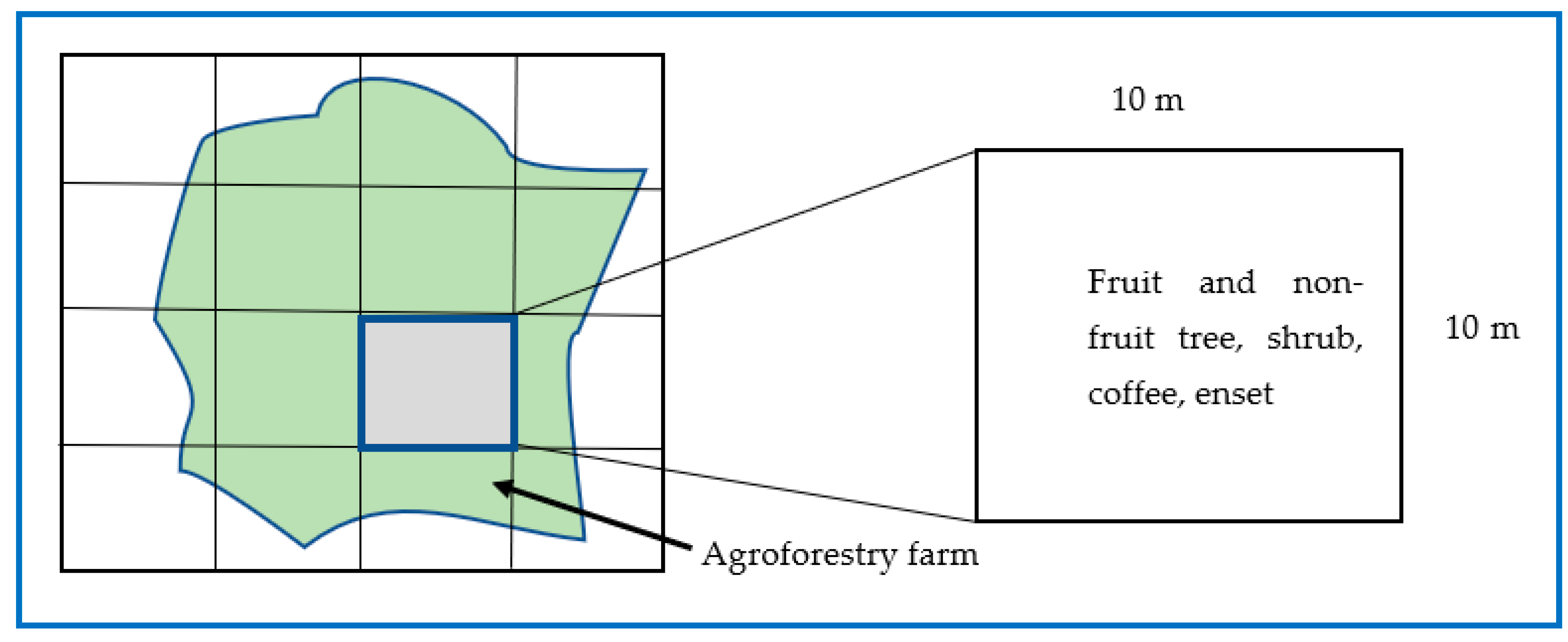
2.2.1. Sampling Design and Data Collection
2.2.2. Plant Species Inventory
2.3. Data Analysis
2.3.1. Stand Characteristics of Plant Species and Diversity Analysis
2.3.2. Analysis of Species Conservation Concern
3. Results and Discussion
3.1. Plant Diversity and Conservation in Indigenous Agroforestry Systems
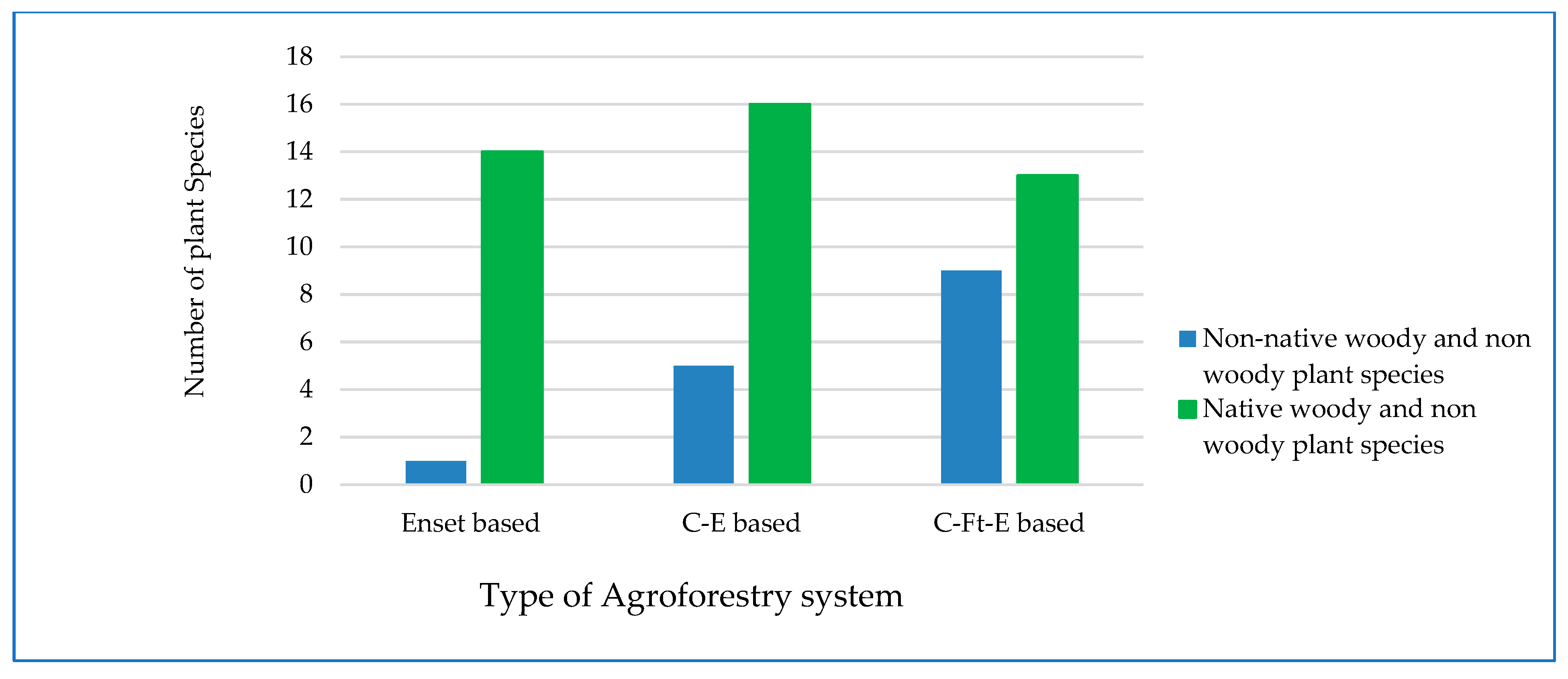
3.1.1. Perennial Plant Species Composition
3.1.2. Plant Species Endemism and Conservation Concern
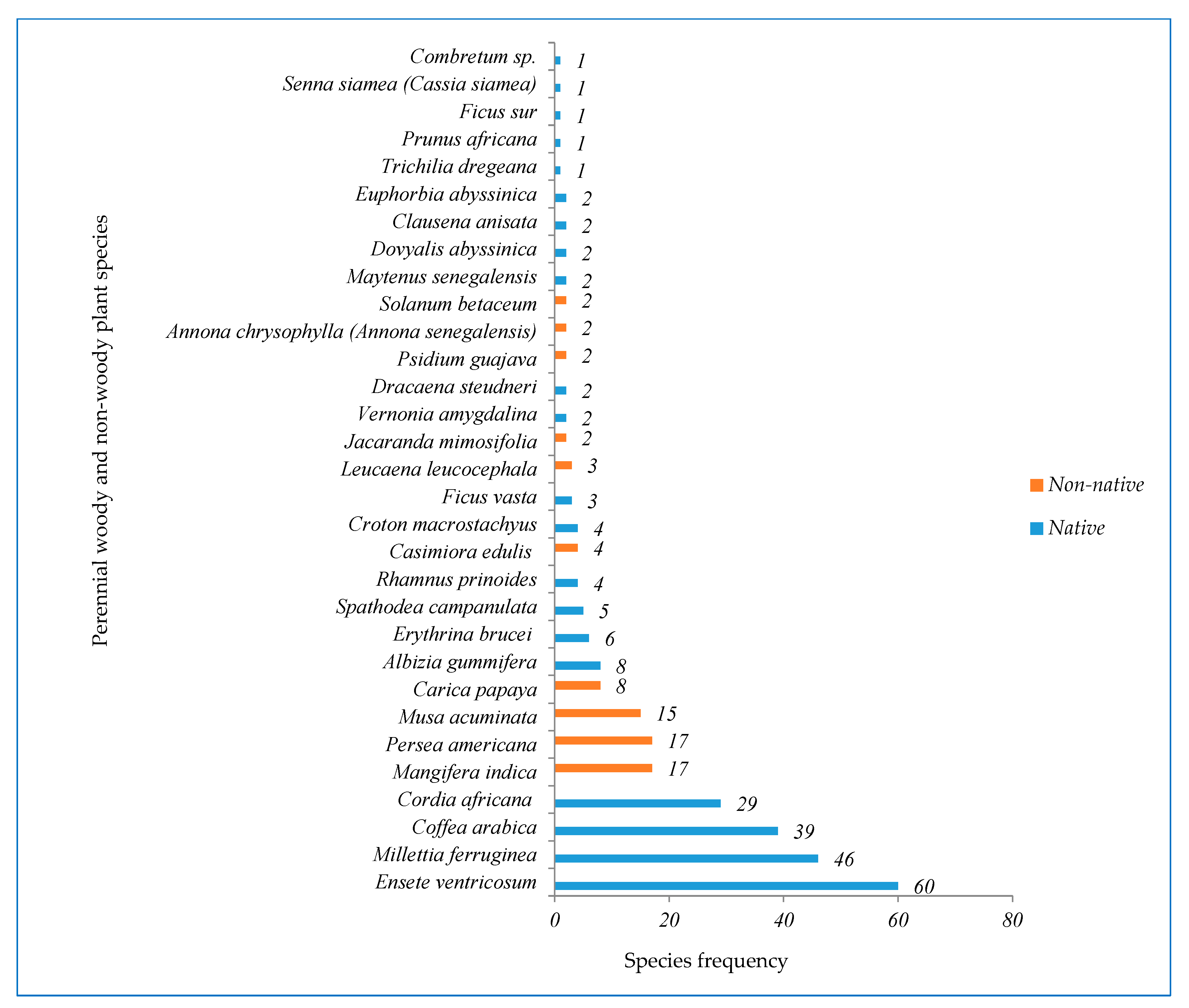
3.1.3. Plant Species Frequency and Important Value Index
3.1.4. Stand Structure, Diversity, and Richness Status of Agroforestry Systems
3.1.5. Relationship of Altitude with Species Richness and Species Abundance
4. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Number | Vernacular Name | Scientific Name | Family | Origin |
|---|---|---|---|---|
| 1 | Gorbe | Albizia gummifera (J.F. Gmel.) C.A.Sm | Fabaceae | Native |
| 2 | Geshita | Annona chrysophylla Bojer | Annonaceae | Non-native |
| 3 | Papaya | Carica papaya L. | Caricaceae | Non-native |
| 4 | Abukere | Casimiroa edulis Lal lave and Lex | Rutaceae | Non-native |
| 5 | Godere | Clausena anisata (Willd.) Benth. | Rutaceae | Native |
| 6 | NI | Combretum sp. | Combretaceae | Native |
| 7 | Wedesa | Cordia africana Lam. | Boraginaceae | Native |
| 8 | Buno | Coffea arabica L. | Rubiaceae | Native |
| 9 | Mokonisa | Croton macrostachyus Hochst. ex Delile | Euphorbiaceae | Native |
| 10 | NI | Dovyalis abyssinica (A.Rich.) Warb. | Salicaceae | Native |
| 11 | Cho’e | Dracaena steudneri Schweinf. Ex Engl. | Dracaenaceae | Native |
| 12 | Ensete | Ensete ventricosum (Welw.) Cheesman | Musaceae | Native |
| 13 | Welale/Gedogna | Erythrina brucei Schweinf. | Leguminosae | Native |
| 14 | Kulkal | Euphorbia abyssinica J.F.Gmel. | Euphorbiaceae | Native |
| 15 | wagela | Ficus sur Forssk. | Moraceae | Native |
| 16 | Kilto | Ficus vasta Forssk. | Moraceae | Native |
| 17 | NI | Jacaranda mimosifolia D.Don | Bignoniaceae | Non-native |
| 18 | Lusina | Leucaena leucocephala (Lam.) de Wit | Mimosoideae | Non-native |
| 19 | Mango | Mangifera indica L. | Anacardiaceae | Non-native |
| 20 | Kobo/gulo | Maytenus senegalensis (Lam.) Exell | Celastraceae | Native |
| 21 | Tatato | Millettia ferruginea (Hochst.) Baker | Leguminosae | Native |
| 22 | Muse | Musa acuminata Colla | Musaceae | Non-native |
| 23 | Avocato | Persea americana Mill. | Lauraceae | Non-native |
| 24 | Gorbe | Prunus africana (Hook.f.) Kalkman | Rosaceae | Native |
| 25 | Sholla | Psidium guajava L. | Myrtaceae | Non-native |
| 26 | Gesho | Rhamnus prinoides L. Herit. | Rhamnaceae | Native |
| 27 | NI | Senna siamea (Cassia siamea) (Lam.) H.S.Irwin and Barneby | Fabaceae | Native |
| 28 | Timatim zaf | Solanum betaceum Cav. | Solanaceae | Non-native |
| 29 | NI | Spathodea campanulata P.Beauv. | Bignoniaceae | Native |
| 30 | NI | Trichilia dregeana Sond. | Meliaceae | Native |
| 31 | Hebicha | Vernonia amygdalina Delile | Asteraceae | Native |
| List of perennial woody and non-woody plant species recorded out of the study plots | ||||
| 32 | NI | Albizia grandibracteata Taub. | Fabaceae | Native |
| 33 | NI | Azadirachta indica var. | Meliaceae | Non-native |
| 34 | Tibero/Sessa | Bersama abyssinica Fresen | Francoaceae | Native |
| 35 | Yebelo | Bridelia micrantha (Hochst.) Baill. | Phyllanthaceae | Native |
| 36 | Tilo | Cassipourea malosana (Baker) Alst | Rhizophoraceae | Native |
| 37 | Chate | Catha edulis (Vahl) Forssk. ex Endl. | Celastraceae | Native |
| 38 | Motokomo | Celtis africana Burm.f. | Ulmaceae | Native |
| 39 | Motokomo | Celtis sp. | Ulmaceae | Native |
| 40 | Lomie | Citrus limon (L.) Osbeck | Rutaceae | Non-native |
| 41 | Birtukan | Citrus sinensis (L.) Osbeck | Rutaceae | Non-native |
| 42 | NI | Cupressus lusitanica Miller. | Cupressaceae | Non-native |
| 43 | Bahirzaf | Eucalyptus camaldulensis Dehnh. | Myrtaceae | Non-native |
| 44 | Bahirzaf | Eucalyptus globules Labill. | Myrtaceae | Non-native |
| 45 | Bahirzaf | Eucalyptus grandis W.Hill ex Maiden | Myrtaceae | Non-native |
| 46 | NI | Grevillea robusta A. Cunn. ex R. Br. | Proteaceae | Non-native |
| 47 | NI | Faidherbia albida (Delile) A.Chev. | Fabaceae | Native |
| 48 | Kilto | Ficus elastica Roxb. ex Hornem. | Moraceae | Native |
| 49 | NI | Hagenia abyssinica (Bruce) J.F.Gmel. | Rosaceae | Native |
| 50 | NI | Melia azedarach L. | Meliaceae | Non-native |
| 51 | NI | Ricinus communis L. | Euphorbiaceae | Native |
| 52 | NI | Sesbania sesban (L.) Merr. | Fabaceae | Non-native |
| Scientific Name | Family | Fre n | RF (%) | Tot Dom | RD (%) | AB | RA (%) | IVI (%) |
|---|---|---|---|---|---|---|---|---|
| Albizia gummifera (J.F. Gmel.) C.A.Sm | Fabaceae | 2.0 | 3.0 | 0.2 | 0.3 | 6.0 | 0.6 | 3.9 |
| Combretum sp. | Combretaceae | 1.0 | 1.5 | 0.0 | 0.0 | 4.0 | 0.4 | 1.9 |
| Cordia africana Lam. | Boraginaceae | 11.0 | 16.7 | 0.9 | 1.4 | 39.0 | 4.1 | 22.2 |
| Crot macrostachyus Hochst. ex Delile | Euphorbiacee | 2.0 | 3.0 | 0.2 | 0.4 | 6.0 | 0.6 | 4.0 |
| Dovyalis abyssinica (A.Rich.) Warb. | Salicaceae | 1.0 | 1.5 | 0.0 | 0.0 | 3.0 | 0.3 | 1.9 |
| Dracaena steudneri Schweinf. ex Engl | Dracaenaceae | 1.0 | 1.5 | 0.0 | 0.0 | 11.0 | 1.2 | 2.7 |
| Ensete ventricosum (Welw.) Cheesman | Musaceae | 20.0 | 30.3 | 61.3 | 96.1 | 743.0 | 78.2 | 204.6 |
| Erythrina brucei Schweinf. | Leguminosae | 3.0 | 4.5 | 0.0 | 0.1 | 14.0 | 1.5 | 6.1 |
| Maytenus senegalensis (Lam.) Exell | Celastraceae | 1.0 | 1.5 | 0.0 | 0.0 | 1.0 | 0.1 | 1.6 |
| Millettia ferruginea (Hochst.) Baker | Leguminosae | 19.0 | 28.8 | 0.9 | 1.3 | 102.0 | 10.7 | 40.9 |
| Prunus africana (Hook.f.) Kalkman | Rosaceae | 1.0 | 1.5 | 0.0 | 0.0 | 4.0 | 0.4 | 1.9 |
| Rhamnus prinoides L. Herit. | Rhamnaceae | 1.0 | 1.5 | 0.0 | 0.0 | 6.0 | 0.6 | 2.1 |
| Senna siamea (Cassia siamea) (Lam.) H.S.Irwin and Barneby | Fabaceae | 1.0 | 1.5 | 0.0 | 0.0 | 4.0 | 0.4 | 2.0 |
| Solanum betaceum Cav. | Solanaceae | 1.0 | 1.5 | 0.0 | 0.0 | 3.0 | 0.3 | 1.9 |
| Vernonia amygdalina Delile | Asteraceae | 1.0 | 1.5 | 0.2 | 0.3 | 4.0 | 0.4 | 2.2 |
| Scientific Name | Family | Fre n | RF (%) | Tot Dom | RD (%) | AB | RA (%) | IVI (%) |
|---|---|---|---|---|---|---|---|---|
| Albizia gummifera (J.F. Gmel.) C.A.Sm | Fabaceae | 5.0 | 5.2 | 0.1 | 0.3 | 14.0 | 1.1 | 6.7 |
| Clausena anisata (Willd.) Benth. | Rutaceae | 2.0 | 2.1 | 0.0 | 0.1 | 8.0 | 0.7 | 2.8 |
| Coffea arabica L. | Rubiaceae | 20.0 | 20.8 | 1.3 | 2.7 | 400.0 | 32.7 | 56.3 |
| Cordia africana Lam. | Boraginaceae | 13.0 | 13.5 | 1.7 | 3.8 | 48.0 | 3.9 | 21.3 |
| Croton macrostachyus Hochst. ex Delile | Euphorbiacee | 2.0 | 2.1 | 0.0 | 0.1 | 8.0 | 0.7 | 2.8 |
| Dovyalis abyssinica | Salicaceae | 1.0 | 1.0 | 0.0 | 0.0 | 2.0 | 0.2 | 1.2 |
| Dracaena steudneri Schweinf. Ex Engl. | Dracaenaceae | 1.0 | 1.0 | 0.0 | 0.0 | 8.0 | 0.7 | 1.7 |
| Ensete ventricosum (Welw.) Cheesman | Musaceae | 20.0 | 20.8 | 41.3 | 89.8 | 594.0 | 48.6 | 159.2 |
| Erythrina brucei Schweinf. | Leguminosae | 3.0 | 3.1 | 0.0 | 0.0 | 13.0 | 1.1 | 4.2 |
| Euphorbia abyssinica J.F.Gmel. | Euphorbiaceae | 2.0 | 2.1 | 0.0 | 0.0 | 6.0 | 0.5 | 2.6 |
| Ficus sur Forssk. | Moraceae | 1.0 | 1.0 | 0.1 | 0.3 | 2.0 | 0.2 | 1.5 |
| Ficus vasta Forssk. | Moraceae | 1.0 | 1.0 | 0.1 | 0.2 | 1.0 | 0.1 | 1.4 |
| Jacaranda mimosifolia D.Don | Bignoniaceae | 1.0 | 1.0 | 0.0 | 0.1 | 5.0 | 0.4 | 1.5 |
| Leucaena leucocephala (Lam.) de Wit | Mimosoideae | 1.0 | 1.0 | 0.0 | 0.0 | 5.0 | 0.4 | 1.5 |
| Mangifera indica L. | Anacardiaceae | 1.5 | 1.55 | 0.1 | 0.2 | 4.0 | 0.3 | 2.1 |
| Millettia ferruginea (Hochst.) Baker | Leguminosae | 19.0 | 28.8 | 0.9 | 1.3 | 102.0 | 10.7 | 40.9 |
| Persea americana Mill. | Lauraceae | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 |
| Psidium guajava L. | Myrtaceae | 2.0 | 2.0 | 0.0 | 0.0 | 10.0 | 0.4 | 1.6 |
| Rhamnus prinoides L. Herit. | Rhamnaceae | 2.0 | 2.1 | 0.0 | 0.0 | 16.0 | 1.3 | 3.4 |
| Spathodea campanulata P.Beauv. | Bignoniaceae | 1.5 | 1.55 | 0.1 | 0.2 | 4.0 | 0.3 | 2.1 |
| Vernonia amygdalina Delile | Asteraceae | 1.0 | 1.0 | 0.0 | 0.0 | 4.0 | 0.3 | 1.4 |
| Scientific Name | Family | Fre n | RF (%) | Tot Dom | RD (%) | AB | RA (%) | IVI (%) |
|---|---|---|---|---|---|---|---|---|
| Albizia gummifera (J.F. Gmel.) C.A.Sm | Fabaceae | 1 | 0.8 | 0.0 | 0.0 | 4.0 | 0.3 | 1.1 |
| Annona chrysophylla Bojer | Annonaceae | 2 | 1.5 | 0.0 | 0.2 | 22.0 | 1.8 | 3.5 |
| Casimiroa edulis Lal lave and Lex | Rutaceae | 4 | 3.1 | 0.0 | 0.0 | 11.0 | 0.9 | 4.0 |
| Carica papaya L. | Caricaceae | 8 | 6.2 | 0.1 | 0.2 | 22.0 | 1.8 | 8.2 |
| Coffea arabica L. | Rubiaceae | 19 | 14.6 | 2.0 | 7.0 | 310.0 | 25.1 | 46.7 |
| Cordia africana Lam. | Boraginaceae | 5 | 3.8 | 0.1 | 0.5 | 16.0 | 1.3 | 5.7 |
| Ensete ventricosum (Welw.) Cheesman | Musaceae | 20 | 15.4 | 16.9 | 58.6 | 363.0 | 29.4 | 103.4 |
| Erythrina brucei Schweinf. | Leguminosae | 1 | 0.8 | 0.0 | 0.2 | 2.0 | 0.2 | 1.1 |
| Ficus sur Forssk. | Moraceae | 1 | 0.75 | 1.5 | 5.15 | 1 | 0.1 | 6.0 |
| Ficus vasta Forssk. | Moraceae | 1 | 0.75 | 1.5 | 5.15 | 1.0 | 0.1 | 6.0 |
| Leucaena leucocephala (Lam.) de Wit | Mimosoideae | 1 | 0.8 | 0.0 | 0.0 | 2.0 | 0.2 | 0.9 |
| Mangifera indica L. | Anacardiaceae | 16 | 12.3 | 0.9 | 3.1 | 108.0 | 8.8 | 24.1 |
| Maytenus senegalensis (Lam.) Exell | Celastraceae | 1 | 0.8 | 0.0 | 0.0 | 2.0 | 0.2 | 0.9 |
| Millettia ferruginea (Hochst.) Baker | Leguminosae | 12 | 9.2 | 0.6 | 2.1 | 64.0 | 5.2 | 16.6 |
| Musa acuminata Colla | Musaceae | 15 | 11.5 | 3.4 | 11.8 | 234.0 | 19.0 | 42.3 |
| Persea americana Mill. | Lauraceae | 16 | 12.3 | 1.3 | 4.5 | 59.0 | 4.8 | 21.6 |
| Prunus africana (Hook.f.) Kalkman | Rosaceae | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Psidium guajava L. | Myrtaceae | 1 | 0.8 | 0.0 | 0.0 | 1.0 | 0.1 | 0.9 |
| Rhamnus prinoides L. Herit. | Rhamnaceae | 1 | 0.8 | 0.0 | 0.0 | 4.0 | 0.3 | 1.1 |
| Solanum betaceum Cav. | Solanaceae | 1 | 0.8 | 0.0 | 0.0 | 1.0 | 0.1 | 0.9 |
| Spathodea campanulata P.Beauv. | Bignoniaceae | 3 | 2.3 | 0.4 | 1.5 | 5.0 | 0.4 | 4.2 |
| Trichilia dregeana Sond. | Meliaceae | 1 | 0.8 | 0.0 | 0.0 | 1.0 | 0.1 | 0.9 |
References
- Duguma, L.; Atela, J.; Minang, P.; Ayana, A.; Gizachew, B.; Nzyoka, J.; Bernard, F. Deforestation and Forest Degradation as an Environmental Behavior: Unpacking Realities Shaping Community Actions. Land 2019, 8, 26. [Google Scholar] [CrossRef]
- Vargas Zeppetello, L.; Parsons, L.; Spector, J.; Naylor, R.; Battisti, D.; Masuda, Y.; Wolff, N.H. Large scale tropical deforestation drives extreme warming. Environ. Res. Lett. 2020, 15, 084012. [Google Scholar] [CrossRef]
- Haggar, J.; Pons, D.; Saenz, L.; Vides, M. Contribution of agroforestry systems to sustaining biodiversity in fragmented forest landscapes. Agric. Ecosyst. Environ. 2019, 283, 106567. [Google Scholar] [CrossRef]
- Jose, S. Agroforestry for ecosystem services and environmental benefits: An overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Das, T.; Das, A.K. Inventorying plant biodiversity in homegardens: A case study in Barak Valley, Assam, North East India. Curr. Sci. 2005, 89, 155–163. [Google Scholar]
- Harvey, C.A. and Gonzalez -villalobos, J.A. Agroforestry systems conserve species-rich but modified assemblages of tropical birds and bats. Biodivers. Conserv. 2007, 16, 2257–2292. [Google Scholar] [CrossRef]
- Jose, S. Agroforestry for conserving and enhancing biodiversity. Agrofor. Syst. 2012, 85, 1–8. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Rankoth, L.M.; Jose, S. Agroforestry for Biodiversity Conservation. In Agroforestry and Ecosystem Services; Udawatta, R.P., Jose, S., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- McNeely, J.A.; Schroth, G. Agroforestry and Biodiversity Conservation—Traditional Practices, Present Dynamics, and Lessons for the Future. Biodivers. Conserv. 2006, 15, 549–554. [Google Scholar] [CrossRef]
- Bhagwat, S.A.; Willis, K.J.; Birks, H.J.B.; Whittaker, R.J. Agroforestry: A refuge for tropical biodiversity? Trends Ecol. Evol. 2008, 23, 261–267. [Google Scholar] [CrossRef]
- Pantera, A.; Mosquera-Losada, M.R.; Herzog, F.; Den Herder, M. Agroforestry and the environment. Agrofor. Syst. 2021, 95, 767–774. [Google Scholar] [CrossRef]
- Kumar, B.M.; Nair, P.K.R. The enigma of tropical homegardens. Agrofor. Syst. 2004, 61–62, 135–152. [Google Scholar] [CrossRef]
- Negash, M.; Yirdaw, E.; Luukkanen, O. Potential of indigenous multistrata agroforests for maintaining native floristic diversity in the south-eastern Rift-Valley escarpment, Ethiopia. Agrofor. Syst. 2012, 85, 9–28. [Google Scholar] [CrossRef]
- Negash, M. The Indigenous Agroforestry Systems of the South-Eastern Rift–Valley Escarpment, Ethiopia: Their Biodiversity, Carbon Stocks, and Litterfall. Ph.D. Dissertation, University of Helsinki, Helsinki, Finland, 2013; 62p. [Google Scholar]
- Hemp, A. The Banana forests of Kilimanjaro: Biodiversity and conservation of the Chagga homegardens. Biodivers. Conserv. 2006, 15, 1193–1217. [Google Scholar] [CrossRef]
- Kabir, M.E.; Webb, E.L. Household and homegarden characteristics in southwestern Bangladesh. Agrofor. Syst. 2009, 75, 129–145. [Google Scholar] [CrossRef]
- Kehlenbeck, K.; Kindt, R.; Sinclair, F.L.; Simons, A.J.; Jamnadass, R. Non-native tree species displace indigenous ones on farms at intermediate altitudes around Mount Kenya. Agrofor. Syst. 2011, 83, 133–147. [Google Scholar] [CrossRef]
- Abebe, T.; Wiersum, K.F.; Bongers, F.J.J.M.; Sterck, F. Diversity and dynamics in homegardens of southern Ethiopia. In Tropical Homegardens: A Time-Tested Example of Sustainable Agroforestry; Springer: Berlin/Heidelberg, Germany, 2006; pp. 123–142. [Google Scholar] [CrossRef]
- Tamrat, S. Study of Useful Plants in and around Gate Uduma (Traditional Gedeo Homegardens) in Kochere Woreda of Gedeo Zone, SNNPR, Ethiopia: An Ethnobotanical Approach. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2011; 144p. [Google Scholar]
- Negash, M.; Achalu, N. History of indigenous agroforestry in Gedeo, southern Ethiopia, Based on local community interviews: Vegetation diversity and structure in the land use systems. Ethiop. J. Nat. Resour. 2008, 10, 31–52. [Google Scholar]
- Woldeyes, F. Homegardens and Spices of Basketo and Kafa (Southwest Ethiopia): Plant Diversity, Product Valorization and Implications to Biodiversity Conservation. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2011; 222p. [Google Scholar]
- Duguma, L.A.; Hager, H. Woody plants diversity and possession, and their future prospects in small-scale tree and shrub growing in agricultural landscapes in central highlands of Ethiopia. Small-Scale For. 2010, 9, 153–174. [Google Scholar] [CrossRef]
- Mengesha, B. Alternative Technologies for Sustainable Agricultural Production and Agroecosystem Conservation in Arsi highlands, Southeastern Ethiopia. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2010; 198p. [Google Scholar]
- Kebede, T.M. Homegardens Agrobiodiversity Conservation in Sebeta-Hawas Woreda, Southwestern Shewa Zone of Oromia Region, Ethiopia. Master’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2010; 78p. [Google Scholar]
- Fentahun, M.; Hager, H. Integration of indigenous wild woody perennial edible fruit bearing species in the agricultural landscapes of Amhara region, Ethiopia. Agrofor. Syst. 2009, 78, 79–95. [Google Scholar] [CrossRef]
- Haileselasie, T.H.; Gebrehiwot, M.T. Agroforestry practices and flora composition in backyards in Hiwane, Hintalo Wejerat of Tigray, Northern Ethiopia. Int. J. Biodivers. Conserv. 2012, 4, 294–303. [Google Scholar] [CrossRef]
- Degefa, S. Home garden agroforestry practices in the Gedeo zone, Ethiopia: A sustainable land management system for socio-ecological benefits. In Socio-Ecological Production Landscapes and Seascapes (SEPLS) in Africa; United Nations University Institute for the Advanced Study of Sustainability: Tokyo, Japan, 2016; Volume 28. [Google Scholar]
- Mulugeta, G. Production and Ecological Potentials of Gedeo’s Indigenous Agroforestry Practices in Southern Ethiopia. J. Resour. Dev. Manag. 2017, 40, 68–76. [Google Scholar]
- Garedew, B. Composition, Structure and Status of Woody Plants along Dilla Zuria rivers, Southern Ethiopia. Res. Rev. J. Bot. 2015, 4, 1–15. [Google Scholar]
- Tefera, Y.; Abebe, W.; Teferi, B. Woody plants species diversity of home garden agroforestry in three agroecological Zones of Dilla Zuria District, Gedeo Zone, Southern Ethiopia. Int. J. Fauna Biol. 2016, 3, 98–106. [Google Scholar]
- Tesfay, H.M.; Negash, M.; Godbold, D.L.; Hager, H. Assessing Carbon Pools of Three Indigenous Agroforestry Systems in the Southeastern Rift–Valley Landscapes, Ethiopia. Sustainability 2022, 14, 4716. [Google Scholar] [CrossRef]
- Maru, Y.; Gebrekirstos, A.; Haile, G. Indigenous Sacred Forests as a Tool for Climate Change Mitigation: Lessons from Gedeo Community, Southern Ethiopia. J. Sustain. For. 2023, 42, 260–287. [Google Scholar] [CrossRef]
- Abebe, T. Determinants of crop diversity and composition in enset-coffee agroforestry homegardens of Southern Ethiopia. J. Agric. Rural. Dev. Trop. Subtrop. 2013, 114, 29–38. [Google Scholar]
- Seta, T.; Demissew, S. Diversity and standing carbon stocks of native agroforestry trees in Wenago district, Ethiopia. J. Emerg. Trends Eng. Appl. Sci. 2014, 5, 125–132. [Google Scholar]
- National Metreology Agency. Climatic Data of South Nations and Nationalities Peoples Regional State; National Metreology Agency: Hawassa, Ethiopia, 2019.
- Mebrate, B.T. Agroforestry Practices in Gedeo Zone, Ethiopia: A Geographical Analysis. Ph.D. Dissertation, Panjab University, Chandigarh, India, 2007; 188p. [Google Scholar]
- Food and Agriculture Organization (FAO). Global Forest Resource Assessment; Main Report No. 163.FAO; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Mulualem, T.; Semman, N. Assessment of Enset Farming Systems, Production constraints and Breeding primacies in Southwest Ethiopia: Implication for Conservation. Res. J. Pharmacogn. Phytochem. 2021, 13, 18–26. [Google Scholar]
- Abebe, T. Diversity in Homegarden Agroforestry Systems of Southern Ethiopia. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2005; 143p. [Google Scholar]
- Shank, R.; Ertiro, C. A Linear Model for Predicting Enset Plant Yield and Assessment of Kocho Production in Ethiopia; World Food Program, Ministry of Agriculture, Southern Nation Nationalities, People Regional State, UNDP Emergencies Unit for Ethiopia: Addis Ababa, Ethiopia, 1996; 62p. [Google Scholar]
- Tsegaye, A.; Struik, P.C. Enset (Ensete ventricosum (Welw.) Cheesman) kocho yield under different crop establishment methods as compared to yields of other carbohydrate-rich food crops. Neth. J. Agri. Sci. 2001, 49, 81–94. [Google Scholar] [CrossRef]
- Negash, A.; Niehof, A. The significance of enset culture and biodiversity for rural household food and livelihood security in southwestern Ethiopia. Agric. Human Values 2004, 21, 61–71. [Google Scholar] [CrossRef]
- Abate Feyissa Senbeta, A.F.; Getachew Sime, G.; Struik, P. Enset farming system—A resilient, climate-robust production system in South and South-Western Ethiopia. Cogent Food Agric. 2022, 8, 14. [Google Scholar] [CrossRef]
- Garedew, B.; Ayiza, A.; Haile, B.; Kasaye, H. Indigenous knowledge of Enset (Ensete ventricosum (Welw.) Cheesman) cultivation and management practice by Shekicho people, southwest Ethiopia. J. Plant Sci. 2017, 5, 6–18. [Google Scholar]
- Negash, A. Diversity and Conservation of Enset (Ensete ventricosum (Welw.) Cheesman) and Its Relation to Household Food and Livelihood Security in South-Western Ethiopia. Ph.D. Dissertation, Wageningen University, Wageningen, The Netherlands, 2001; 247p. [Google Scholar]
- Tsegaye, A. On Indigenous Production, Genetic Diversity and Crop Ecology of Enset (Ensete ventricosum (Welw.) Cheesman). Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2002; 198p. [Google Scholar]
- NBE (National Bank of Ethiopia) Annual Report. Available online: https://nbe.gov.et/wp-content/uploads/2023/07/2021-22-Annual-report.pdf (accessed on 12 January 2024).
- Labouisse, J.P.; Bellachew, B.; Kotecha, S.; Bertrand, B. Current status of coffee (Coffea arabica L.) genetic resources in Ethiopia: Implications for conservation. Genet. Resour. Crop. Evol. 2008, 55, 1079–1093. [Google Scholar] [CrossRef]
- Abebe, T.; Wiersum, K.F.; Bongers, F. Spatial and temporal variation in crop diversity in agroforestry homegardens of southern Ethiopia. Agrofor. Syst. 2010, 78, 309–322. [Google Scholar] [CrossRef]
- Asfaw, Z.; Mulata, Y.; Assefa, B.; Abebe, T.; Duna, S.; Mulugeta, G.; Mebrahten, H.; Kassa, H. Enhancing the Role of Forestry in Building Climate Resilient Green Economy in Ethiopia: Strategy for Scalling up Effective Forest Management Practices in Southern Nations, Nationalities and Peoples Regional State with Particular an Emphasis on Agroforestry; Center for International Forestry Research (CIFOR): Addis Ababa, Ethiopia, 2015; 66p. [Google Scholar]
- Negash, M.; Starr, M. Biomass and soil carbon stocks of indigenous agroforestry systems on the south-eastern Rift Valley escarpment, Ethiopia. Plant Soil 2015, 393, 95–107. [Google Scholar] [CrossRef]
- Dytham, C. Choosing and Using Statistics: A Biologist’s Guide, 2nd ed.; Blackwell: Oxford, UK, 2003. [Google Scholar]
- Snowdon, P.; Raison, J.; Keith, H.; Ritson, P.; Grierson, P.; Adams, M.; Montagu, K.; Bi, H.Q.; Burrows, W.; Eamus, D. Protocol for Sampling Tree and Stand Biomass; National Carbon Accounting System, Technical Report No. 31; Australian Greenhouse Office: Canberra, Austria, 2002. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; 117p. [Google Scholar]
- Kent, M.; Coker, K. Vegetation Description and Analysis: A Practical Approach; Belhaven Press: London, UK, 1992; 363p. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Sciences: Oxford, UK, 2004. [Google Scholar]
- Pielou, E.C. An Introduction to Mathematical Ecology; Wiley: New York, NY, USA, 1969; 286p. [Google Scholar]
- Caiafa, A.N.; Martins, F.R. Forms of rarity of tree species in the southern Brazilian Atlantic rainforest. Biodivers. Conserv. 2010, 19, 2597–2618. [Google Scholar] [CrossRef]
- Espeland, E.K.; Emam, T.M. The value of structuring rarity: The seven types and links to reproductive ecology. Biodivers. Conserv. 2011, 20, 963–985. [Google Scholar] [CrossRef]
- Vivero, L.J.; Kelbessa, E.; Demissew, S. The Red List of Endemic Trees and Shrubs of Ethiopia and Eritrea; Fauna and Flora International: Cambridge, UK, 2005; 23p. [Google Scholar]
- Bekele, T.; Haase, G.; Soromessa, T. Forest genetic resources of Ethiopia: Status and proposed actions. In The National Forest Resources Conservations Strategy Development Workshop, Proceedings of the National Workshop, Addis Ababa, Ethiopia, 21–22 June 1999; Edwards, S., Demissie, A., Bekele, T., Haase, G., Eds.; Institute of Biodiversity Conservation and Research (IBCR), GTZ: Addis Ababa, Ethiopia, 1999; pp. 39–48. [Google Scholar]
- Gebremariam, A.H.; Bekele, M.; Ridgewell, A. Small and Medium Forest Enterprises in Ethiopia; IIED Small and Medium Forest Enterprise Series No. 26; FARM-Africa and International Institute for Environment and Development: London, UK, 2009; 52p. [Google Scholar]
- Kassa, K.; Abebe, T.; Ewnetu, Z. Diversity, density and management of trees in different agro-forestry practices of Yem Special District, Southern Ethiopia. Ethiop. J. Sci. 2015, 38, 1–16. [Google Scholar]
- Legesse, A.; Negash, M. Species diversity, composition, structure and management in agroforestry systems: The case of Kachabira district, Southern Ethiopia. Heliyon 2021, 7, 10. [Google Scholar] [CrossRef]
- Molla, A.; Asfaw, Z. Woody species diversity under natural forest patches and adjacent enset-coffee based Agroforestry in the Midland of Sidama Zone, Ethiopia. Biodivers. Conserv. 2014, 6, 708–723. [Google Scholar]
- Bajigo, A.; Tadesse, M. Woody species diversity of traditional agroforestry practices in Gununo Watershed in Wolayitta Zone, Ethiopia. Forest Res. 2015, 4, 155. [Google Scholar] [CrossRef]
- Negawo, W.J.; Beyene, D.N. The Role of coffee Based Agroforestry System in Tree Diversity Conservation in Eastern Uganda. J. Landsc. Ecol. 2017, 10, 1–18. [Google Scholar] [CrossRef]
- Molla, A.; Kewessa, G. Woody species diversity in traditional agroforestry practices of Dellomenna District, Southeastern Ethiopia: Implication for maintaining native woody species. Int. J. Biodivers. 2015, 2015, 643031. [Google Scholar] [CrossRef]
- Ngo Bieng, M.A.; Delgado-Rodríguez, D.; Vilchez-Mendoza, S.; López-Sampson, A.; García, E.; Sepúlveda, N.; Somarriba, E. Tree diversity in a tropical agricultural-forest mosaic landscape in Honduras. Sci. Rep. 2022, 12, 18544. [Google Scholar] [CrossRef]
- Mattsson, E.; Ostwald, M.; Nissanka, S.P.; Pushpakumara, D.K.N.G. Quantification of carbon stock and tree diversity of homegardens in a dry zone area of Moneragala district, Sri Lanka. Agrofor. Syst. 2015, 89, 435–445. [Google Scholar] [CrossRef]
- Tibebu Enkossa, T.; Nemomissa, S.; Lemessa, D. Woody species diversity and the carbon stock potentials of different land use types in agroecosystem of Jimma Ganati District, Western Ethiopia. Environ. Chall. 2023, 13, 11. [Google Scholar] [CrossRef]
- Vebrova, H.; Lojka, B.; Husband, T.P.; Zans ME, C.; Van Damme, P.; Rollo, A.; Kalousova, M. Tree diversity in cacao agroforests in San Alejandro, Peruvian Amazon. Agrofor. Syst. 2014, 88, 1101–1115. [Google Scholar] [CrossRef]
- Dayamba, S.D.; Djoudi, H.; Zida, M.; Sawadogo, L.; Verchot, L. Biodiversity and carbon stocks in different land use types in the Sudanian Zone of Burkina Faso, West Africa. Agric. Ecosyst. Environ. 2016, 216, 61–72. [Google Scholar] [CrossRef]
- Kumari, M.S.; Kansuntisukmongkol, K.; Brockelman, W.Y. Plant diversity in home gardens and its contribution to household economy in suburban areas in Sri Lanka. Environ. Nat. Resour. J. 2009, 7, 12–29. [Google Scholar]
- Oginosako, Z.; Simitu, P.; Orwa, C.; Mathenge, S. Are They Competing or Compensating on Farm?: Status of Indigenous and Non-Native Tree Species in a Wide Range of Agro-Ecological Zones of Eastern and Central Kenya, Surrounding Mt. Kenya; Results of Vegetation, Farmer, and Nursery Surveys (No. 16); World Agroforestry Centre: Nairobi, Kenya, 2006; 45p. [Google Scholar]
- Nogués-Bravo, D.; Araújo, M.B.; Romdal, T.; Rahbek, C. Scale effects and human impact on the elevational species richness gradients. Nature 2008, 453, 216–219. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Sharma, M. Conservation of plant diversity in agroforestry systems in a biodiversity hotspot region of northeast India. Agric. Res. 2021, 10, 569–581. [Google Scholar]
- O’Neill, G.A.; Dawson, I.; Sotelo-Montes, C.; Guarino, L.; Guariguata, M.; Current, D.; Weber, J.C. Strategies for genetic conservation of trees in the Peruvian Amazon basin. Biodivers. Conserv. 2001, 10, 837–850. [Google Scholar] [CrossRef]
- Hailu, T.; Negash, L.; Olsson, M. Millettia ferruginea from southern Ethiopia: Impacts on soil fertility and growth of maize. Agrofor. Syst. 2000, 48, 9–24. [Google Scholar] [CrossRef]
- Birhane, E.; Ahmed, S.; Hailemariam, M.; Negash, M.; Rannestad, M.M.; Norgrove, L. Carbon stock 833 and woody species diversity in homegarden agroforestry along an elevation gradient in southern Ethiopia. Agrofor. Syst. 2020, 94, 1099–1110. [Google Scholar] [CrossRef]
- Graham, S.; Ihli, H.J.; Gassner, A. Agroforestry, Indigenous Tree Cover and Biodiversity Conservation: A Case Study of Mount Elgon in Uganda. Eur. J. Dev. Res. 2022, 34, 1893–1911. [Google Scholar] [CrossRef]
- Berhanu, A.; Asfaw, Z. The role of home gardens for conservation and sustainable utilization of plant biodiversity of Ethiopia. In Coffee Production, Variety and Trading Ways to Maximize Ethiopia’s Benefits, Proceedings of the 24th Annual Conference of the Biological Society of Ethiopia, Addis Ababa, Ethiopia, 24–26 March 2014; Girma, A., Wube, T., Eds.; 2014; pp. 35–81. Available online: https://www.researchgate.net/profile/Abiyot-Berhanu/publication/293822093_THE_ROLE_OF_HOME_GARDENS_FOR_CONSERVATION_AND_SUSTAINABLE_UTILIZATION_OF_PLANT_BIODIVERSITY_OF_ETHIOPIA/links/56bc4b2a08ae7be8798bef8e/THE-ROLE-OF-HOME-GARDENS-FOR-CONSERVATION-AND-SUSTAINABLE-UTILIZATION-OF-PLANT-BIODIVERSITY-OF-ETHIOPIA.pdf (accessed on 12 January 2024).
- Schroth, G.; Krauss, U.; Gasparotto, L.J.A.D.; Duarte Aguilar, J.A.; Vohland, K. Pests and diseases in agroforestry systems of the humid tropics. Agrofor. Syst. 2000, 50, 199–241. [Google Scholar] [CrossRef]
- Fan, C.; Johnston, M.; Darling, L.; Scott, L.; Liao, F.H. Land use and socio-economic determinants of urban forest structure and diversity. Landsc. Urban Plan. 2019, 181, 10–21. [Google Scholar] [CrossRef]
- Correia, M.; Diabaté, M.; Beavogui, P.; Guilavogui, K.; Lamanda, N.; de Foresta, H. Conserving Forest tree diversity in Guinée Forestière (Guinea, West Africa): The role of coffee-based agroforests. Biodivers. Conserv. 2010, 19, 1725–1747. [Google Scholar] [CrossRef]
- Asase, A.; Tetteh, D.A. The role of complex agroforestry systems in the conservation of forest tree diversity and structure in Southeastern Ghana. Agrofor. Syst. 2010, 79, 355–368. [Google Scholar] [CrossRef]
- Abreha, A.; Gebrekidan, W. Woody plant inventory and diversity in traditional agroforestry of selected peasant association of South Gonder Zone, North West Ethiopia. J. Environ. Earth Sci. 2014, 4, 1–16. [Google Scholar]
- Körner, C. Why are there global gradients in species richness? Mountains might hold the answer. Correspondence 2000, 15, 513–514. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q.; Li, S.; Wang, G. Distribution and Species Diversity of Plant Communities along Transect on the Northeastern Tibetan Plateau. Biodivers. Conserv. 2006, 15, 1811–1828. [Google Scholar] [CrossRef]
- Shimono, A.; Zhou, H.; Shen, H.; Hirota, M.; Ohtsuka, T.; Tang, Y. Patterns of plant diversity at high altitudes on the Qinghai-Tibetan Plateau. Plant Ecol. 2010, 3, 1–7. [Google Scholar] [CrossRef]
- Luzuriaga, A.L.; Escudero, A. What Factors Affect Diversity and Species Composition of Endangered Tumbesian Dry Forests in Southern Ecuador? Biotropica 2011, 43, 15–22. [Google Scholar] [CrossRef]
- Ma, M. Species richness vs. evenness: Independent relationship and different responses to edaphic factors. Oikos 2005, 111, 192–198. [Google Scholar] [CrossRef]




| Number | Scientific Name | Origin | Reference |
|---|---|---|---|
| 1 | Acokanthera schimperi (A.Dc) Schweinf. | Native | [29] |
| 2 | Albizia grandibracteata Taub. | Native | Present study |
| 3 | Albizia gummifera (J.F. Gmel.) C.A.Sm | Native | Present study |
| 4 | Annona chrysophylla Bojer | Non-native | Present study |
| 5 | Annona reticulate Sieber ex A.DC. | Non-native | [30] |
| 6 | Apodytes dimidiata E. Mey. ex Arn. | Native | [29] |
| 7 | Arundinaria alpine K.Schum. | Native | [30] |
| 8 | Azadirachta indica var. | Non-native | Present study |
| 9 | Bersama abyssinica Fresen | Native | Present study |
| 10 | Bridelia atroviridis Muell.Arg. | Native | [29] |
| 11 | Bridelia micrantha (Hochst.) Baill. | Native | Present study |
| 12 | Brucea sp. | Native | [30] |
| 13 | Cadaba longifolia DC. | Non-native | [29] |
| 14 | Calpurnia aurea (Aiton) Benth. | Native | [29] |
| 15 | Canthium oligocarpum Hiern | Native | [30] |
| 16 | Carica papaya L. | Non-native | Present study |
| 17 | Carissa spinarum L. | Native | [29] |
| 18 | Casimiroa edulis Lal lave and Lex | Non-native | Present study |
| 19 | Cassipourea malosana (Baker) Alst | Native | Present study |
| 20 | Catha edulis (Vahl) Forssk. ex Endl. | Native | Present study |
| 21 | Celtis africana N.L. Burm | Native | Present study |
| 22 | Celtis sp. | Native | Present study |
| 23 | Chionanthus mildbraedii (Gilg and G.Schellenb.) Stearn | Native | [29] |
| 24 | Citrus limon (L.) Osbeck | Non-native | Present study |
| 25 | Citrus sinensis (L.) Osbeck | Non-native | Present study |
| 26 | Clausena anisata (Willd.) Benth. | Native | Present study |
| 27 | Clutia abyssinica Jaub. and Spach. | Native | [29] |
| 28 | Combretum adenogonium Steud.ex A.Rich. | Native | [29] |
| 29 | Combretum molle (Klotzsch) Engl. and Diels | Native | [29] |
| 30 | Cordia africana Lam. | Native | Present study |
| 31 | Coffea arabica L. | Native | Present study |
| 32 | Croton macrostachyus Hochst. ex Delile | Native | Present study |
| 33 | Cupressus lusitanica Miller. | Non-native | Present study |
| 34 | Dalbergia lactea Vatke | Native | [29] |
| 35 | Dichrostachys cinerea (L.) Wight and Arrn. | Native | [29] |
| 36 | Diospyros abyssinica (Hiern.) F. White | Native | [29] |
| 37 | Dodonaea angustifolia L.f. | Native | [29] |
| 38 | Dovyalis abyssinica (A.Rich.) Warb. | Native | Present study |
| 39 | Dracaena steudneri Schweinf. Ex Engl. | Native | Present study |
| 40 | Ensete ventricosum (Welw.) Cheesman | Native | Present study |
| 41 | Ehretia cymosa Thonn. | Native | [29] |
| 42 | Ekebergia capensis Sparrm | Native | [29] |
| 43 | Erythrina brucei Schweinf. | Native | Present study |
| 44 | Eucalyptus camaldulensis Dehnh | Non-native | Present study |
| 45 | Eucalyptus globules Labill. | Non-native | Present study |
| 46 | Eucalyptus grandis W.Hill ex Maiden | Non-native | Present study |
| 47 | Euclea racemosa L. | Native | [29] |
| 48 | Euphorbia abyssinica J.F.Gmel. | Native | Present study |
| 49 | Euphorbia candelabrum Welw | Native | [29] |
| 50 | Euphorbia tirucalli L. | Native | [29] |
| 51 | Faidherbia albida (Delile) A.Chev. | Native | Present study |
| 52 | Faurea rochetiana (A.Rich.) Chiov. ex Pic.Serm. | Native | [29] |
| 53 | Faurea speciosa Welw | Native | [30] |
| 54 | Ficus elastica Roxb. ex Hornem. | Native | Present study |
| 55 | Ficus ovata Vahl. | Native | [29] |
| 56 | Ficus sur Forssk. | Native | Present study |
| 57 | Ficus sycomorus L. | Native | [29] |
| 58 | Ficus thonningii Blume | Native | [29] |
| 59 | Ficus vasta Forssk. | Native | Present study |
| 60 | Grevillea robusta A. Cunn. ex R. Br. | Non-native | Present study |
| 61 | Hagenia abyssinica (Bruce) J.F.Gmel. | Native | Present study |
| 62 | Hibiscus macranthus Hochst.ex A. Rich. | Native | [29] |
| 63 | Jacaranda mimosifolia D.Don | Non-native | Present study |
| 64 | Justicia schimperiana (Hochst. ex Nees) T.Anderson | Native | [29] |
| 65 | Lantana camara L. | Non-native | [29] |
| 66 | Lepisanthes senegalensis (Poir.) Leenh. | Native | [29] |
| 67 | Leucaena leucocephala (Lam.) de Wit | Non-native | Present study |
| 68 | Maesa lanceolata Forssk. | Native | [29] |
| 69 | Mangifera indica L. | Non-native | Present study |
| 70 | Manilkara butugi Chiov. | Native | [29] |
| 71 | Maytenus arbutifolia (Hochst. ex A.Rich.) R.Wilczek | Native | [29] |
| 72 | Maytenus senegalensis (Lam.) Exell | Native | Present study |
| 73 | Melia azedarach L. | Non-native | Present study |
| 74 | Millettia ferruginea (Hochst.) Baker | Native | Present study |
| 75 | Mimusops kummel Bruce ex A.DC. | Native | [29] |
| 76 | Musa acuminata Colla | Non-native | Present study |
| 77 | Ochna schweinfurthiana F.Hoffim. | Native | [29] |
| 78 | Olea welwitschii (Knobl.) Gilg and Schellenb. | Native | [29] |
| 79 | Osyris quadripartita Salzm. ex Decne. | Native | [29] |
| 80 | Pavetta oliveriana Heirn | Native | [29] |
| 81 | Persea americana Mill. | Non-native | Present study |
| 82 | Phyllanthus ovalifolius Forssk | Native | [29] |
| 83 | Pittosporum viridiflorum Sims | Native | [29] |
| 84 | Polyscias fulva Harms | Native | [30] |
| 85 | Pouteria adolfi-friederici (Engl.) A.Meeuse | Native | [29] |
| 86 | Pouteria alnifolia (Baker) Pierre | Native | [30] |
| 87 | Prunus africana (Hook.f.) Kalkman | Native | Present study |
| 88 | Prunus persica (L.) Batsch | Non-native | [30] |
| 89 | Psidium guajava L. | Non-native | Present study |
| 90 | Rhamnus prinoides L. Herit. | Native | Present study |
| 91 | Rhus vulgaris Meikle | Native | [29] |
| 92 | Ricinus communis L. | Native | Present study |
| 93 | Sapium ellipticum (Hochst.) Pax | Native | [29] |
| 94 | Senna siamea (Cassia siamea) (Lam.) H.S.Irwin and Barneby | Native | Present study |
| 95 | Sesbania sesban (L.) Merr. | Non-native | Present study |
| 96 | Solanum incanum L. | Native | [29] |
| 97 | Spathodea campanulata P.Beauv. | Native | Present study |
| 98 | Spathodea nilotica Seem. | Native | [29] |
| 99 | Solanum betaceum Cav. | Non-native | Present study |
| 100 | Solanum macrocarpon L. | Non-native | [30] |
| 101 | Suregada procera (Prain) Croizat. | Native | [29] |
| 102 | Syzygium guineense (Willd.) DC.Subsp.afromontanumF. White. | Native | [29] |
| 103 | Terminalia schimperiana Hochst. | Native | [29] |
| 104 | Trichilia dregeana Sond. | Native | Present study |
| 105 | Vangueria madagascariensis J.F.Gmel. | Native | [29] |
| 106 | Vernonia amygdalina Delile | Native | Present study |
| 107 | Vernonia auriculifera Hiern. | Native | [29] |
| Agroforestry System | Species Scientific Name | IVI% |
|---|---|---|
| Enset based | Ensete ventricosum (Welw.) Cheesman | 204.6 |
| Millettia ferruginea (Hochst.) Baker | 40.9 | |
| Cordia africana Lam. | 22.2 | |
| Erythrina brucei Schweinf. | 6.1 | |
| Croton macrostachyus Hochst. ex Delile | 4.0 | |
| Coffee–enset based | Ensete ventricosum (Welw.) Cheesman | 159.2 |
| Coffea arabica L. | 56.3 | |
| Millettia ferruginea (Hochst.) Baker | 23.9 | |
| Cordia africana Lam. | 21.3 | |
| Albizia gummifera (J.F. Gmel.) C.A.Sm | 6.7 | |
| Coffee–fruit tree–enset based | Ensete ventricosum (Welw.) Cheesman | 103.4 |
| Coffea arabica L. | 46.7 | |
| Musa acuminata Colla. | 42.3 | |
| Mangifera indica L. | 24.1 | |
| Persea americana Mill. | 21.6 |
| Agroforestry System | n | Stem Number (No/100 m2) | BA (m2 ha−1) | Height (m) | DBH (cm) |
|---|---|---|---|---|---|
| Enset based AF | 20 | 34.7 (2.7) (b) | 306.4 (28.8) (b) | 4.4 (0.2) (b) | 31.0 (1.7) (b) |
| C–E based AF | 20 | 29.3 (2.8) (b) | 207.0 (15.1) (c) | 4.1 (0.2) (b) | 28.8 (1.8) (ab) |
| C–Ft–E based AF | 20 | 13.1 (2.0) (a) | 81.2 (9.3) (a) | 3.6 (0.2) (a) | 24.2 (1.4) (a) |
| p-value | <0.05 | <0.05 | <0.05 | <0.05 |
| Agroforestry System | n | Stem Number (No/100 m2) | BA (m2 ha−1) | Height (m) | DBH (cm) |
|---|---|---|---|---|---|
| Enset based AF | 20 | 9.3 (1.7) (b) | 11.3 (2.5) (b) | 6.0 (0.8) (b) | 11.2 (1.3) (a) |
| C–E based AF | 20 | 22.0 (1.1) (c) | 21.9 (4.1) (bc) | 3.6 (0.2) (ac) | 8.1 (0.3) (b) |
| C–Ft–E based AF | 20 | 31.2 (3.5) (a) | 53.8 (10.4) (a) | 4.2 (0.2) (a) | 11.8 (0.5) (a) |
| p-value | <0.05 | <0.05 | <0.05 | <0.05 |
| Agroforestry System | N | Stem Number (No/100 m2) | BA (m2 ha−1) | Height (m) | DBH (cm) |
|---|---|---|---|---|---|
| Enset based AF | 20 | 46.9 (3.0) (b) | 317.7 (28.1) (b) | 4.6 (0.1) (a) | 26.7 (1.5) (b) |
| C–E based AF | 20 | 53.8 (2.6) (b) | 228.5 (14.8) (c) | 4.3 (0.2) (a) | 18.9 (0.7) (c) |
| C–Ft–E based AF | 20 | 71.2 (3.2) (a) | 149.2 (17.6) (a) | 4.3 (0.1) (a) | 15.7 (0.7) (a) |
| p-value | <0.05 | <0.05 | NS | <0.05 |
| Agroforestry System | N | Abundance per 100 m2 | Shannon Diversity Index | Margalef’s Richness Index | Pielou’s Eveness Index |
|---|---|---|---|---|---|
| Enset based AF | 20 | 44.6 (3.0) (a) | 0.7 ± 0.2 (b) | 0.6 ± 0.2 (b) | 0.6 ± 0.1 (a) |
| C–E based AF | 20 | 51.3 (2.6) (a) | 1.0 ± 0.1 (c) | 1.0 ± 0.3 (c) | 0.6 ± 0.1 (a) |
| C–Ft–E based AF | 20 | 48.5 (3.2) (a) | 1.1 ± 0.2 (a) | 1.2 ± 0.3 (a) | 0.6 ± 0.1 (a) |
| p-value | NS | <0.05 | <0.05 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesfay, H.M.; Oettel, J.; Lapin, K.; Negash, M. Plant Diversity and Conservation Role of Three Indigenous Agroforestry Systems of Southeastern Rift-Valley Landscapes, Ethiopia. Diversity 2024, 16, 64. https://doi.org/10.3390/d16010064
Tesfay HM, Oettel J, Lapin K, Negash M. Plant Diversity and Conservation Role of Three Indigenous Agroforestry Systems of Southeastern Rift-Valley Landscapes, Ethiopia. Diversity. 2024; 16(1):64. https://doi.org/10.3390/d16010064
Chicago/Turabian StyleTesfay, Hafte Mebrahten, Janine Oettel, Katharina Lapin, and Mesele Negash. 2024. "Plant Diversity and Conservation Role of Three Indigenous Agroforestry Systems of Southeastern Rift-Valley Landscapes, Ethiopia" Diversity 16, no. 1: 64. https://doi.org/10.3390/d16010064
APA StyleTesfay, H. M., Oettel, J., Lapin, K., & Negash, M. (2024). Plant Diversity and Conservation Role of Three Indigenous Agroforestry Systems of Southeastern Rift-Valley Landscapes, Ethiopia. Diversity, 16(1), 64. https://doi.org/10.3390/d16010064








