Near-Infrared Spectroscopy as a Potential COVID-19 Early Detection Method: A Review and Future Perspective
Abstract
:1. Introduction
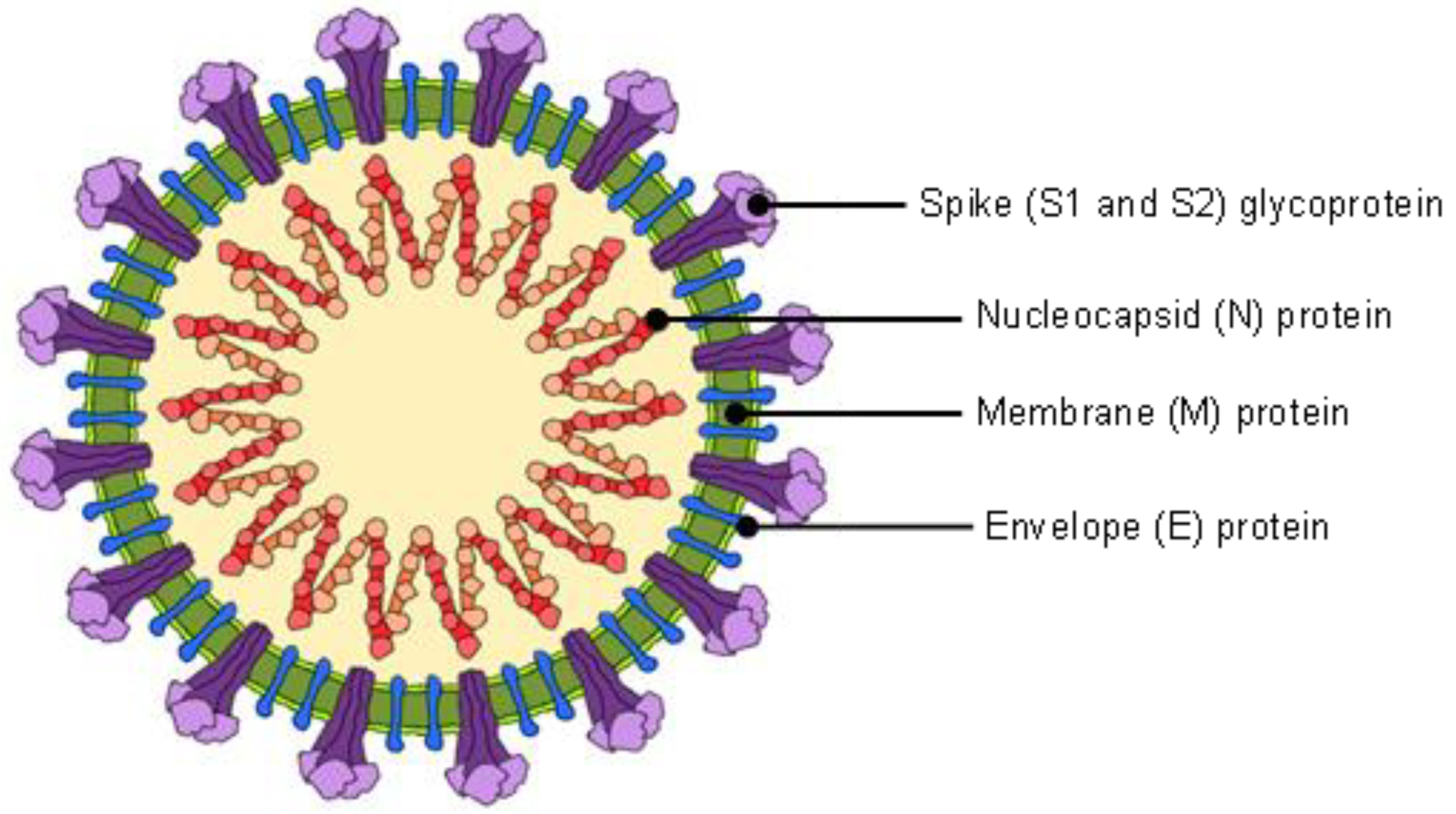
2. Structural Properties of Coronavirus and Relation to COVID-19 Diagnosis
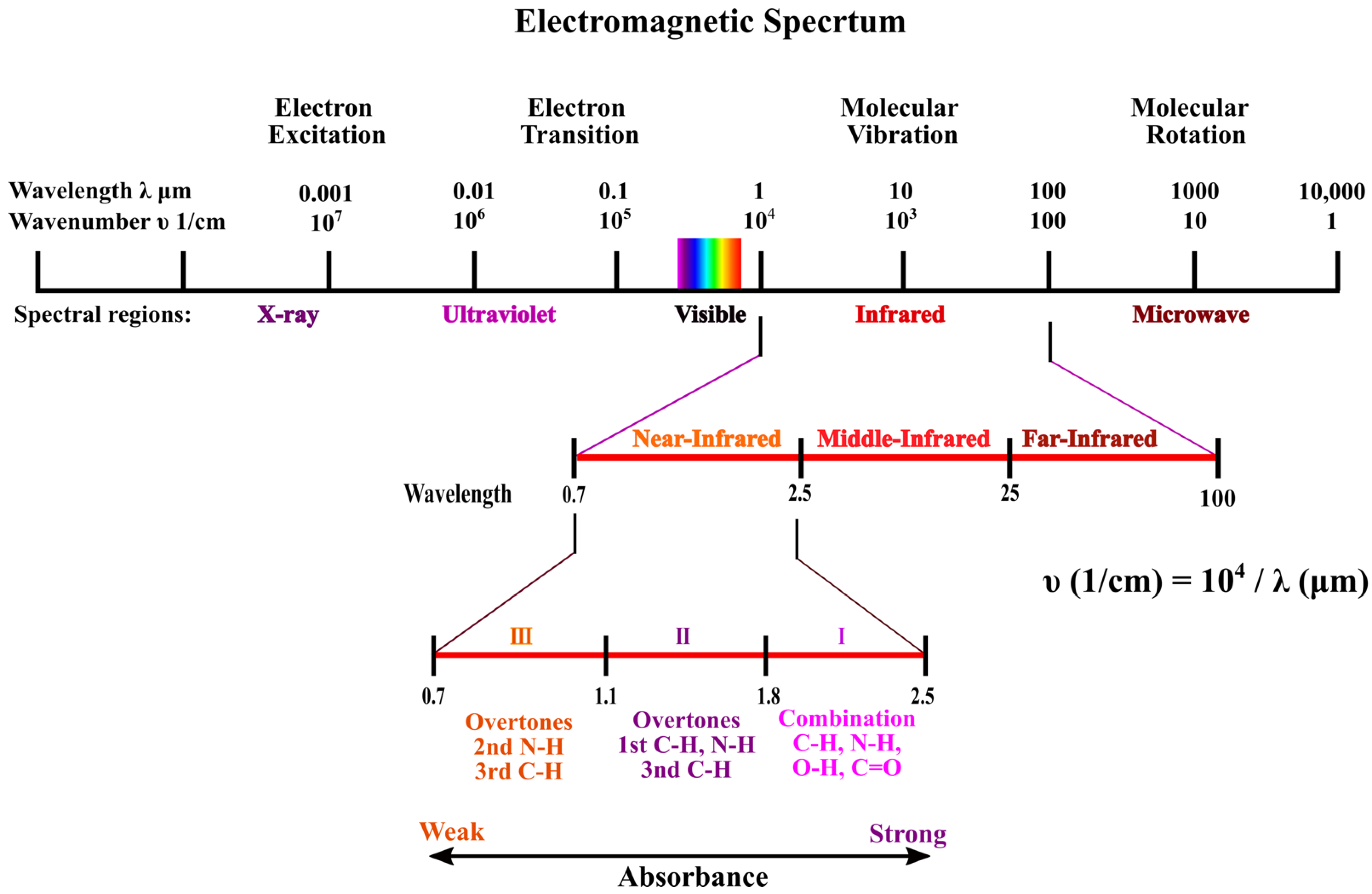
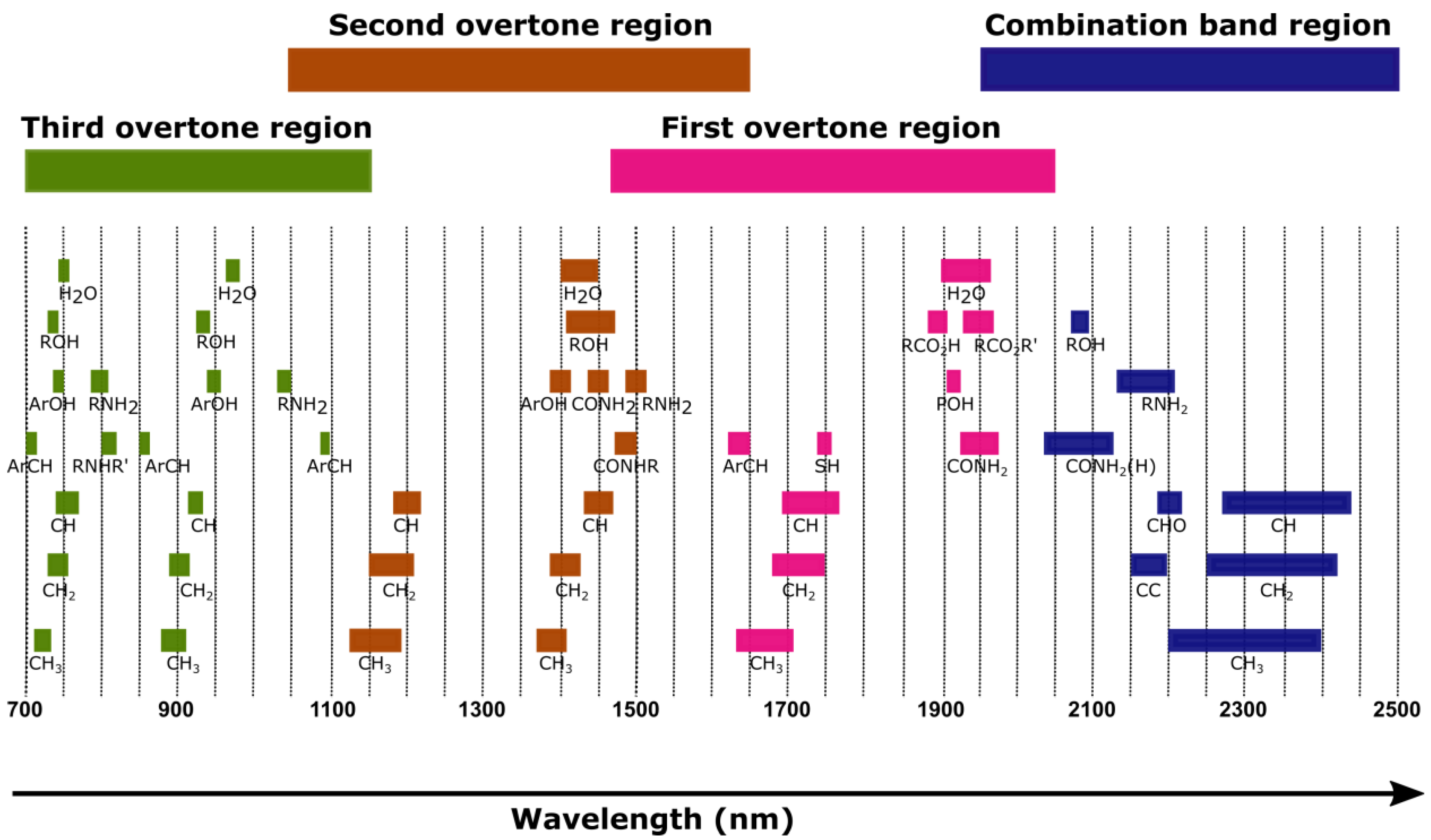
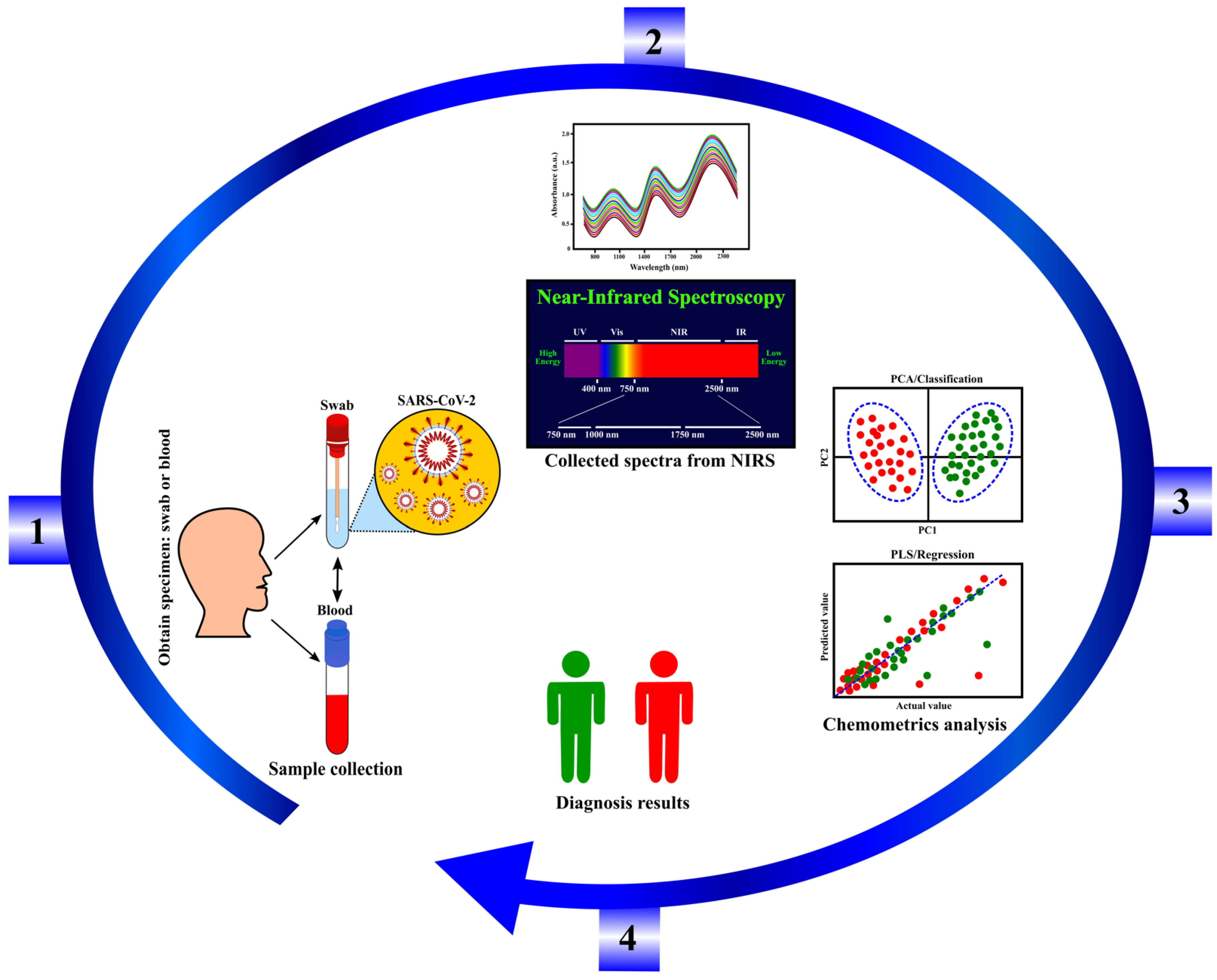
3. Background on Near-Infrared Spectroscopy
3.1. Instrumentation and Sample Presentation
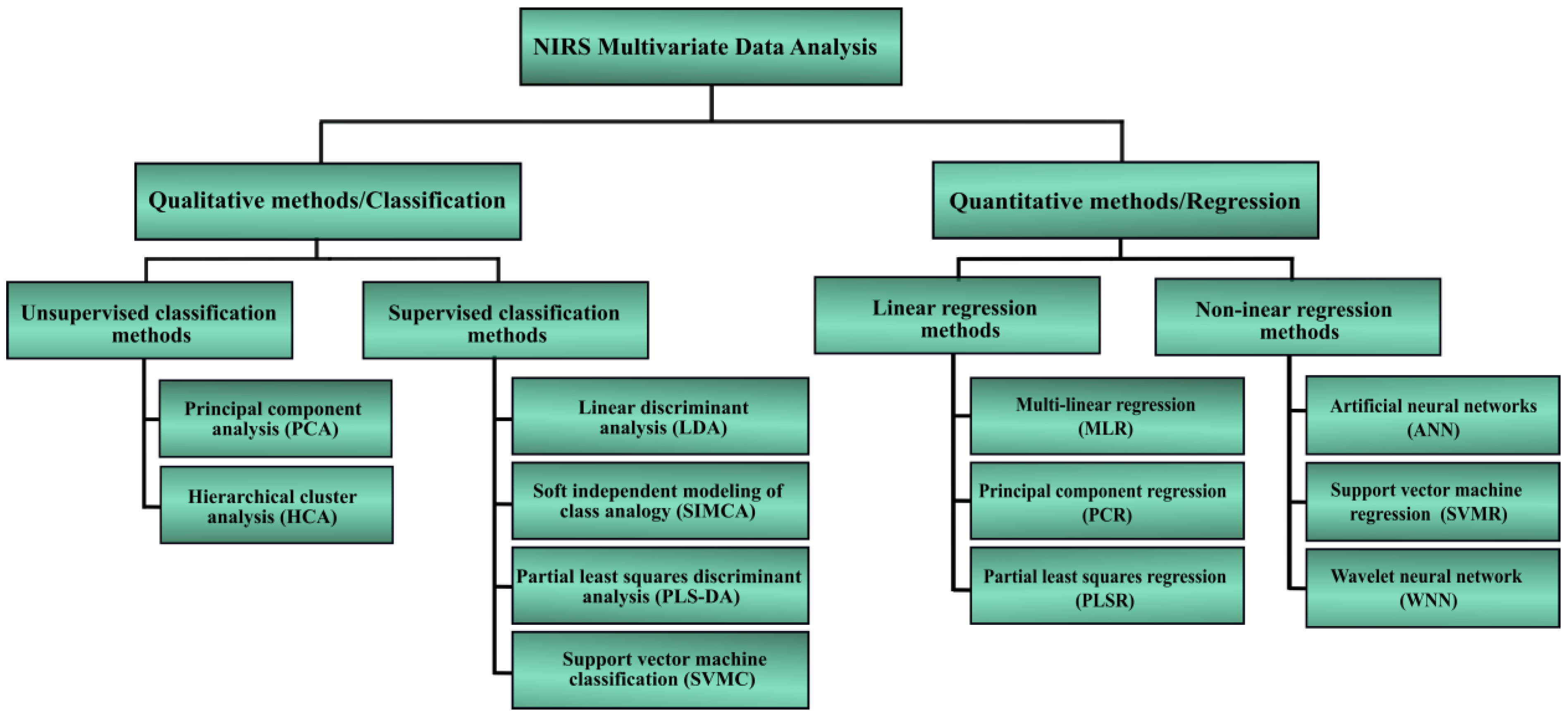
3.2. Literature Review of Viruses Detection Using NIRS Technique
| Detected Virus | Samples | Chemometric Analysis | Reference Methods | Wavelength Range (nm) | Limit of Detection | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| HIV-1 [24] | Plasma | PLS and leave-out cross-validation | PCR and ELISA | 600–1000 | Not applicable | Sensitive at 970 nm, R2 = 0.8555 | - |
| HIV-1 [66] | Plasma | PCA and SIMCA | PCR | 600–1100 | Not applicable | Sensitive at 970 nm and between 1000 nm to 1100 nm | Could differentiate between HIV-1 patients and healthy |
| Human influenza virus [27] | Nasal aspirates | PCA and SIMCA | Immunochromatography | 600–1100 | Not applicable | Sensitive at 970 nm | >93% |
| Human influenza virus [28] | Nasal mucosal | Kruskal–Wallis test and Dunn’s | Immunochromatography | 600–1100 | Not applicable | Sensitive at 970 nm | - |
| DENV [70] | Human blood | Not applicable_ | Serological test | 400–900 | Not applicable | Sensitive at 540 nm and 580 nm | Obvious difference between DENV, normal, and other viruses from 500–600 nm |
| Hepatitis C [70] | Human blood | Not applicable | Serological test | 400–900 | Not applicable | Sensitive at 700 nm | Obvious difference between hepatitis C, normal, and other viruses from 700–1000 nm |
| Wolbachia pipientis in Ae. aegypti mosquito [71] | Heads and thoraces | PLS | Not applicable | 500–2350 | Not applicable | Sensitive at 1400 nm and 1900 nm | >96.6% between different strains |
| ZIKV-infected Ae. aegypti mosquitoes [68] | Heads and thoraces | Cross-validation, Regression coefficient and PLS | RT-qPCR | 350–2500 | Not applicable | Sensitive at 1900 nm | >94% |
| ZIKV-, CHIKV, and Wolbachia-infection [69] | Heads and thoraces | PLS and PLSDA | RT-qPCR | 350–2500 | Not applicable | Sensitive at 1400 nm and 1900 nm | >96% |
3.3. Potential of Coronavirus Detection Using NIRS Technology
| Samples | Chemometric Analysis | Reference Methods | Wavelength Range (nm) | Limit of Detection | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| RNA from swab test [75] | PLS, PCA, machine learning | RT-PCR | 1250–16,667 nm | 10 copies/μL | 97%, Sensitive at 2352 nm and at Mid IR region | 98.3% between positive and negative samples |
| SARS-CoV-2 from saliva [76] | PCA, PLS-DA, Monte Carlo Double Cross Validation | RT-qPCR | 2500–10,000 nm | Not applicable | 93% | 82% between positive and negative samples |
| Swab fluid [77] | PLS and cosine k-nearest neighbors (KNN) | RT-PCR | Mid IR | Not applicable | 84–87% | 64–66% between positive and negative samples |
| SARS-CoV-2 isolate [78] | Not applicable | Not applicable | UV-VIS | Not applicable | Sensitive at 280 nm | Not applicable |
| RNA from swab test [79] | Genetic Algorithm-LDA | RT-PCR | Mid IR | 1582 copies/mL | 89% between positive and negative samples |
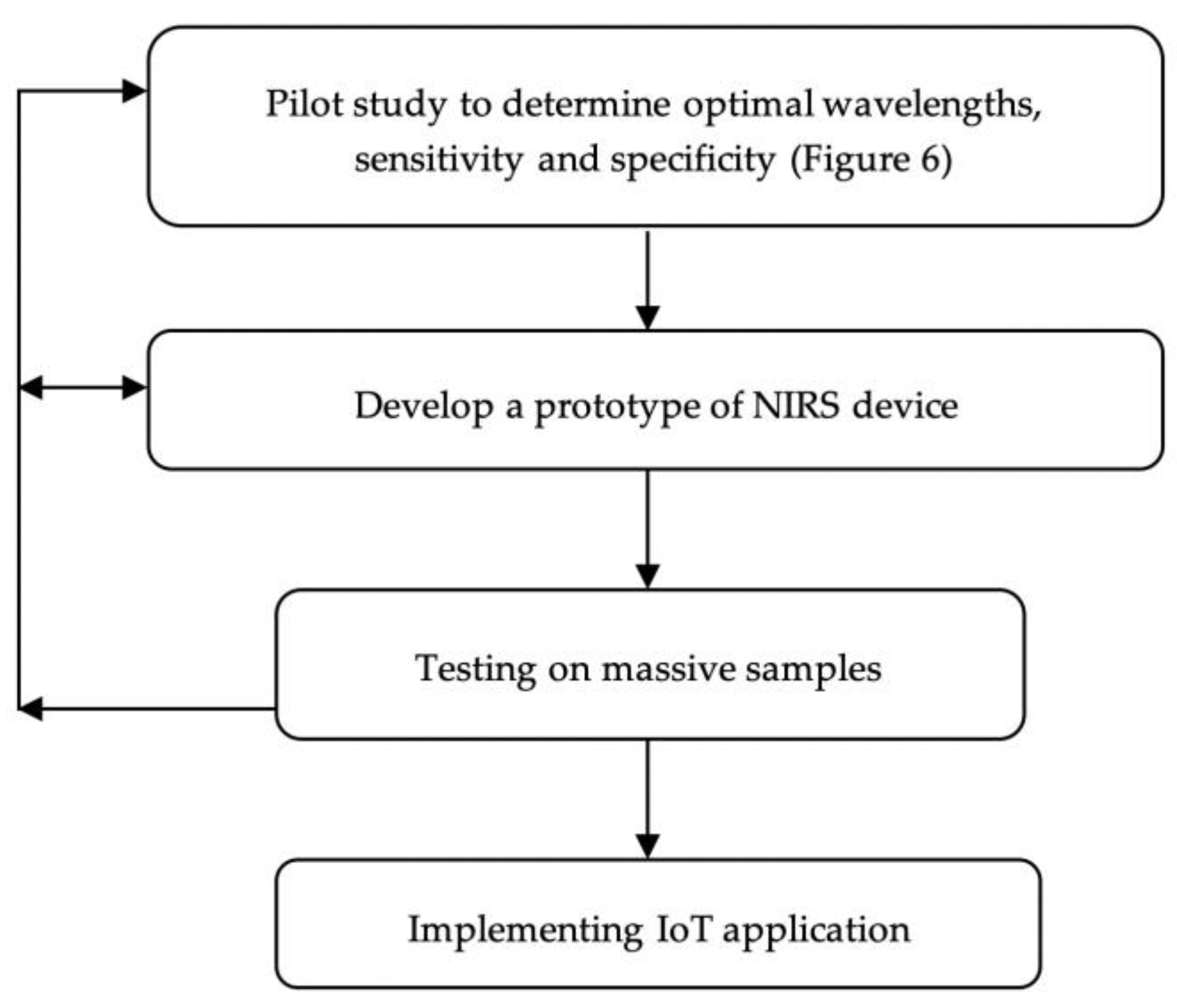
4. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, T.; Duong Bang, D.; Wolff, A. 2019 novel coronavirus disease (COVID-19): Paving the road for rapid detection and point-of-care diagnostics. Micromachines 2020, 11, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.-C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, G.; Chen, G.; Zhu, N.; Wu, D.; Wu, Y.; James, T.D. Recent progresses and remaining challenges for the detection of Zika virus. Med. Res. Rev. 2021, 41, 2039–2108. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.P.; Ghimire, T.R.; Subramanya, S.H. Access to and equitable distribution of COVID-19 vaccine in low-income countries. Npj Vaccines 2021, 6, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M. COVID-19 vaccines for low-and middle-income countries. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Nie, S.; Zhang, Z.; Zhang, Z. Longitudinal change of severe acute respiratory syndrome coronavirus 2 antibodies in patients with coronavirus disease 2019. J. Infect. Dis. 2020, 222, 183–188. [Google Scholar] [CrossRef]
- Ribeiro, B.V.; Cordeiro, T.A.R.; e Freitas, G.R.O.; Ferreira, L.F.; Franco, D.L. Biosensors for the detection of respiratory viruses: A review. Talanta Open 2020, 2, 100007. [Google Scholar] [CrossRef]
- Tang, Y.-W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. Laboratory diagnosis of COVID-19: Current issues and challenges. J. Clin. Microbiol. 2020, 58, e00512-20. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, Y.; Chen, Y.; Qin, Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020, 92, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yu, H.; Horby, P.W.; Cao, B.; Wu, P.; Yang, S.; Gao, H.; Li, H.; Tsang, T.K.; Liao, Q. Comparison of patients hospitalized with influenza A subtypes H7N9, H5N1, and 2009 pandemic H1N1. Clin. Infect. Dis. 2014, 58, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yang, C.; He, Q.; Chen, J.; Yu, D.; Li, J.; Zhai, S.; Qin, Z.; Du, K.; Chu, Z. Current and perspective diagnostic techniques for COVID-19. ACS Infect. Dis. 2020, 6, 1998–2016. [Google Scholar] [CrossRef] [PubMed]
- Petisco, C.; Garcia-Criado, B.; Zabalgogeazcoa, I.; Vázquez-de-Aldana, B.R. A spectroscopy approach to the study of virus infection in the endophytic fungus Epichloë festucae. Virol. J. 2011, 8, 286. [Google Scholar] [CrossRef] [Green Version]
- Sakudo, A.; Suganuma, Y.; Kobayashi, T.; Onodera, T.; Ikuta, K. Near-infrared spectroscopy: Promising diagnostic tool for viral infections. Biochem. Biophys. Res. Commun. 2006, 341, 279–284. [Google Scholar] [CrossRef]
- Soler, M.; Scholtz, A.; Zeto, R.; Armani, A.M. Engineering photonics solutions for COVID-19. APL Photonics 2020, 5, 090901. [Google Scholar] [CrossRef]
- Bhalla, N.; Pan, Y.; Yang, Z.; Payam, A.F. Opportunities and challenges for biosensors and nanoscale analytical tools for pandemics: COVID-19. ACS Nano 2020, 14, 7783–7807. [Google Scholar] [CrossRef]
- Santos, M.C.; Morais, C.L.; Nascimento, Y.M.; Araujo, J.M.; Lima, K.M. Spectroscopy with computational analysis in virological studies: A decade (2006–2016). TrAC Trends Anal. Chem. 2017, 97, 244–256. [Google Scholar] [CrossRef]
- Lukose, J.; Chidangil, S.; George, S.D. Optical technologies for the detection of viruses like COVID-19: Progress and prospects. Biosens. Bioelectron. 2021, 178, 113004. [Google Scholar] [CrossRef]
- Soler, M.; Estevez, M.C.; Cardenosa-Rubio, M.; Astua, A.; Lechuga, L.M. How nanophotonic label-free biosensors can contribute to rapid and massive diagnostics of respiratory virus infections: COVID-19 case. ACS Sens. 2020, 5, 2663–2678. [Google Scholar] [CrossRef]
- Taha, B.A.; Al Mashhadany, Y.; Bachok, N.N.; Bakar, A.A.; Hafiz Mokhtar, M.H.; Dzulkefly Bin Zan, M.S.; Arsad, N. Detection of COVID-19 virus on surfaces using photonics: Challenges and perspectives. Diagnostics 2021, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, F.; Zang, L.; Zang, H.; Alcalà, M.; Nie, L.; Wang, M.; Li, L. Near infrared spectroscopy combined with multivariate analysis for monitoring the ethanol precipitation process of fraction I + II + III supernatant in human albumin separation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 175, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, Z.; Wu, H.; Wang, L.; Wu, T.; Tan, C. Diagnosis of colorectal cancer by near-infrared optical fiber spectroscopy and random forest. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Sakudo, A.; Tsenkova, R.; Onozuka, T.; Morita, K.; Li, S.; Warachit, J.; Iwabu, Y.; Li, G.; Onodera, T.; Ikuta, K. A novel diagnostic method for human immunodeficiency virus type-1 in plasma by near-infrared spectroscopy. Microbiol. Immunol. 2005, 49, 695–701. [Google Scholar] [CrossRef] [Green Version]
- Sakudo, A.; Suganuma, Y.; Sakima, R.; Ikuta, K. Diagnosis of HIV-1 infection by near-infrared spectroscopy: Analysis using molecular clones of various HIV-1 subtypes. Clin. Chim. Acta 2012, 413, 467–472. [Google Scholar] [CrossRef]
- Abe, K.; Wada, A.; Oshima, S.; Kono, S.; Takahashi, A.; Kanno, Y.; Imaizumi, H.; Hayashi, M.; Okai, K.; Niwa, S.I. Reduced frontal activation during verbal fluency task in chronic hepatitis C patients with interferon-based therapy as measured by near-infrared spectroscopy. Hepatol. Res. 2017, 47, E55–E63. [Google Scholar] [CrossRef] [Green Version]
- Sakudo, A.; Baba, K.; Ikuta, K. Discrimination of influenza virus-infected nasal fluids by Vis-NIR spectroscopy. Clin. Chim. Acta 2012, 414, 130–134. [Google Scholar] [CrossRef]
- Sakudo, A.; Baba, K.; Ikuta, K. Analysis of Vis–NIR spectra changes to measure the inflammatory response in the nasal mucosal region of influenza A and B virus-infected patients. J. Clin. Virol. 2012, 55, 334–338. [Google Scholar] [CrossRef]
- Pasquini, C. Near infrared spectroscopy: Fundamentals, practical aspects and analytical applications. J. Braz. Chem. Soc. 2003, 14, 198–219. [Google Scholar] [CrossRef] [Green Version]
- Manley, M. Near-infrared spectroscopy and hyperspectral imaging: Non-destructive analysis of biological materials. Chem. Soc. Rev. 2014, 43, 8200–8214. [Google Scholar] [CrossRef] [Green Version]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis—a review of current methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef] [PubMed]
- Yelin, I.; Aharony, N.; Tamar, E.S.; Argoetti, A.; Messer, E.; Berenbaum, D.; Shafran, E.; Kuzli, A.; Gandali, N.; Shkedi, O. Evaluation of COVID-19 RT-qPCR test in multi sample pools. Clin. Infect. Dis. 2020, 71, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wen, K.; Zhang, J.; Chen, J.; Han, C.; Chen, Y.; Wang, S.; Deng, G.; Zhou, H.; Wu, Y. Accuracy of a nucleocapsid protein antigen rapid test in the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2021, 27, 289.e1–289.e4. [Google Scholar] [CrossRef] [PubMed]
- Tozetto-Mendoza, T.R.; Kanunfre, K.A.; Vilas-Boas, L.S.; Espinoza, E.P.S.; Paião, H.G.O.; Rocha, M.C.; de Paula, A.V.; de Oliveira, M.S.; Zampelli, D.B.; Vieira, J.M., Jr. Nucleoprotein-based ELISA for detection of SARS-COV-2 IgG antibodies: Could an old assay be suitable for serodiagnosis of the new coronavirus? J. Virol. Methods 2021, 290, 114064. [Google Scholar] [CrossRef]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives–A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Pellicer, A.; del Carmen Bravo, M. Near-infrared spectroscopy: A methodology-focused review. Semin. Fetal Neonatal Med. 2011, 16, 42–49. [Google Scholar] [CrossRef]
- Cheong, W.-F.; Prahl, S.A.; Welch, A.J. A review of the optical properties of biological tissues. IEEE J. Quantum Electron. 1990, 26, 2166–2185. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, F.H.; Jones, P.W.; McEwan, A.L. Near infrared spectroscopy for body fat sensing in neonates: Quantitative analysis by GAMOS simulations. Biomed. Eng. Online 2017, 16, 14. [Google Scholar] [CrossRef] [Green Version]
- Pogue, B.W.; Patterson, M.S. Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. J. Biomed. Opt. 2006, 11, 041102. [Google Scholar] [CrossRef]
- Mustafa, F.H.; Bek, E.J.; Huvanandana, J.; Jones, P.W.; Carberry, A.E.; Jeffery, H.E.; Jin, C.T.; McEwan, A.L. Length-free near infrared measurement of newborn malnutrition. Sci. Rep. 2016, 6, 36052. [Google Scholar] [CrossRef] [Green Version]
- Sandorfy, C.; Buchet, R.; Lachenal, G. Principles of molecular vibrations for near-infrared spectroscopy. In Near-Infrared Spectroscopy in Food Science and Technology; Ozaki, Y., McClure, W.F., Christy, A.A., Eds.; Wiley: Hoboken, NJ, USA, 2007; pp. 11–46. [Google Scholar]
- Siddiqui, S.A.; Dwivedi, A.; Pandey, A.; Singh, P.; Hasan, T.; Jain, S.; Misra, N. Molecular structure, vibrational spectra and potential energy distribution of colchicine using ab initio and density functional theory. J. Comput. Chem. Jpn. 2009, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Lister, T.; Wright, P.A.; Chappell, P.H. Optical properties of human skin. J. Biomed. Opt. 2012, 17, 090901. [Google Scholar] [CrossRef] [PubMed]
- Troy, T.L.; Thennadil, S.N. Optical properties of human skin in the near infrared wavelength range of 1000 to 2200 nm. J. Biomed. Opt. 2001, 6, 167–177. [Google Scholar] [CrossRef]
- Ozaki, Y. Near-Infrared Spectroscopy: Theory, Spectral Analysis, Instrumentation, and Applications; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef] [PubMed]
- Turzhitsky, V.; Qiu, L.; Itzkan, I.; Novikov, A.A.; Kotelev, M.S.; Getmanskiy, M.; Vinokurov, V.A.; Muradov, A.V.; Perelman, L.T. Spectroscopy of scattered light for the characterization of micro and nanoscale objects in biology and medicine. Appl. Spectrosc. 2014, 68, 133–154. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Hou, Y.; Qiu, C.; Yang, S.; Li, C.; Nie, L. Sparse representation classification of tobacco leaves using near-infrared spectroscopy and a deep learning algorithm. Anal. Lett. 2018, 51, 1029–1038. [Google Scholar] [CrossRef]
- Workman, J., Jr.; Weyer, L. Practical Guide and Spectral Atlas for Interpretive Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Hibbert, D.B. Vocabulary of concepts and terms in chemometrics (IUPAC Recommendations 2016). Pure Appl. Chem. 2016, 88, 407–443. [Google Scholar] [CrossRef]
- López, M.G.; García-González, A.S.; Franco-Robles, E. Carbohydrate analysis by NIRS-Chemometrics. Dev. Near-Infrared Spectrosc. 2017, 10, 67208. [Google Scholar]
- Gemperline, P. Practical Guide to Chemometrics; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Beć, K.B.; Huck, C.W. Breakthrough potential in near-infrared spectroscopy: Spectra simulation. A review of recent developments. Front. Chem. 2019, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Elmasry, G.; Kamruzzaman, M.; Sun, D.-W.; Allen, P. Principles and applications of hyperspectral imaging in quality evaluation of agro-food products: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 999–1023. [Google Scholar] [CrossRef]
- Mark, H.; Workman, J., Jr. Chemometrics in Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Brereton, R.G. Chemometrics: Data Analysis for the Laboratory and Chemical Plant; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- ElMasry, G.; Wang, N.; ElSayed, A.; Ngadi, M. Hyperspectral imaging for nondestructive determination of some quality attributes for strawberry. J. Food Eng. 2007, 81, 98–107. [Google Scholar] [CrossRef]
- Heise, H.; Winzen, R. Chemometrics in near-infrared spectroscopy. In Near-Infrared Spectroscopy: Principles, Instruments, Applications; Wiley: Hoboken, NJ, USA, 2001; pp. 125–162. [Google Scholar]
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, H.M. Near-Infrared Spectroscopy: Principles, Instruments, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Cen, H.; He, Y. Theory and application of near infrared reflectance spectroscopy in determination of food quality. Trends Food Sci. Technol. 2007, 18, 72–83. [Google Scholar] [CrossRef]
- Nilsen, H.; Esaiassen, M.; Heia, K.; Sigernes, F. Visible/near-infrared spectroscopy: A new tool for the evaluation of fish freshness? J. Food Sci. 2002, 67, 1821–1826. [Google Scholar] [CrossRef]
- Manoto, S.L.; El-Hussein, A.; Malabi, R.; Thobakgale, L.; Ombinda-Lemboumba, S.; Attia, Y.A.; Kasem, M.A.; Mthunzi-Kufa, P. Exploring optical spectroscopic techniques and nanomaterials for virus detection. Saudi J. Biol. Sci. 2021, 28, 78–89. [Google Scholar] [CrossRef]
- Miller, J.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry; Pearson education: London, UK, 2018. [Google Scholar]
- Adams, M.J. Chemometrics in Analytical Spectroscopy; Royal Society of Chemistry: London, UK, 2004. [Google Scholar]
- Bec, K.B.; Grabska, J.; Huck, C.W. NIR spectroscopy of natural medicines supported by novel instrumentation and methods for data analysis and interpretation. J. Pharm. Biomed. Anal. 2021, 193, 113686. [Google Scholar] [CrossRef]
- Bahmani, M.K.; Khosravi, A.; Miri, R.; Iwabu, Y.; Ikuta, K.; Sakudo, A. Spectroscopic characterization of human immunodeficiency virus type-1-infected plasma by principal component analysis and soft independent modeling of class analogy of visible and near-infrared spectra. Mol. Med. Rep. 2009, 2, 805–809. [Google Scholar]
- Ellwanger, J.H.; de Lima Kaminski, V.; Chies, J.A. Emerging infectious disease prevention: Where should we invest our resources and efforts? J. Infect. Public Health 2019, 12, 313–316. [Google Scholar] [CrossRef]
- Fernandes, J.N.; Dos Santos, L.M.; Chouin-Carneiro, T.; Pavan, M.G.; Garcia, G.A.; David, M.R.; Beier, J.C.; Dowell, F.E.; Maciel-de-Freitas, R.; Sikulu-Lord, M.T. Rapid, noninvasive detection of Zika virus in Aedes aegypti mosquitoes by near-infrared spectroscopy. Sci. Adv. 2018, 4, eaat0496. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.M.; Mutsaers, M.; Garcia, G.A.; David, M.R.; Pavan, M.G.; Petersen, M.T.; Corrêa-Antônio, J.; Couto-Lima, D.; Maes, L.; Dowell, F. High throughput estimates of Wolbachia, Zika and chikungunya infection in Aedes aegypti by near-infrared spectroscopy to improve arbovirus surveillance. Commun. Biol. 2021, 4, 67. [Google Scholar] [CrossRef]
- Firdous, S.; Ahmed, M.; Rehman, A.; Nawaz, M.; Anwar, S.; Murtaza, S. Transmission spectroscopy of dengue viral infection. Laser Phys. Lett. 2012, 9, 317. [Google Scholar] [CrossRef]
- Sikulu-Lord, M.T.; Maia, M.F.; Milali, M.P.; Henry, M.; Mkandawile, G.; Kho, E.A.; Wirtz, R.A.; Hugo, L.E.; Dowell, F.E.; Devine, G.J. Rapid and non-destructive detection and identification of two strains of Wolbachia in Aedes aegypti by near-infrared spectroscopy. PLoS Negl. Trop. Dis. 2016, 10, e0004759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.D.; Shin, K.; Ho, D.-S.; Kim, B.-M. Evaluation of Chip LED Sensor Module for Fat Thickness Measurement using Tissue Phantoms. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; IEEE: Piscataway Township, NJ, USA, 2006; pp. 5993–5996. [Google Scholar]
- Varghese, J.M.; Megalingam, R.K. Study and analysis of embedded system based indoor navigation on multiple platforms. In Proceedings of the 2016 International Conference on Communication and Signal Processing (ICCSP), Melmaruvathur, Tamilnadu, India, 6–8 April 2016; IEEE: Piscataway Township, NJ, USA, 2016; pp. 1203–1207. [Google Scholar]
- Kitane, D.L.; Loukman, S.; Marchoudi, N.; Fernandez-Galiana, A.; El Ansari, F.Z.; Jouali, F.; Badir, J.; Gala, J.-L.; Bertsimas, D.; Azami, N. A simple and fast spectroscopy-based technique for Covid-19 diagnosis. Sci. Rep. 2021, 11, 16740. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.R.; Kochan, K.; Bedolla, D.E.; Salazar-Quiroz, N.; Grimley, S.L.; Perez-Guaita, D.; Baker, M.J.; Vongsvivut, J.; Tobin, M.J.; Bambery, K.R. Infrared based saliva screening test for COVID-19. Angew. Chem. 2021, 133, 17239–17244. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Leal, L.B.; Marcarini, W.D.; Pimentel, R.L.; Muller, M.; Vassallo, P.F.; Campos, L.C.G.; Dos Santos, L.; Luiz, W.B.; Mill, J.G. Rapid diagnosis of COVID-19 using FT-IR ATR spectroscopy and machine learning. Sci. Rep. 2021, 11, 15409. [Google Scholar] [CrossRef]
- Minamikawa, T.; Koma, T.; Suzuki, A.; Mizuno, T.; Nagamatsu, K.; Arimochi, H.; Tsuchiya, K.; Matsuoka, K.; Yasui, T.; Yasutomo, K. Quantitative evaluation of SARS-CoV-2 inactivation using a deep ultraviolet light-emitting diode. Sci. Rep. 2021, 11, 5070. [Google Scholar] [CrossRef]
- Barauna, V.G.; Singh, M.N.; Barbosa, L.L.; Marcarini, W.D.; Vassallo, P.F.; Mill, J.G.; Ribeiro-Rodrigues, R.; Campos, L.C.; Warnke, P.H.; Martin, F.L. Ultrarapid on-site detection of SARS-CoV-2 infection using simple ATR-FTIR spectroscopy and an analysis algorithm: High sensitivity and specificity. Anal. Chem. 2021, 93, 2950–2958. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raypah, M.E.; Faris, A.N.; Mohd Azlan, M.; Yusof, N.Y.; Suhailin, F.H.; Shueb, R.H.; Ismail, I.; Mustafa, F.H. Near-Infrared Spectroscopy as a Potential COVID-19 Early Detection Method: A Review and Future Perspective. Sensors 2022, 22, 4391. https://doi.org/10.3390/s22124391
Raypah ME, Faris AN, Mohd Azlan M, Yusof NY, Suhailin FH, Shueb RH, Ismail I, Mustafa FH. Near-Infrared Spectroscopy as a Potential COVID-19 Early Detection Method: A Review and Future Perspective. Sensors. 2022; 22(12):4391. https://doi.org/10.3390/s22124391
Chicago/Turabian StyleRaypah, Muna E., Asma Nadia Faris, Mawaddah Mohd Azlan, Nik Yusnoraini Yusof, Fariza Hanim Suhailin, Rafidah Hanim Shueb, Irneza Ismail, and Fatin Hamimi Mustafa. 2022. "Near-Infrared Spectroscopy as a Potential COVID-19 Early Detection Method: A Review and Future Perspective" Sensors 22, no. 12: 4391. https://doi.org/10.3390/s22124391






