Feasibility Analysis of ECG-Based pH Estimation for Asphyxia Detection in Neonates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset and Selection Criteria
2.2. pH Threshold in Neonates
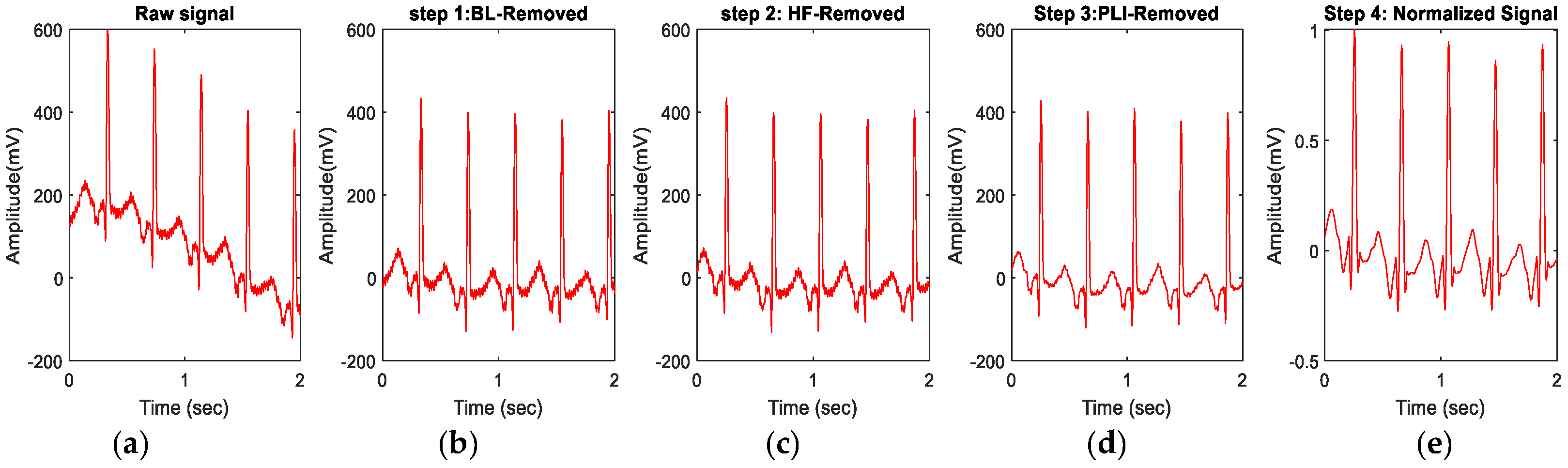
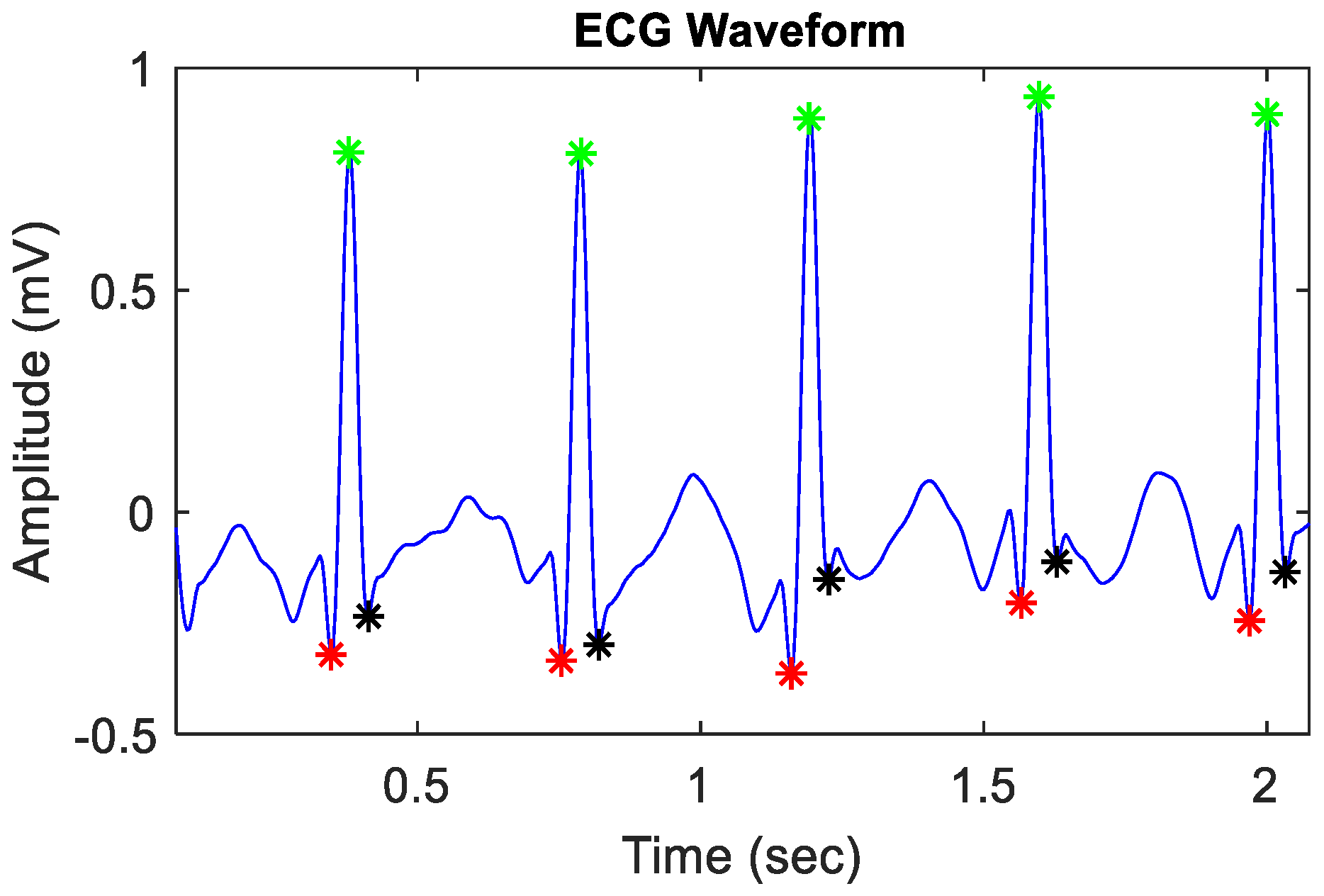
2.3. Signal Preprocessing and Extraction of ECG Features
2.4. Statistical Analysis of ECG Features
2.4.1. Descriptive Analysis
2.4.2. Statistical Analysis
3. Results and Discussion
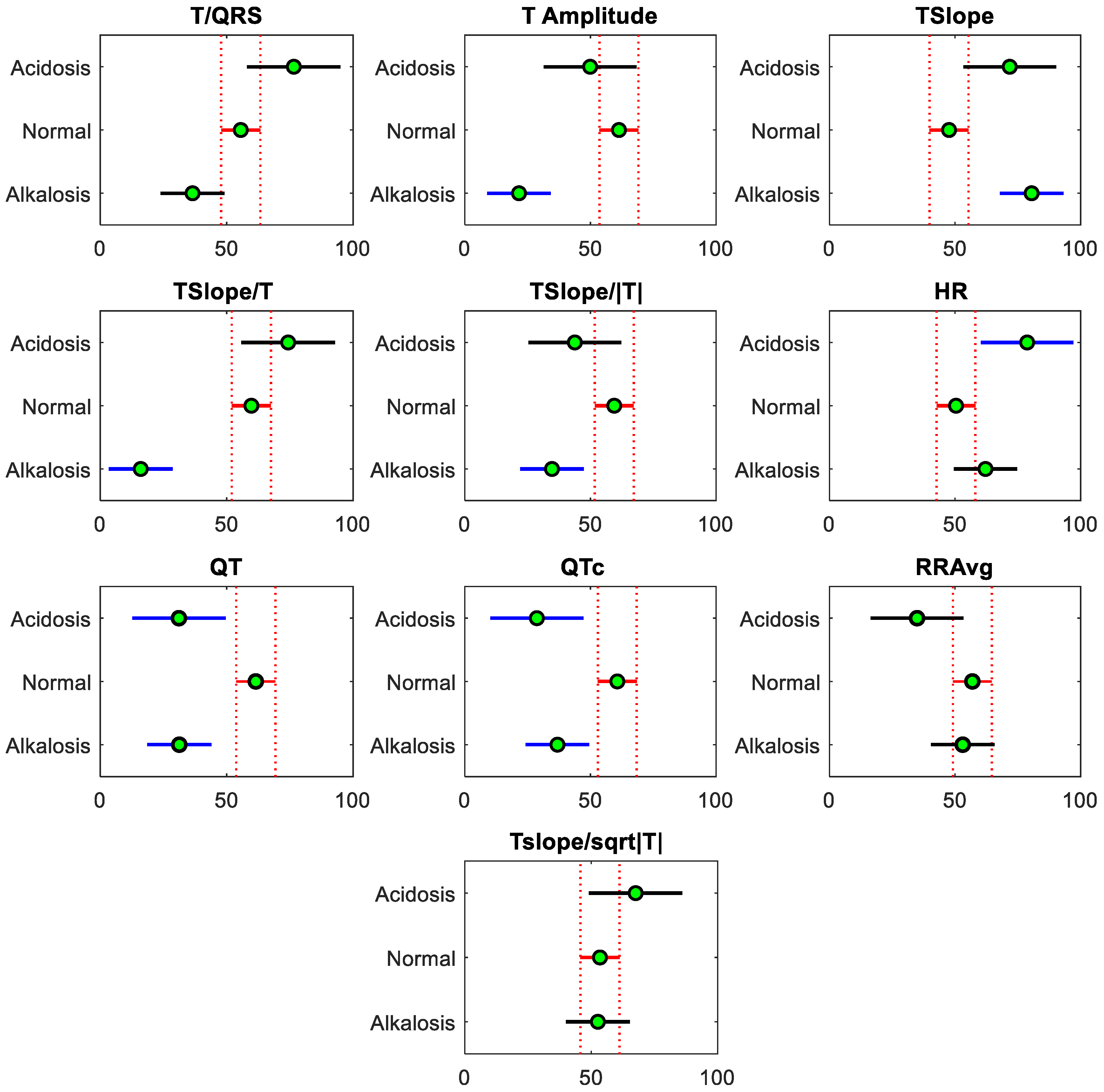
3.1. Results from the Descriptive Analysis
3.2. Results from the Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNICEF. “Newborn Mortality”, UNICEF Data: Monitoring the Situation of Children and Women. 2024. Available online: https://data.unicef.org/topic/child-survival/neonatal-mortality/ (accessed on 26 March 2024).
- World Health Organization. Guidelines on basic newborn resuscitation. In WHO Library Cataloguing-in-Publication Data; WHO Press: Geneva, Switzerland, 2012; Available online: www.who.int/publications/i/item/9789241503693 (accessed on 4 December 2023).
- Lawn, J.E.; Cousens, S.; Zupan, J. 4 million neonatal deaths: When? Where? Why? Lancet 2005, 365, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Abdo, R.A.; Halil, H.M.; Kebede, B.A.; Anshebo, A.A.; Gejo, N.G. Prevalence and contributing factors of birth asphyxia among the neonates delivered at Nigist Eleni Mohammed memorial teaching hospital, Southern Ethiopia: A cross-sectional study. BMC Pregnancy Childbirth 2019, 19, 536. [Google Scholar] [CrossRef] [PubMed]
- Workineh, Y.; Semachew, A.; Ayalew, E.; Animaw, W.; Tirfie, M.; Birhanu, M. Prevalence of perinatal asphyxia in East and Central Africa: Systematic review and meta-analysis. Heliyon 2020, 6, e03793. [Google Scholar] [CrossRef] [PubMed]
- Moshiro, R.; Mdoe, P.; Perlman, J.M. A Global View of Neonatal Asphyxia and Resuscitation. Front. Pediatr. 2019, 7, 489. [Google Scholar] [CrossRef]
- LaRosa, D.A.; Ellery, S.J.; Walker, D.W.; Dickinson, H. Understanding the Full Spectrum of Organ Injury Following Intrapartum Asphyxia. Front. Pediatr. 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.M.; Amin, B.; O’halloran, M.; Elahi, A. Development and Characterization of Interstitial-Fluid-Mimicking Solutions for Pre-Clinical Assessment of Hypoxia. Diagnostics 2023, 13, 3125. [Google Scholar] [CrossRef]
- Satriano, A.; Pluchinotta, F.; Gazzolo, F.; Serpero, L.; Gazzolo, D. The potentials and limitations of neuro-biomarkers as predictors of outcome in neonates with birth asphyxia. Early Hum. Dev. 2017, 105, 63–67. [Google Scholar] [CrossRef]
- Wosenu, L.; Worku, A.G.; Teshome, D.F.; Gelagay, A.A. Determinants of birth asphyxia among live birth newborns in University of Gondar referral hospital, northwest Ethiopia: A case-control study. PLoS ONE 2018, 13, e0203763. [Google Scholar] [CrossRef] [PubMed]
- Mannan, M.A.; Dey, S.; Karim, S.M.R.; Iqbal, S.; Yasmin, S.; Ferdous, N. Neonatal arterial blood gases & immediate outcome following perinatal asphyxia. Bangladesh J. Med. Sci. 2019, 18, 238–243. [Google Scholar] [CrossRef]
- Cavaliere, T.A.; Arias-Oliveras, A. Neonatal Blood Gas Interpretation. Newborn Infant Nurs. Rev. 2016, 16, 119–121. [Google Scholar] [CrossRef]
- Koehn, A.R. The Delicate Balance: Managing Oxygen Treatment in Neonates. Adv. Neonatal Care 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Racinet, C.; Ouellet, P.; Muraskas, J.; Daboval, T. Neonatal cord blood eucapnic pH: A potential biomarker predicting the need for transfer to the NICU. Arch. Pédiatrie 2020, 27, 6–11. [Google Scholar] [CrossRef]
- Morton, S.; Avery, P.; Payne, J.; Omeara, M. Arterial Blood Gases and Arterial Lines in the Prehospital Setting: A Systematic Literature Review and Survey of Current United Kingdom Helicopter Emergency Medical Services. Air Med. J. 2022, 41, 201–208. [Google Scholar] [CrossRef]
- Tan, R.N.G.B.; Mulder, E.E.M.; Lopriore, E.; Pas, A.B.T. Monitoring Oxygenation and Gas Exchange in Neonatal Intensive Care Units: Current Practice in the Netherlands. Front. Pediatr. 2015, 3, 94. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Morley, C. Monitoring oxygen saturation and heart rate in the early neonatal period. Semin. Fetal Neonatal Med. 2010, 15, 203–207. [Google Scholar] [CrossRef]
- Hussain, N.M.; Amin, B.; O’Halloran, M.; Elahi, A. Dielectric Characterization of Interstitial Fluid Phantoms for Hypoxia Monitoring at Microwave Frequencies. In Proceedings of the 2023 Photonics & Electromagnetics Research Symposium (PIERS), Prague, Czech Republic, 3–6 July 2023; pp. 1732–1737. [Google Scholar] [CrossRef]
- Plana, M.N.; Zamora, J.; Suresh, G.; Fernandez-Pineda, L.; Thangaratinam, S.; Ewer, A.K. Pulse oximetry screening for critical congenital heart defects. Cochrane Database Syst. Rev. 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Poets, C.F. Noninvasive Monitoring and Assessment of Oxygenation in Infants. Clin. Perinatol. 2019, 46, 417–433. [Google Scholar] [CrossRef]
- Sola, A.; Golombek, S.G. Early Detection with Pulse Oximetry of Hypoxemic Neonatal Conditions. Development of the IX Clinical Consensus Statement of the Ibero-American Society of Neonatology (SIBEN). Int. J. Neonatal Screen. 2018, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.M.; O’Halloran, M.; McDermott, B.; Elahi, M.A. Fetal Monitoring Technologies for the Detection of Intrapartum Hypoxia—Challenges and Opportunities. Biomed. Phys. Eng. Express 2024, 10, 22002. [Google Scholar] [CrossRef]
- Shiao, S.Y.P.K.; Ou, C.N. Validation of oxygen saturation monitoring in neonates. Am. J. Crit. Care 2007, 16, 168–178. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D. The Effects of Sodium Phosphocreatine on ECG Abnormalities and Cardiac Markers of Neonatal Asphyxia Based on Big Data Statistics. J. Phys. Conf. Ser. 2020, 1574, 012073. [Google Scholar] [CrossRef]
- Yellanthoor, R.B.; Dineshkumar, R. Electrocardiographic and echocardiographic findings in asphyxiated neonates. Iran. Heart J. 2021, 22, 51–57. [Google Scholar]
- Koether, K.; Ulian, C.M.; Lourenço, M.L.; Gonçalves, R.S.; Sudano, M.J.; Cruz, R.K.; da Silva, B.N.; Alfonso, A.; Chiacchio, S.B. The normal electrocardiograms in the conscious newborn lambs in neonatal period and its progression. BMC Physiol. 2016, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.E. Neonatal arrhythmias: Diagnosis, treatment, and clinical outcome. Korean J. Pediatr. 2017, 60, 344–352. [Google Scholar] [CrossRef]
- Pærregaard, M.M.; Hvidemose, S.O.; Pihl, C.; Sillesen, A.S.; Parvin, S.B.; Pietersen, A.; Iversen, K.K.; Bundgaard, H.; Christensen, A.H. Defining the normal QT interval in newborns: The natural history and reference values for the first 4 weeks of life. EP Eur. 2021, 23, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Amoozgar, H.; Barekati, M.; Farhani, N.; Pishva, N. Effect of Birth Asphyxia on P Wave Dispersion. Indian J. Pediatr. 2014, 81, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Hermans, B.J.M.; Vink, A.S.; Bennis, F.C.; Filippini, L.H.; Meijborg, V.M.F.; Wilde, A.A.M.; Pison, L.; Postema, P.G.; Delhaas, T. The development and validation of an easy to use automatic QT-interval algorithm. PLoS ONE 2017, 12, e0184352. [Google Scholar] [CrossRef]
- Zhao, X.-B.; Wang, H.; Wu, H.-B.; Gong, X. Correlation of QT dispersion with serum potassium or blood sodium levels post-neonatal asphyxia. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7448–7452. [Google Scholar] [CrossRef]
- Rosén, K.G.; Amer-Wåhlin, I.; Luzietti, R.; Norén, H. Fetal ECG waveform analysis. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 485–514. [Google Scholar] [CrossRef]
- Oudijk, M.A.; Kwee, A.; Visser, G.H.; Blad, S.; Meijboom, E.J.; Rosén, K.G. The effects of intrapartum hypoxia on the fetal OT interval. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 656–660. [Google Scholar] [CrossRef]
- Wibbens, B.; Bennet, L.; Westgate, J.A.; De Haan, H.H.; Wassink, G.; Gunn, A.J. Preexisting hypoxia is associated with a delayed but more sustained rise in T/QRS ratio during prolonged umbilical cord occlusion in near-term fetal sheep. Am. J. Physiol. Integr. Comp. Physiol. 2007, 293, R1287–R1293. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Hermans, B.J.; Andriessen, P.; Niemarkt, H.J.; Jellema, R.K.; Ophelders, D.R.; Vullings, R.; Wolfs, T.G.; Kramer, B.W.; Delhaas, T. Comparison of ECG-based physiological markers for hypoxia in a preterm ovine model. Pediatr. Res. 2016, 79, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Martinek, R.; Kahankova, R.; Martin, B.; Nedoma, J.; Fajkus, M. A novel modular fetal ECG STAN and HRV analysis: Towards robust hypoxia detection. Technol. Health Care 2019, 27, 257–287. [Google Scholar] [CrossRef]
- Luttkus, A.K.; Norén, H.; Stupin, J.H.; Blad, S.; Arulkumaran, S.; Erkkola, R.; Hagberg, H.; Lenstrup, C.; Visser, G.H.; Tamazian, O.; et al. Fetal scalp pH and ST analysis of the fetal ECG as an adjunct to CTG. A multi-center, observational study. JPME 2004, 32, 486–494. [Google Scholar] [CrossRef]
- Goulding, R.M.; Stevenson, N.J.; Murray, D.M.; Livingstone, V.; Filan, P.M.; Boylan, G.B. Heart rate variability in hypoxic ischemic encephalopathy: Correlation with EEG grade and 2-y neurodevelopmental outcome. Pediatr. Res. 2015, 77, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Gholinezhadasnefestani, S.; Temko, A.; Stevenson, N.; Boylan, G.; Lightbody, G.; Marnane, W. Assessment of quality of ECG for accurate estimation of Heart Rate Variability in newborns. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 5863–5866. [Google Scholar] [CrossRef]
- Armstrong, L.; Stenson, B.J. Use of umbilical cord blood gas analysis in the assessment of the newborn. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Thorborg, P.A.J. Blood Gas Analysis. In Mechanical Ventilation: Clinical Applications and Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 457–470. [Google Scholar] [CrossRef]
- Tan, S.; Campbell, M. Acid–base physiology and blood gas interpretation in the neonate. Paediatr. Child Health 2008, 18, 172–177. [Google Scholar] [CrossRef]
- Davison, J.E. Ammonia, lactate and blood gases—A user’s guide. Paediatr. Child Health 2019, 29, 142–145. [Google Scholar] [CrossRef]
- Darmawahyuni, A.; Nurmaini, S.; Yuwandini, M.; Rachmatullah, M.N.; Firdaus, F.; Tutuko, B. Congestive heart failure waveform classification based on short time-step analysis with recurrent network. Inform. Med. Unlocked 2020, 21, 100441. [Google Scholar] [CrossRef]
- Meidani, M.; Mashoufi, B. Introducing new algorithms for realising an FIR filter with less hardware in order to eliminate power line interference from the ECG signal. IET Signal Process. 2016, 10, 709–716. [Google Scholar] [CrossRef]
- Lastre-Domínguez, C.; Shmaliy, Y.S.; Ibarra-Manzano, O.; Munoz-Minjares, J.; Morales-Mendoza, L.J. ECG Signal Denoising and Features Extraction Using Unbiased FIR Smoothing. BioMed Res. Int. 2019, 2019, 2608547. [Google Scholar] [CrossRef] [PubMed]
- Kawala-Sterniuk, A.; Podpora, M.; Pelc, M.; Blaszczyszyn, M.; Gorzelanczyk, E.J.; Martinek, R.; Ozana, S. Comparison of Smoothing Filters in Analysis of EEG Data for the Medical Diagnostics Purposes. Sensors 2020, 20, 807. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.A.; Colletta, G.D.; Ferreira, L.H.C.; Dutra, O.O. Utilization of Savitzky-Golay filter for power line interference cancellation in an embedded electrocardiographic monitoring platform. In Proceedings of the 2017 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rochester, MN, USA, 7–10 May 2017; pp. 227–232. [Google Scholar] [CrossRef]
- Hamilton, P.S.; Tompkins, W.J. Quantitative investigation of QRS detection rules using the MIT/BIH arrhythmia database. IEEE Trans. Biomed. Eng. 1986, 33, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.; Mexicano, A.; Bila, J.; Cervantes, S.; Ponce, R. Feature Extraction of Electrocardiogram Signals by Applying Adaptive Threshold and Principal Component Analysis. J. Appl. Res. Technol. 2015, 13, 261–269. [Google Scholar] [CrossRef]
- Kalpana, V.; Hamde, S.T.; Waghmare, L.M. ECG feature extraction using principal component analysis for studying the effect of diabetes. J. Med. Eng. Technol. 2013, 37, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Castells, F.; Laguna, P.; Sörnmo, L.; Bollmann, A.; Roig, J.M. Principal Component Analysis in ECG Signal Processing. EURASIP J. Adv. Signal Process. 2007, 2007, 074580. [Google Scholar] [CrossRef]
- Szymankiewicz, M.; Matuszczak-Wleklak, M.; Hodgman, J.E.; Gadzinowski, J. Usefulness of Cardiac Troponin T and Echocardiography in the Diagnosis of Hypoxic Myocardial Injury of Full-Term Neonates. Biol. Neonate 2005, 88, 19–23. [Google Scholar] [CrossRef]
- Dhayabarani, R.; Balachandar, P.; Arunkumar, R.; Elakkiyaselvan, M. Design of FIR Filter for Reduction of Power Line Interference from ECG Signal. In Proceedings of the 2018 Second International Conference on Inventive Communication and Computational Technologies (ICICCT), Coimbatore, India, 20–21 April 2018; pp. 1205–1208. [Google Scholar] [CrossRef]
- Fariha, M.A.Z.; Ikeura, R.; Hayakawa, S.; Tsutsumi, S. Analysis of Pan-Tompkins Algorithm Performance with Noisy ECG Signals. J. Phys. Conf. Ser. 2020, 1532, 012022. [Google Scholar] [CrossRef]
- Sommo, L.; Laguna, P. Chapter 7: ECG Signal Processing. In Bioelectrical Signal Processing in Cardiac and Neurological Applications; Academic Press: Cambridge, MA, USA, 2005; pp. 453–566. [Google Scholar] [CrossRef]
- Al Mahamdy, M.; Riley, H.B. Performance Study of Different Denoising Methods for ECG Signals. Procedia Comput. Sci. 2014, 37, 325–332. [Google Scholar] [CrossRef]
- Kher, R. Signal Processing Techniques for Removing Noise from ECG Signals. J. Biomed. Eng. Res. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Krupa, A.J.D.; Dhanalakshmi, S.; Kumar, R. Joint time-frequency analysis and non-linear estimation for fetal ECG extraction. Biomed. Signal Process. Control. 2022, 75, 103569. [Google Scholar] [CrossRef]
- Rahul, J.; Sora, M.; Sharma, L.D. A novel and lightweight P, QRS, and T peaks detector using adaptive thresholding and template waveform. Comput. Biol. Med. 2021, 132, 104307. [Google Scholar] [CrossRef] [PubMed]
- Borowska, M.; Brzozowska, E.; Kuć, P.; Oczeretko, E.; Mosdorf, R.; Laudański, P. Identification of preterm birth based on RQA analysis of electrohysterograms. Comput. Methods Programs Biomed. 2018, 153, 227–236. [Google Scholar] [CrossRef]
- Chaitanya, M.K.; Sharma, L.D. Electrocardiogram signal filtering using circulant singular spectrum analysis and cascaded Savitzky-Golay filter. Biomed. Signal Process. Control. 2022, 75, 103583. [Google Scholar] [CrossRef]
- Bazett, H.C. An analysis of the time-relations of electrocardiograms. Ann. Noninvasive Electrocardiol. 1997, 2, 177–194. [Google Scholar] [CrossRef]
- Glass, G.F.; Sudhir, A.; Anil, A.; Pandit, K. The ECG and Metabolic Abnormalities. In Electrocardiogram in Clinical Medicine; Wiley: Hoboken, NJ, USA, 2021; pp. 307–313. [Google Scholar] [CrossRef]
- Brown, B.; Eilerman, B. Understanding Blood Gas Interpretation. Newborn Infant Nurs. Rev. 2006, 6, 57–62. [Google Scholar] [CrossRef]
- Varsha, A.V.; George, G.; Sahajanandan, R. Descriptive Statistics and Normality Tests for Statistical Data Abstract. Ann. Card. Anaesth. 2017, 20, 456–458. [Google Scholar] [CrossRef]
- Dinno, A. Nonparametric Pairwise Multiple Comparisons in Independent Groups using Dunn’s Test. Stata J. Promot. Commun. Stat. Stata 2015, 15, 292–300. [Google Scholar] [CrossRef]
- Divine, G.W.; Norton, H.J.; Barón, A.E.; Juarez-Colunga, E. The Wilcoxon–Mann–Whitney Procedure Fails as a Test of Medians. Am. Stat. 2018, 72, 278–286. [Google Scholar] [CrossRef]
- Miziolek, B.; Bergler-Czop, B.; Kucharz, E.; Kotyla, P.; Kopec-Medrek, M.; Widuchowska, M.; Sienczyk, M.; Brzezinska-Wcislo, L. Significance of the angiotensin I/angiotensin II/angiotensin-(1-7) axis in the pathogenesis of systemic sclerosis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.-X. New applications of electrical impedance of human blood. J. Med. Eng. Technol. 1996, 20, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Amer-Wåhlin, I.; Bördahl, P.; Eikeland, T.; Hellsten, C.; Norén, H.; Sörnes, T.; Rosén, K.G. ST analysis of the fetal electrocardiogram during labor: Nordic observational multicenter study. J. Matern. Neonatal Med. 2002, 12, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Javorka, K.; Lehotska, Z.; Kozar, M.; Uhrikova, Z.; Kolarovszki, B.; Zibolen, M. Heart Rate Variability in Newborns. Physiol. Res. 2017, 66, S203–S214. [Google Scholar] [CrossRef] [PubMed]
- Sbrollini, A.; Mancinelli, M.; Marcantoni, I.; Morettini, M.; Carnielli, V.P.; Burattini, L. Adaptive bradycardia assessment in preterm infants. Biomed. Signal Process. Control. 2021, 68, 102816. [Google Scholar] [CrossRef]
- Bersani, I.; Piersigilli, F.; Gazzolo, D.; Campi, F.; Savarese, I.; Dotta, A.; Tamborrino, P.P.; Auriti, C.; Di Mambro, C. Correction to: Heart rate variability as possible marker of brain damage in neonates with hypoxic ischemic encephalopathy: A systematic review. Eur. J. Pediatr. 2021, 180, 1347. [Google Scholar] [CrossRef]
- Yap, C.Y.; Aw, T.C. Arterial Blood Gases. Proc. Singap. Healthc. 2011, 20, 227–235. [Google Scholar] [CrossRef]



| ECG Features | Chi-Square Value | p-Value |
|---|---|---|
| T/QRS | 9.85 | 0.0073 |
| T Amplitude | 21.86 | 0.0000 |
| T slope | 17.78 | 0.0001 |
| Tslope/T | 30.14 | 0.0000 |
| Tslope/|T| | 9.56 | 0.0084 |
| HR | 7.80 | 0.0202 |
| QT | 18.02 | 0.0001 |
| QTc | 14.44 | 0.0007 |
| RRAvg | 4.05 | 0.1319 |
| Tslope/√|T| | 1.72 | 0.4242 |
| ECG Features | Groups | Mean Ranks | Interval | p-Value | ||
|---|---|---|---|---|---|---|
| T/QRS | 1 * | 3 *** | 8.8955 | 40.0556 | 71.2156 | 0.0064 |
| T Amplitude | 2 ** | 3 | 19.3682 | 39.7869 | 60.2056 | 0.0000 |
| Tslope | 2 | 3 | −53.3043 | −32.8855 | −12.4668 | 0.0004 |
| Tslope/T | 1 | 3 | 27.1733 | 58.3333 | 89.4934 | 0.0000 |
| 2 | 3 | 23.3524 | 43.7711 | 64.1898 | 0.0000 | |
| Tslope/|T| | 2 | 3 | 4.3757 | 24.7944 | 45.2131 | 0.0112 |
| HR | 1 | 2 | 2.1611 | 28.3186 | 54.4761 | 0.0289 |
| QT | 1 | 2 | −56.6424 | −30.4070 | −4.1715 | 0.0169 |
| 2 | 3 | 9.8567 | 30.2681 | 50.6795 | 0.0012 | |
| QTc | 1 | 2 | −58.2769 | −32.0321 | −5.7873 | 0.0107 |
| 2 | 3 | 3.4051 | 23.8238 | 44.2425 | 0.0159 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, N.M.; Amin, B.; McDermott, B.J.; Dunne, E.; O’Halloran, M.; Elahi, A. Feasibility Analysis of ECG-Based pH Estimation for Asphyxia Detection in Neonates. Sensors 2024, 24, 3357. https://doi.org/10.3390/s24113357
Hussain NM, Amin B, McDermott BJ, Dunne E, O’Halloran M, Elahi A. Feasibility Analysis of ECG-Based pH Estimation for Asphyxia Detection in Neonates. Sensors. 2024; 24(11):3357. https://doi.org/10.3390/s24113357
Chicago/Turabian StyleHussain, Nadia Muhammad, Bilal Amin, Barry James McDermott, Eoghan Dunne, Martin O’Halloran, and Adnan Elahi. 2024. "Feasibility Analysis of ECG-Based pH Estimation for Asphyxia Detection in Neonates" Sensors 24, no. 11: 3357. https://doi.org/10.3390/s24113357







