Open-Path Cavity Ring-Down Spectroscopy for Simultaneous Detection of Hydrogen Chloride and Particles in Cleanroom Environment
Abstract
:1. Introduction
2. Overview of Cavity Ring-Down Spectroscopy (CRDS)
3. Experimental Methods
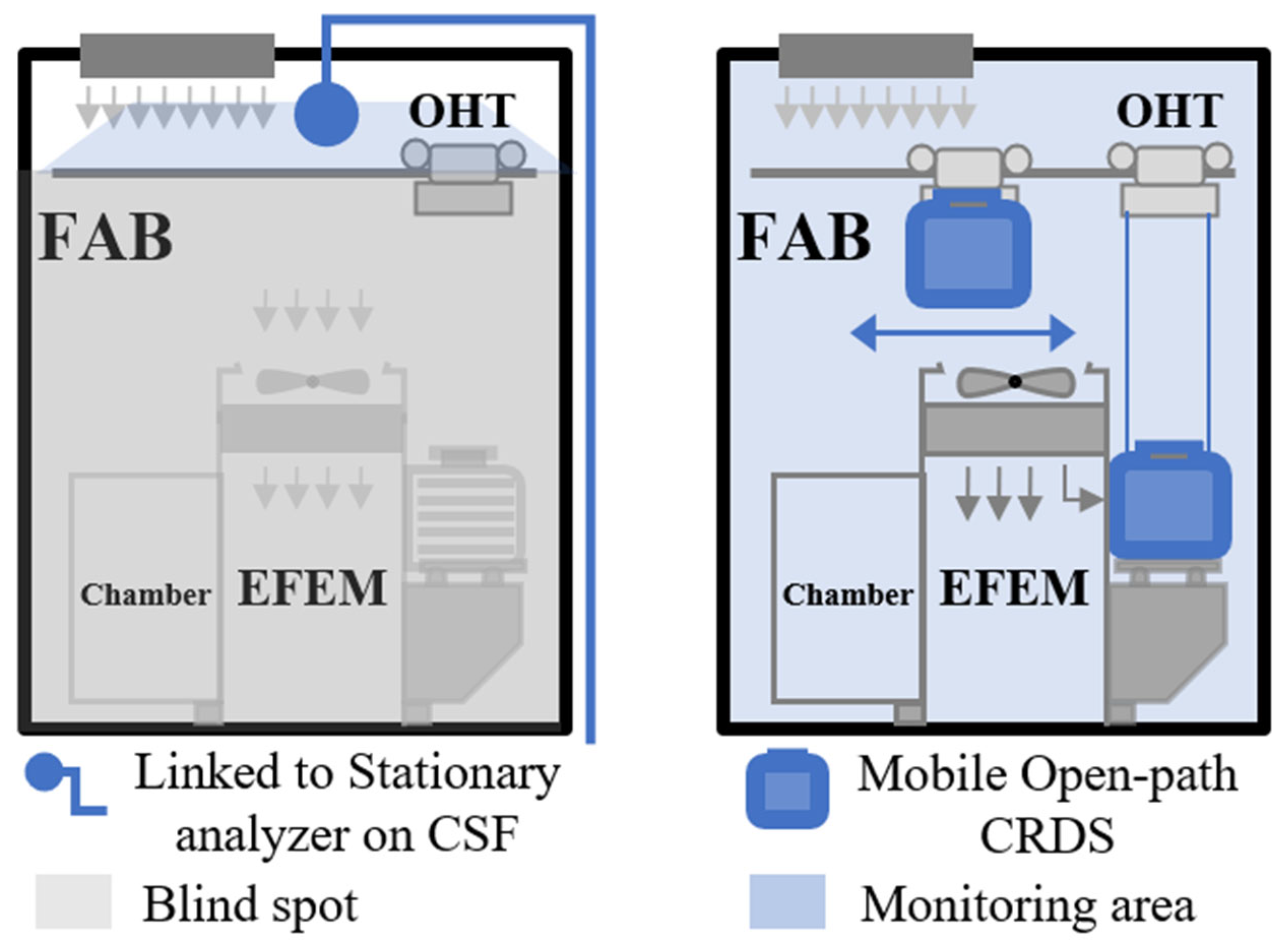
3.1. AMC and PM Testbed Chamber
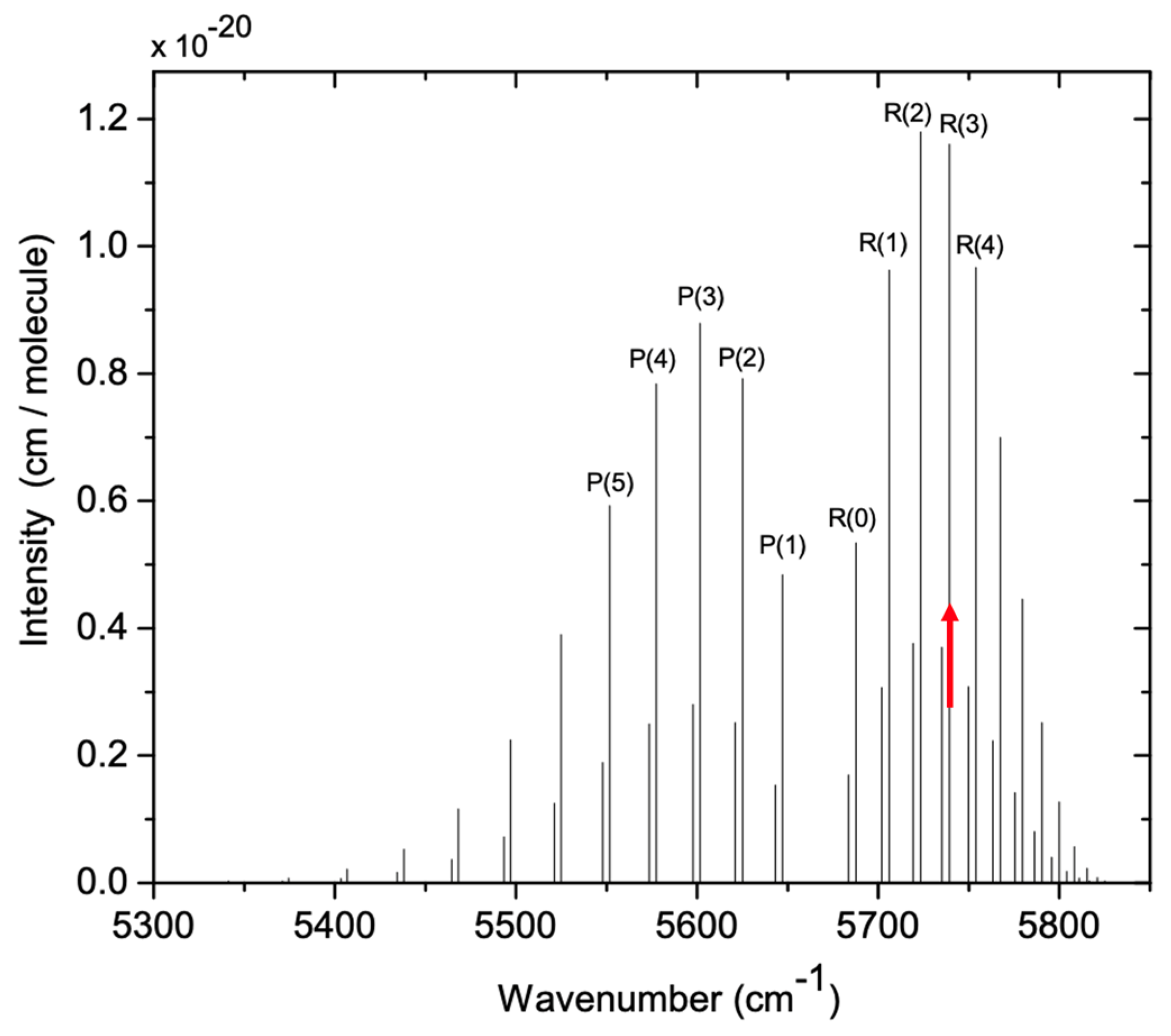
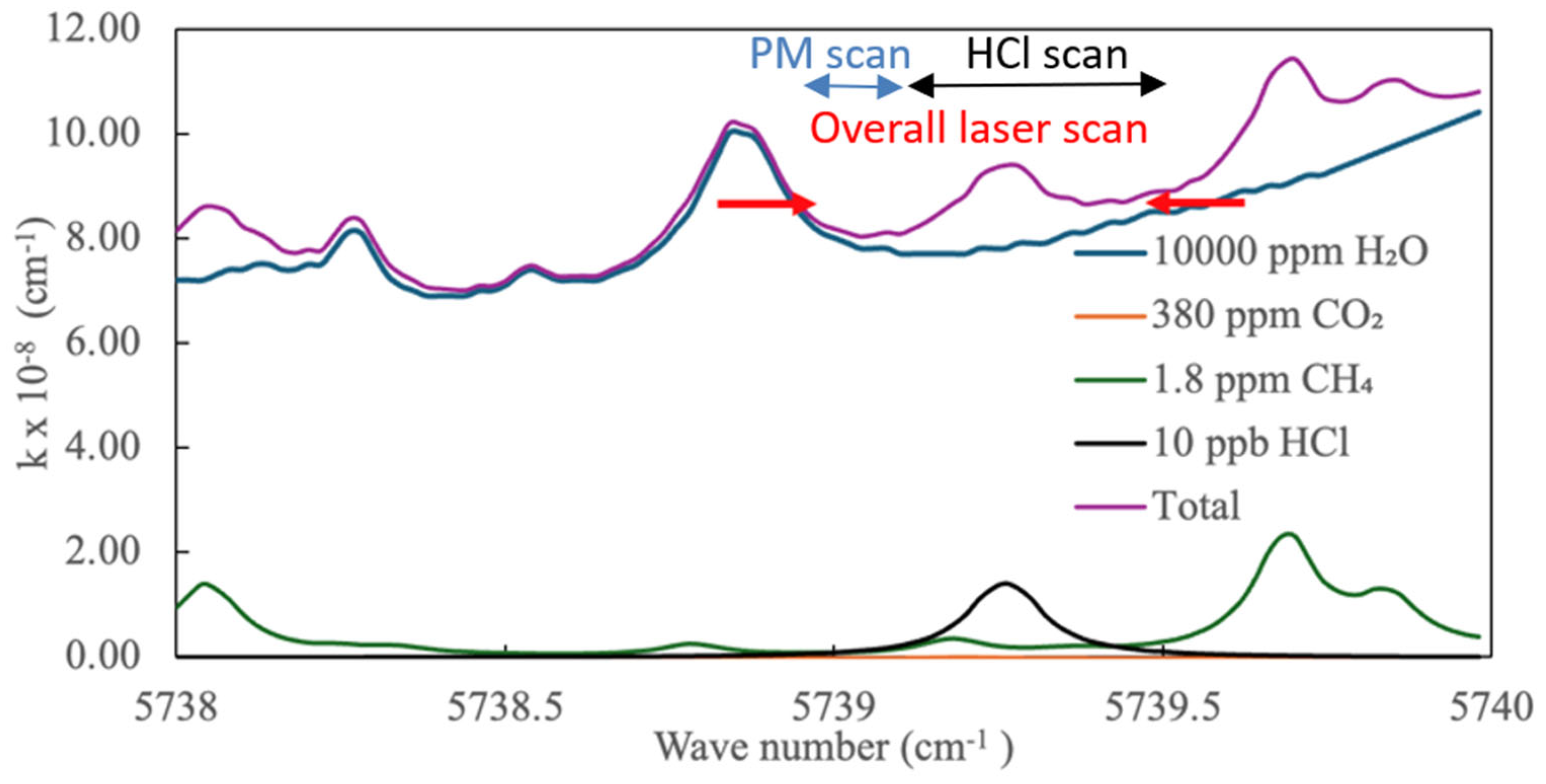
3.2. Open-Path CRDS Instrument for HCl and PM Detection
4. Results and Discussion
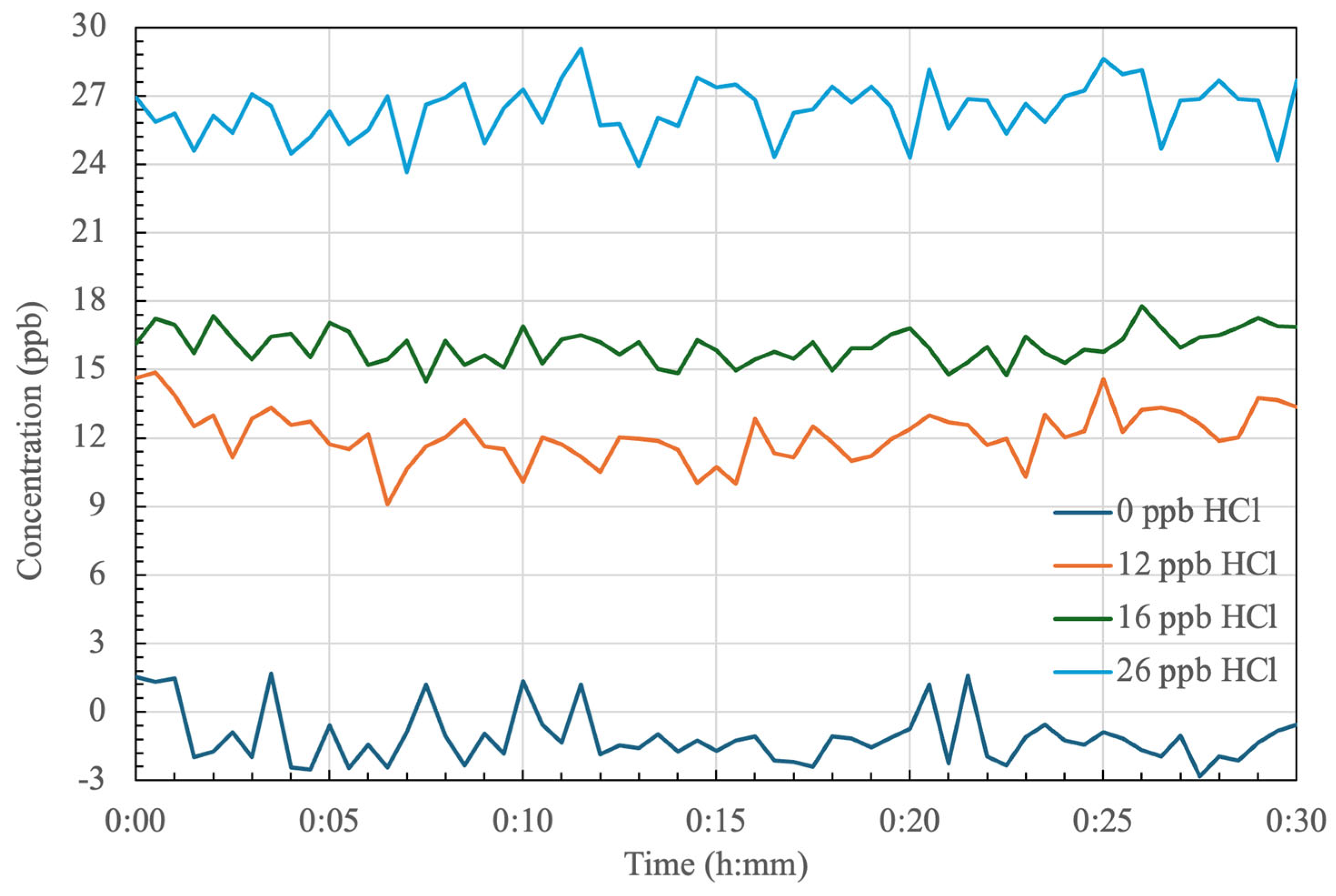
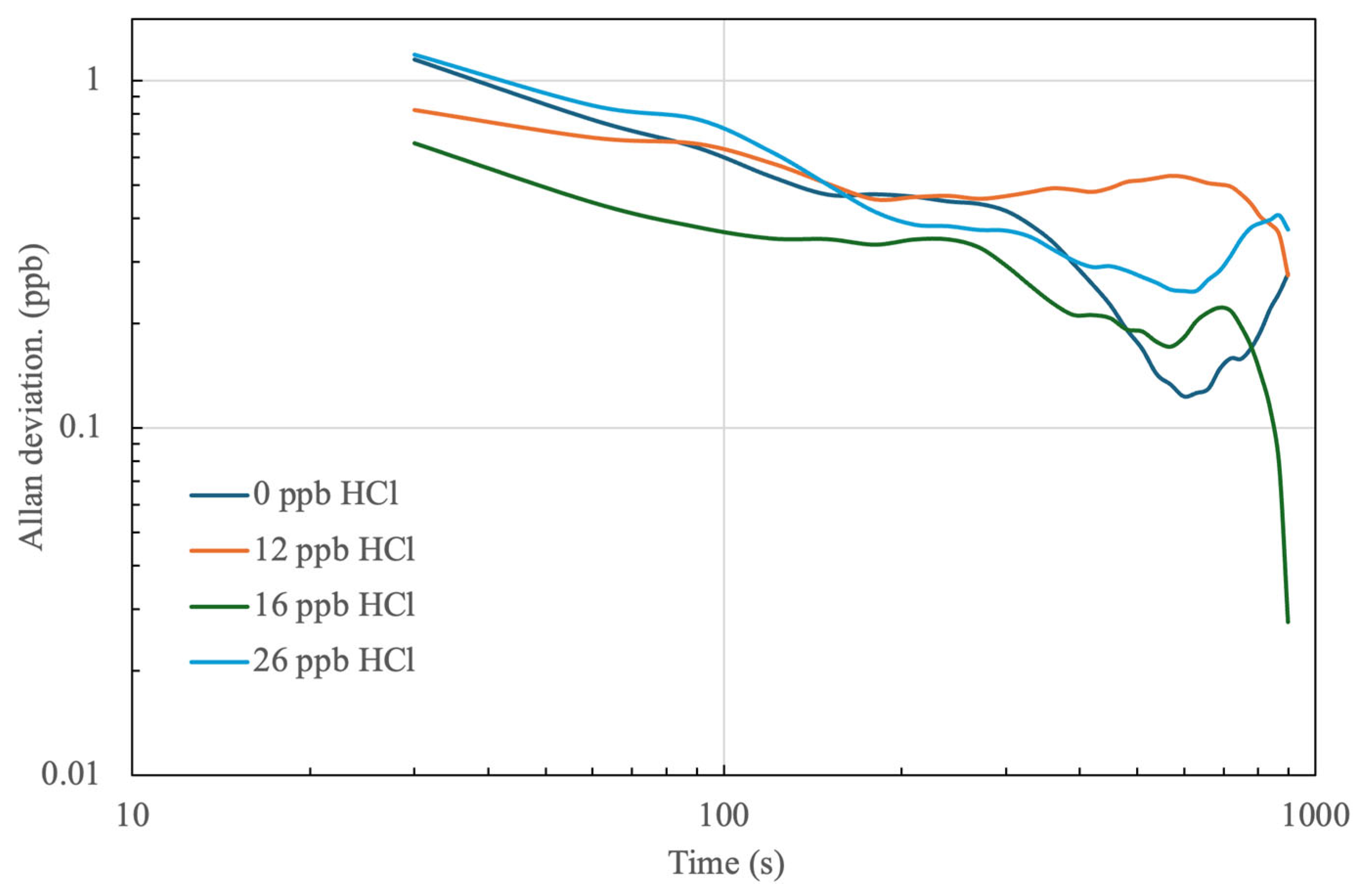
4.1. Stability of HCl Detection
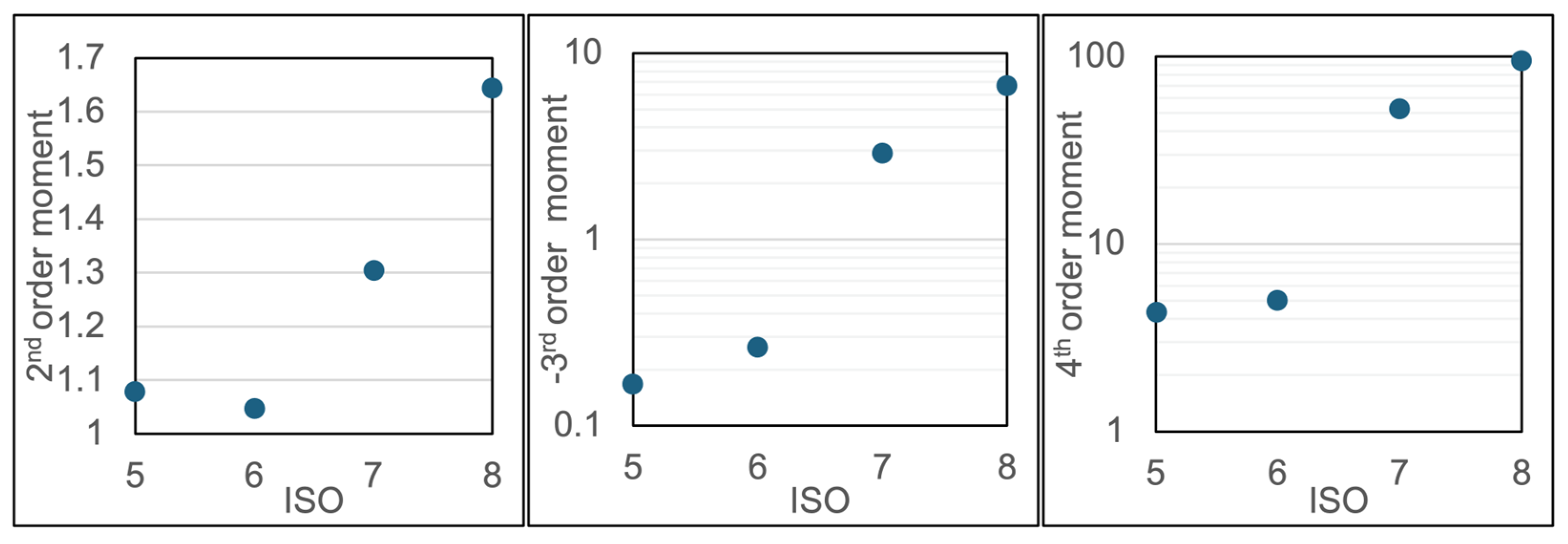
4.2. Particle Detection
5. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, T.; Wang, W. From Cleanroom to Desktop: Emerging Micro-Nanofabrication Technology for Biomedical Applications. Ann. Biomed. Eng. 2011, 39, 600–620. [Google Scholar] [CrossRef]
- Naughton, P. History of Cleanrooms. ASHRAE 2019, 61, 38–54. [Google Scholar]
- International Organization for Standardization. Cleanrooms and Associated Controlled Environments—Part 1: Classification of Air Cleanliness by Particle Concentration; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- IEEE. IEEE International Roadmap for Devices and Systems Yield Enhancement; IEEE: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Conte, T. International Roadmap for Devices and Systems; IEEE: New York, NY, USA, 2018. [Google Scholar]
- International Technology Roadmap for Semiconductors; Semiconductor Industry Association: Washington, DC, USA, 2015.
- Sreenivasan, S.V. Nanoimprint Lithography Steppers for Volume Fabrication of Leading-Edge Semiconductor Integrated Circuits. Microsyst. Nanoeng. 2017, 3, 17075. [Google Scholar] [CrossRef]
- Weineck, G.; Zastera, D.; Dallas, A.J. AMC Control in Photolithography: The Past Decade in Review; Raymond, C.J., Ed.; SPIE Advanced Lithography: San Jose, CA, USA, 2010; p. 76383H. [Google Scholar]
- Pic, N.; Martin, C.; Vitalis, M.; Calarnou, T.; Camlay, D.; Grosjean, C.; Lanier, A.; Kames, J.; Acksel, A.; Galvez, C. Defectivity Decrease in the Photolithography Process by AMC Level Reduction through Implementation of Novel Filtration and Monitoring Solutions; Raymond, C.J., Ed.; SPIE Advanced Lithography: San Jose, CA, USA, 2010; p. 76380M. [Google Scholar]
- Li, J.; Zhou, Y.-F. Occupational Hazards Control of Hazardous Substances in clean Room of Semiconductor Manufacturing Plant Using CFD Analysis. Toxicol. Ind. Health 2015, 31, 123–139. [Google Scholar] [CrossRef]
- Garnier, P.; Borde, Y.; Sevilla, G. Airborne Molecular Contamination: Mechanism and Consequences on Devices. ECS Trans. 2022, 108, 61–67. [Google Scholar] [CrossRef]
- Wu, B.-J.; Bai, H.; Lin, I.-K.; Liu, S.S. Al–Cu Pattern Wafer Study on Metal Corrosion Due to Chloride Ion Contaminants. IEEE Trans. Semicond. Manufact. 2010, 23, 553–558. [Google Scholar] [CrossRef]
- Kamoshima, T.; Fujii, Y.; Noguchi, T.; Saeki, T.; Takata, Y.; Ochi, H.; Koiwa, A. Controlling Ambient Gas in Slot-to-Slot Space Inside FOUP to Suppress Cu-Loss After Dual Damascene Patterning. IEEE Trans. Semicond. Manufact. 2008, 21, 573–577. [Google Scholar] [CrossRef]
- Pelissier, B.; Beaurain, A.; Fontaine, H.; Danel, A.; Joubert, O. Investigations on HCl Contaminated Cu 200 Mm Wafers Using Parallel Angle Resolved XPS. Microelectron. Eng. 2009, 86, 1013–1016. [Google Scholar] [CrossRef]
- Lin, I.-K.; Bai, H.; Wu, B.-J. Analysis of Relationship between Inorganic Gases and Fine Particles in Cleanroom Environment. Aerosol Air Qual. Res. 2010, 10, 245–254. [Google Scholar] [CrossRef]
- Clement, C. Gas-to-Particle Conversion in the Atmosphere: I. Evidence from Empirical Atmospheric Aerosols. Atmos. Environ. 1999, 33, 475–487. [Google Scholar] [CrossRef]
- Kohli, R.; Mittal, K.L. Developments in Surface Contamination and Cleaning; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-1-4377-7883-0. [Google Scholar]
- Wilcox, D.; Libman, S. Yield Enhancement. In Proceedings of the 2021 IEEE International Roadmap for Devices and Systems Outbriefs, Santa Clara, CA, USA, 30 November 2021; IEEE: New York, NY, USA, 2021; pp. 1–47. [Google Scholar]
- Chen, R.; Shiue, A.; Liu, J.; Zhi, Y.; Zhang, D.; Xia, F.; Leggett, G. Integrated On-Site Collection and off-Site Analysis of Airborne Molecular Contamination in Cleanrooms for Integrated Circuit Manufacturing Processes. Build. Environ. 2022, 214, 108941. [Google Scholar] [CrossRef]
- Lehmann, K.K.; Berden, G.; Engeln, R. An Introduction to Cavity Ring-Down Spectroscopy. In Cavity Ring-Down Spectroscopy; Berden, G., Engeln, R., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 1–26. ISBN 978-1-4443-0825-9. [Google Scholar]
- Berden, G.; Peeters, R.; Meijer, G. Cavity Ring-Down Spectroscopy: Experimental Schemes and Applications. Int. Rev. Phys. Chem. 2000, 19, 565–607. [Google Scholar] [CrossRef]
- Anderson, D.Z.; Frisch, J.C.; Masser, C.S. Mirror Reflectometer Based on Optical Cavity Decay Time. Appl. Opt. 1984, 23, 1238. [Google Scholar] [CrossRef] [PubMed]
- Hagen, C.L.; Lee, B.C.; Franka, I.S.; Rath, J.L.; VandenBoer, T.C.; Roberts, J.M.; Brown, S.S.; Yalin, A.P. Cavity Ring-down Spectroscopy Sensor for Detection of Hydrogen Chloride. Atmos. Meas. Tech. 2014, 7, 345–357. [Google Scholar] [CrossRef]
- Panu, H.; Timo, R.; Thomas, F.; Makkonen, J.; Alyshev, S.; Kharakhordin, A.; Firstov, S. Real-Time HCl Gas Detection at Parts-per-Billion Level Concentrations Utilising a Diode Laser and a Bismuth-Doped Fibre Amplifier. Meas. Sci. Technol. 2021, 32, 055206. [Google Scholar] [CrossRef]
- Smith, J.D.; Atkinson, D.B. A Portable Pulsed Cavity Ring-down Transmissometer for Measurement of the Optical Extinction of the Atmospheric Aerosol. Analyst 2001, 126, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Lovejoy, E.R.; Brock, C.A.; Brown, S.S.; Ravishankara, A.R. Measurement of Aerosol Optical Extinction at with Pulsed Cavity Ring down Spectroscopy. J. Aerosol Sci. 2004, 35, 995–1011. [Google Scholar] [CrossRef]
- Gordon, T.D.; Wagner, N.L.; Richardson, M.S.; Law, D.C.; Wolfe, D.; Eloranta, E.W.; Brock, C.A.; Erdesz, F.; Murphy, D.M. Design of a Novel Open-Path Aerosol Extinction Cavity Ringdown Spectrometer. Aerosol Sci. Technol. 2015, 49, 717–726. [Google Scholar] [CrossRef]
- Wang, C.; Gong, Z.; Pan, Y.-L.; Videen, G. Optical Trap-Cavity Ringdown Spectroscopy as a Single-Aerosol-Particle-Scope. Appl. Phys. Lett. 2015, 107, 241903. [Google Scholar] [CrossRef]
- Riziq, A.A.; Erlick, C.; Dinar, E.; Rudich, Y. Optical Properties of Absorbing and Non-Absorbing Aerosols Retrieved by Cavity Ring down (CRD) Spectroscopy. Atmos. Chem. Phys. 2007, 7, 1523–1536. [Google Scholar] [CrossRef]
- Miles, R.E.H.; Carruthers, A.E.; Reid, J.P. Novel Optical Techniques for Measurements of Light Extinction, Scattering and Absorption by Single Aerosol Particles. Laser Photonics Rev. 2011, 5, 534–552. [Google Scholar] [CrossRef]
- Mason, B.J.; Walker, J.S.; Reid, J.P.; Orr-Ewing, A.J. Deviations from Plane-Wave Mie Scattering and Precise Retrieval of Refractive Index for a Single Spherical Particle in an Optical Cavity. J. Phys. Chem. A 2014, 118, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- McHale, L.E.; Hecobian, A.; Yalin, A.P. Open-Path Cavity Ring-down Spectroscopy for Trace Gas Measurements in Ambient Air. Opt. Express 2016, 24, 5523. [Google Scholar] [CrossRef] [PubMed]
- McHale, L.E.; Martinez, B.; Miller, T.W.; Yalin, A.P. Open-Path Cavity Ring-down Methane Sensor for Mobile Monitoring of Natural Gas Emissions. Opt. Express 2019, 27, 20084. [Google Scholar] [CrossRef] [PubMed]
- Rothman, L.S.; Gordon, I.E.; Babikov, Y.; Barbe, A.; Chris Benner, D.; Bernath, P.F.; Birk, M.; Bizzocchi, L.; Boudon, V.; Brown, L.R.; et al. The HITRAN2012 Molecular Spectroscopic Database. J. Quant. Spectrosc. Radiat. Transf. 2013, 130, 4–50. [Google Scholar] [CrossRef]
- Ortwein, P.; Woiwode, W.; Wagner, S.; Gisi, M.; Ebert, V. Laser-Based Measurements of Line Strength, Self- and Pressure-Broadening Coefficients of the H35Cl R(3) Absorption Line in the First Overtone Region for Pressures up to 1 MPa. Appl. Phys. B 2010, 100, 341–347. [Google Scholar] [CrossRef]
- Kogelnik, H.; Li, T. Laser Beams and Resonators. Appl. Opt. 1966, 5, 1550. [Google Scholar] [CrossRef]
- Huang, H.; Lehmann, K.K. Sensitivity Limits of Continuous Wave Cavity Ring-Down Spectroscopy. J. Phys. Chem. A 2013, 117, 13399–13411. [Google Scholar] [CrossRef]
- Huang, H.; Lehmann, K.K. Noise Caused by a Finite Extinction Ratio of the Light Modulator in CW Cavity Ring-down Spectroscopy. Appl. Phys. B 2009, 94, 355–366. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.B.; L’Orange, C.; Lim, C.; Kwon, D.; Yalin, A.P. Open-Path Cavity Ring-Down Spectroscopy for Simultaneous Detection of Hydrogen Chloride and Particles in Cleanroom Environment. Sensors 2024, 24, 5611. https://doi.org/10.3390/s24175611
Khan MB, L’Orange C, Lim C, Kwon D, Yalin AP. Open-Path Cavity Ring-Down Spectroscopy for Simultaneous Detection of Hydrogen Chloride and Particles in Cleanroom Environment. Sensors. 2024; 24(17):5611. https://doi.org/10.3390/s24175611
Chicago/Turabian StyleKhan, Muhammad Bilal, Christian L’Orange, Cheongha Lim, Deokhyeon Kwon, and Azer P. Yalin. 2024. "Open-Path Cavity Ring-Down Spectroscopy for Simultaneous Detection of Hydrogen Chloride and Particles in Cleanroom Environment" Sensors 24, no. 17: 5611. https://doi.org/10.3390/s24175611





