Electrochemical Interrogation of G3-Poly(propylene thiophenoimine) Dendritic Star Polymer in Phenanthrene Sensing
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagent and Materials
2.2. Instruments
2.3. Synthesis of the G3 Poly(propylene thiophenoimine) Dendrimer (G3PPT)
2.4. Electrochemical Preparation of Generation 3 Poly(propylene thiophenoimine)-co-Poly(3-hexlythiophene) Dendritic Star-Copolymer
2.5. Characterization of Dendritic Star-Copolymer Au|G3PPT-co-P3HT
2.6. Electrochemical Degradation of Phenanthrene
2.7. Sensor Specificity and Selectivity
3. Results and Discussion
3.1. Dendritic Star-Copolymer (Au|G3PPT-co-P3HT) Sensor Modification



3.2. CV and ACV Responses of Sensor


3.3. SWV Responses of Sensor

3.4. Application of the Phenanthrene Sensor




3.5. Sensor Specificity
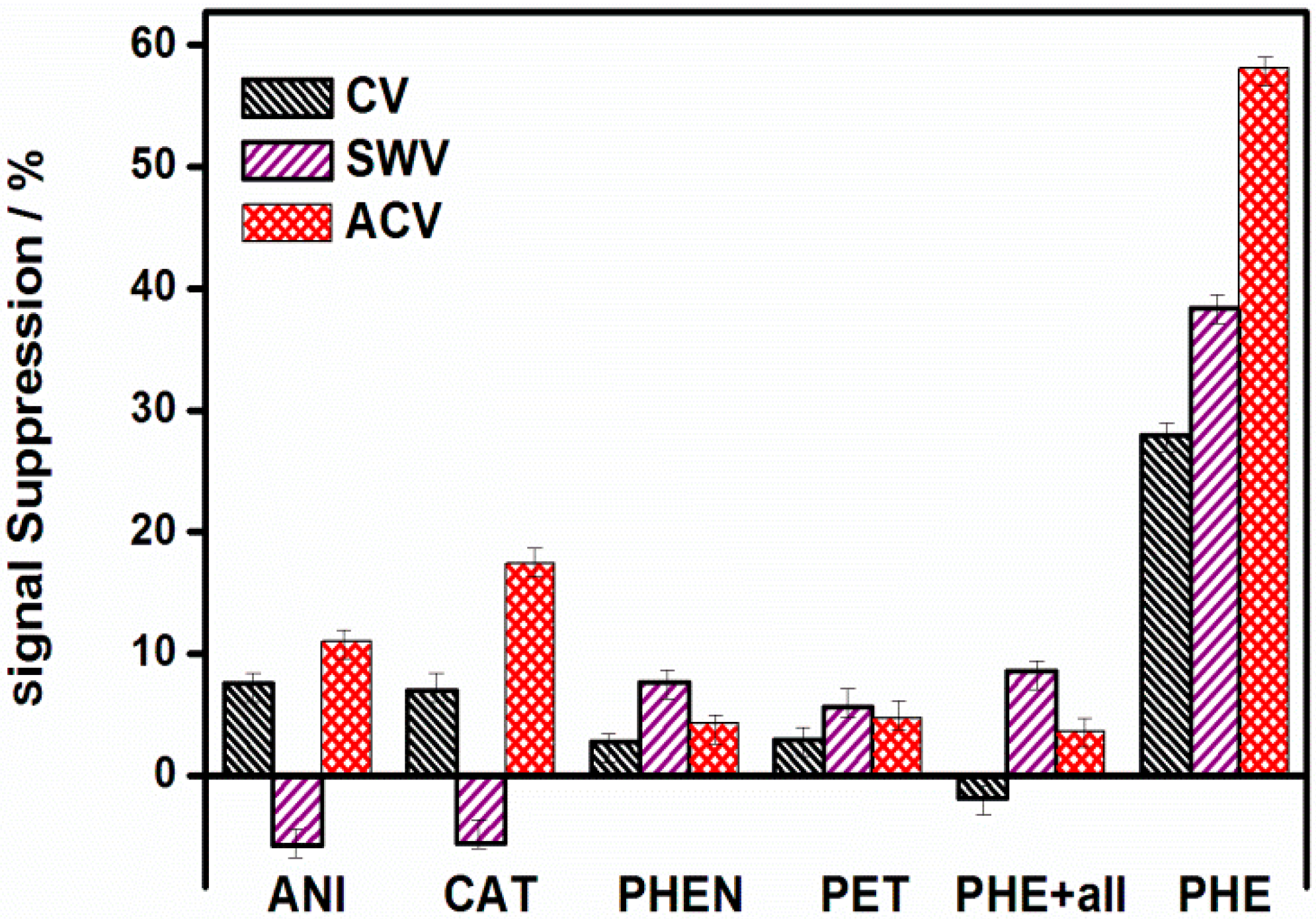
3.6. Sensor Sensitivity and Selectivity
| Techniques | Solutions | R2 | LOD (nmol/L) | Linear Range (nmol/L) | %SE |
|---|---|---|---|---|---|
| CV | 0.1 MBu4NClO4/CH3CN/H2O | 0.990 | 4.74 | 6.68–40.79 | 74 |
| Tap water | 0.998 | 12.62 | 13.35–35.86 | 11 | |
| ACV | 0.1 MBu4NClO4/CH3CN/H2O | 0.997 | 1.42 | 4.78–37.65 | 84 |
| Tap water | 0.983 | 2.99 | 5.46–42.57 | 68 | |
| SWV | 0.1 M Bu4NClO4/CH3CN/H2O | 0.985 | 3.24 | 5.35–38.76 | 20 |
| Tap water | 0.988 | 9.61 | 10.34–40.56 | 7 |
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A Review of Airborne Polycyclic Aromatic Hydrocarbons (Pahs) and Their Human Health Effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Rajput, N.; Lakhani, A. Measurements of Polycyclic Aromatic Hydrocarbons in an Urban Atmosphere of Agra, India. Atmosfera 2010, 23, 165–183. [Google Scholar]
- Liu, L.; Liu, Y.; Lin, J.; Tang, N.; Hayakawa, K.; Maeda, T. Development of Analytical Methods for Polycyclic Aromatic Hydrocarbons (PAHs) in Airborne Particulates: A Review. J. Environ. Sci. 2007, 19, 1–11. [Google Scholar] [CrossRef]
- Karyab, H.; Yunesian, M.; Nasseri, S.; Mahvi, A.H.; Ahmadkhaniha, R.; Rastkari, N.; Nabizadeh, R. Polycyclic Aromatic Hydrocarbons in Drinking Water of Tehran, Iran. J. Environ. Heal. Sci. Eng. 2013, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.; Ali, L.N.; Al-Bloushi, A.; Al-Hashash, H.; Al-Bahloul, M.; Al-Khabbaz, A.; Al-Khayat, A. Effect of Environmental Factors on Photodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) in the Water-Soluble Fraction of Kuwait Crude Oil in Seawater. Mar. Environ. Res. 2011, 72, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Manoli, E.; Samara, C. Polycyclic Aromatic Hydrocarbons in Natural Waters: Sources, Occurrence and Analysis. Trends Anal. Chem. 1999, 18, 417–428. [Google Scholar] [CrossRef]
- Khalili-Fard, V.; Ghanemi, K.; Nikpour, Y.; Fallah-Mehrjardi, M. Application of Sulfur Microparticles for Solid-Phase Extraction of Polycyclic Aromatic Hydrocarbons from Sea Water and Wastewater Samples. Anal. Chim. Acta 2012, 714, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Martorell, I.; Nieto, A.; Nadal, M.; Perelló, G.; Marcé, R.M.; Domingo, J.L. Human Exposure to Polycyclic Aromatic Hydrocarbons (PAHs) Using Data from a Duplicate Diet Study in Catalonia, Spain. Food Chem. Toxicol. 2012, 50, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Udovyk, O.; Rabilloud, L.; Gilek, M.; Karlsson, M. Hazardous Substances: A Case Study of Environmental Risk Governance in the Baltic Sea Region. Södertörn University: Huddinge, Sweden, 2010; pp. 1–77. [Google Scholar]
- Bandowe, B.A.M.; Meusel, H.; Huang, R.-J.; Ho, K.; Cao, J.; Hoffmann, T.; Wilcke, W. PM2.5-Bound Oxygenated PAHs, Nitro-PAHs and Parent-PAHs from the Atmosphere of a Chinese Megacity: Seasonal Variation, Sources and Cancer Risk Assessment. Sci. Total Environ. 2014, 473/474, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Busetti, F.; Heitz, A.; Cuomo, M.; Badoer, S.; Traverso, P. Determination of Sixteen Polycyclic Aromatic Hydrocarbons in Aqueous and Solid Samples from an Italian Wastewater Treatment Plant. J. Chromatogr. A 2006, 1102, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Bruno, P.; Caselli, M.; de Gennaro, G.; Tutino, M. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Particulate Matter Collected with Low Volume Samplers. Talanta 2007, 72, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Cigić, I.K.; Prosen, H. An Overview of Conventional and Emerging Analytical Methods for the Determination of Mycotoxins. Int. J. Mol. Sci. 2009, 10, 62–115. [Google Scholar] [CrossRef] [PubMed]
- Mailu, S.N.; Waryo, T.T.; Ndangili, P.M.; Ngece, F.R.; Baleg, A.A.; Baker, P.G.; Iwuoha, E.I. Determination of Anthracene on Ag-Au Alloy Nanoparticles/Overoxidized-Polypyrrole Composite Modified Glassy Carbon Electrodes. Sensors 2010, 10, 9449–9465. [Google Scholar] [CrossRef] [PubMed]
- Tovide, O.; Jaheed, N.; Mohamed, N.; Nxusani, E.; Sunday, C.E.; Tsegaye, A.; Ajayi, R.F.; Njomo, N.; Makelane, H.; Bilibana, M.; et al. Graphenated Polyaniline-Doped Tungsten Oxide Nanocomposite Sensor for Real Time Determination of Phenanthrene. Electrochim. Acta 2014, 128, 138–148. [Google Scholar] [CrossRef]
- Fähnrich, K.A.; Pravda, M.; Guilbault, G.G. Disposable Amperometric Immunosensor for the Detection of Polycyclic Aromatic Hydrocarbons (PAHs) Using Screen-Printed Electrodes. Biosens. Bioelectron. 2003, 18, 73–82. [Google Scholar] [CrossRef]
- Rassie, C.; Olowu, R.A.; Waryo, T.T.; Wilson, L.; Williams, A.; Baker, P.G.; Iwuoha, E.I. Dendritic 7T-Polythiophene Electro-Catalytic Sensor System for the Determination of Polycyclic Aromatic Hydrocarbons. Int. J. Electrochem. Sci. 2011, 6, 1949–1967. [Google Scholar]
- Malinga, P.; Arotiba, O.A.; Krause, R.W.M.; Mapolie, S.F.; Diallo, M.S.; Mamba, B.B. Composite Polyester Membranes with Embedded Dendrimer Hosts and Bimetallic Fe/Ni Nanoparticles: Synthesis, Characterisation and Application to Water Treatment. J. Nanopart. Res. 2013, 1698, 1–15. [Google Scholar]
- Inoue, K. Functional dendrimers, hyperbranched and star polymers. Prog. Polym. Sci. 2000, 25, 453–571. [Google Scholar] [CrossRef]
- Newkome, G.R.; Shreiner, C.D. Poly(amidoamine), Polypropylenimine, and Related Dendrimers and Dendrons Possessing Different 1→2 Branching Motifs: An Overview of the Divergent Procedures. Polymer 2008, 49, 171–173. [Google Scholar] [CrossRef]
- Akbari, S. The Application of Dentritic Material in Textile. Sci. Bull. Escorena 2013, 7, 11–26. [Google Scholar]
- Maiti, S.; Adivarekar, R.V. Dendrimers—An Auxilliary in Dyeing. J. Text. Assoc. 2013, 74, 26–30. [Google Scholar]
- Kim, D.H.; Jang, Y.; Park, Y.D.; Cho, K. Controlled One-Dimensional Nanostructures in Poly(3-hexylthiophene) Thin Film for High-Performance Organic Field-Effect Transistors. J. Phys. Chem. B 2006, 110, 15763–15768. [Google Scholar] [CrossRef] [PubMed]
- Kline, R.J.; Mcgehee, M.D.; Kadnikova, E.N.; Liu, J.; Jean, M.J. The Dependence of Regioregular Poly(3-hexylthiophene) Film Morphology and Field-Effect Mobility on Molecular Weight. Macromolecules 2005, 38, 3312–3319. [Google Scholar] [CrossRef]
- Lu, C.; Cao, L.; Liu, R.; Lei, Y.; Ding, G. Effect of Common Metal Ions on the Rate of Degradation of 4-Nitrophenol by a Laccase-Cu2+ Synergistic System. J. Environ. Manag. 2012, 113, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, P.L.; Criddle, C.S. Chemical and Biological Processes: The Need for Mixing. In Delivery and Mixing in the Subsurface; Kitanidis, P.K., McCarty, P.L., Eds.; Springer: New York, NY, USA, 2012; pp. 7–52. [Google Scholar]
- Olowu, R.A.; Ndangili, P.M.; Baleg, A.A.; Ikpo, C.O.; Njomo, N.; Baker, P.; Iwuoha, E. Spectroelectrochemical Dynamics of Dendritic Poly(propylene imine)-Polythiophene Star Copolymer Aptameric 17 β -Estradiol Biosensor. Int. J. Electrochem. Sci. 2011, 6, 1686–1708. [Google Scholar]
- Scott, R.W.J.; Wilson, O.M.; Crooks, R.M. Titania-Supported Au and Pd Composites Synthesized from Dendrimer-Encapsulated Metal Nanoparticle Precursors. J. Chem. Mater. 2004, 16, 5682–5688. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.G. A Practical Approach for the Preparation of Regioregular Poly(3-hexylthiophene). Bull. Korean Chem. Soc. 2010, 31, 193–195. [Google Scholar] [CrossRef]
- Nazarudin, M.F.; Zainal, Z.; Tan, W.T.; Hamadneh, I.; Kadri, E.F. Abrasive Stripping Voltammetric (abrsv) Studies of ErBa2Cu3O7−δ Superconductor Synthesised Via Co-Precipitation And Solid State Methods. Int. J. Electrochem. Sci. 2012, 7, 2965–2982. [Google Scholar]
- Pilehvar, S.; Dardenne, F.; Blust, R.; de Wael, K. Electrochemical Sensing of Phenicol Antibiotics at Gold. Int. J. Electrochem. Sci. 2012, 7, 5000–5011. [Google Scholar]
- Zen, J.-M.; Yang, C.-C.; Kumar, A.S. Potential Scan Rate Dependence of Underpotential and Bulk Depositions of Lead on Screen-Printed Silver Electrodes. Electrochim. Acta 2001, 47, 899–904. [Google Scholar] [CrossRef]
- Sharp, M.; Pettersson, M.; Edstrom, K. Preliminary Determinations of Electron Transfer Kinetics Involving Ferrocene Covalently Attached to a Platinum Surface. J. Electroanal. Chem. Interfacial Electrochem. 1979, 95, 123–130. [Google Scholar] [CrossRef]
- Punckt, C.; Pope, M.A.; Aksay, I.A. On the Electrochemical Response of Porous Functionalized Graphene Electrodes. J. Phys. Chem. C 2013, 117, 16076–16086. [Google Scholar] [CrossRef]
- Cohen, Y.; Klein, J.; Rabinovitz, M. The Charge Alternation Concept. Application to Cyclic Conjugated Doubly Charged Systems. J. Am. Chem. Soc. 1988, 110, 4634–4640. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Seetharamappa, J.; Katrahalli, U.; Kandagal, P.B. Electrochemical Studies of Buzepide Methiodide and Their Analytical Applications. Int. J. Electrochem. Sci. 2008, 3, 711–720. [Google Scholar]
- Eckermann, A.L.; Feld, D.J.; Shaw, J.A.; Meade, T.J. Electrochemistry of Redox-Active Self-Assembled Monolayers. Coord. Chem. Rev. 2010, 254, 1769–1802. [Google Scholar] [CrossRef] [PubMed]
- Zeli, M. Electrochemical Reduction of Europium (3+) at Increasing Concentrations of Different Salts. Part I. Voltammetric Measurements. Croat. Chem. Acta 2003, 76, 241–248. [Google Scholar]
- Vasile, E.; Serafim, A.; Petre, D.; Giol, D.; Dubruel, P.; Iovu, H.; Stancu, I.C. Direct Synthesis and Morphological Characterization of Gold-Dendrimer Nanocomposites Prepared Using PAMAM Succinamic Acid Dendrimers: Preliminary Study of the Calcification Potential. Sci. World J. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hourani, R.; Kakkar, A. Advances in the Elegance of Chemistry in Designing Dendrimers. Macromol. Rapid Commun. 2010, 31, 947–974. [Google Scholar] [CrossRef] [PubMed]
- Švorc, Ľ.; Sochr, J.; Tomčík, P.; Rievaj, M.; Bustin, D. Simultaneous Determination of Paracetamol and Penicillin V By Square-Wave Voltammetry at a Bare Boron-Doped Diamond Electrode. Electrochim. Acta 2012, 68, 227–234. [Google Scholar]
- Tommos, C.; Valentine, K.G.; Martínez-rivera, M.C.; Liang, L.; Moorman, V.R. Reversible Phenol Oxidation-Reduction in the Structurally Well-Defined 2-Mercaptophenol-α3C Protein. Biochemistry 2014, 52, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lovrić, M.; Komorsky-Lovrić, Š. Theory of Square-Wave Voltammetry of Two-Step Electrode Reaction Using an Inverse Scan Direction. Int. J. Electrochem. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Mirčeski, V.; Gulaboski, R.; Scholz, F. Square-Wave Thin-Film Voltammetry: Influence of Uncompensated Resistance and Charge Transfer Kinetics. J. Electroanal. Chem. 2004, 566, 351–360. [Google Scholar] [CrossRef]
- Liu, H.; Cao, X.; Yang, J.; Gong, X.Q.; Shi, X. Dendrimer-Mediated Hydrothermal Synthesis of Ultrathin Gold Nanowires. Sci. Rep. 2013, 3, 3181. [Google Scholar] [CrossRef] [PubMed]
- Hatchett, D.W.; Josowicz, M. Composites of Intrinsically Conducting Polymers as Sensing Nanomaterials. Chem. Rev. 2008, 108, 746–769. [Google Scholar] [CrossRef] [PubMed]
- Janmanee, R.; Chuekachang, S.; Sriwichai, S.; Baba, A.; Phanichphant, S. Functional Conducting Polymers in the Application of SPR Biosensors. J. Nanotechnol. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Maingi, V.; Jain, V.; Bharatam, P. V.; Maiti, P.K. Dendrimer Building Toolkit: Model Building and Characterization of Various Dendrimer Architectures. J. Comput. Chem. 2012, 33, 1997–2011. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-S.; Keum, Y.-S.; Li, Q.X. Bacterial Degradation of Aromatic Compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.Y.; Walker, B.; Stormberg, K.; Zaitouna, A.J.; Yang, W. Electrochemical Techniques for Characterization of Stem-Loop Probe and Linear Probe-Based DNA Sensors. Methods 2013, 64, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, G.V.; Zaitouna, A.J.; Lai, R.Y. Characterization of an Electrochemical Mercury Sensor Using Alternating Current, Cyclic, Square Wave and Differential Pulse Voltammetry. Anal. Chim. Acta 2014, 810, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, Y.; Zhou, R. Distribution of Polycyclic Aromatic Hydrocarbons in Water, Sediment and Soil in Drinking Water Resource of Zhejiang Province, China. J. Hazard. Mater. 2008, 150, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Dobrinas, S.; Stanciu, G.; Chirila, E.; Soceanu, A.D.; Paunescu, E.; Epure, D.T. Occurrence of PAHs and PCBs in Petrochemical Wastewater. Ovidius Univ. Ann. Chem. 2011, 22, 21–26. [Google Scholar]
- Law, R.J.; Dawes, V.J.; Woodhead, R.J.; Matthiessen, P. Polycyclic Aromatic Hydrocarbons (PAH) in Seawater around England and Wales. Mar. Pollut. Bull. 1997, 34, 306–322. [Google Scholar] [CrossRef]
- Bopp, S.; Weiß, H.; Schirmer, K. Time-Integrated Monitoring of Polycyclic Aromatic Hydrocarbons (PAHs) in Groundwater Using the Ceramic Dosimeter Passive Sampling Device. J. Chromatogr. A. 2005, 107, 2137–2147. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for Drinking-Water Quality, 3rd ed; WHO Library Cataloguing-in-Publication Data: Geneva, Switzerland, 2004; pp. 1–515. [Google Scholar]
- May, W.E.; Wasik, S.P.; Freeman, D.H. Determination of Solubility Behavior of Some Polycyclic Aromatic-Hydrocarbons in Water. Anal. Chem. 1978, 50, 997–100. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makelane, H.R.; Tovide, O.; Sunday, C.E.; Waryo, T.; Iwuoha, E.I. Electrochemical Interrogation of G3-Poly(propylene thiophenoimine) Dendritic Star Polymer in Phenanthrene Sensing. Sensors 2015, 15, 22343-22363. https://doi.org/10.3390/s150922343
Makelane HR, Tovide O, Sunday CE, Waryo T, Iwuoha EI. Electrochemical Interrogation of G3-Poly(propylene thiophenoimine) Dendritic Star Polymer in Phenanthrene Sensing. Sensors. 2015; 15(9):22343-22363. https://doi.org/10.3390/s150922343
Chicago/Turabian StyleMakelane, Hlamulo R., Oluwakemi Tovide, Christopher E. Sunday, Tesfaye Waryo, and Emmanuel I. Iwuoha. 2015. "Electrochemical Interrogation of G3-Poly(propylene thiophenoimine) Dendritic Star Polymer in Phenanthrene Sensing" Sensors 15, no. 9: 22343-22363. https://doi.org/10.3390/s150922343







