Study of Ion-to-Electron Transducing Layers for the Detection of Nitrate Ions Using FPSX(TDDAN)-Based Ion-Sensitive Electrodes
Abstract
:1. Introduction
2. Materials and Methods
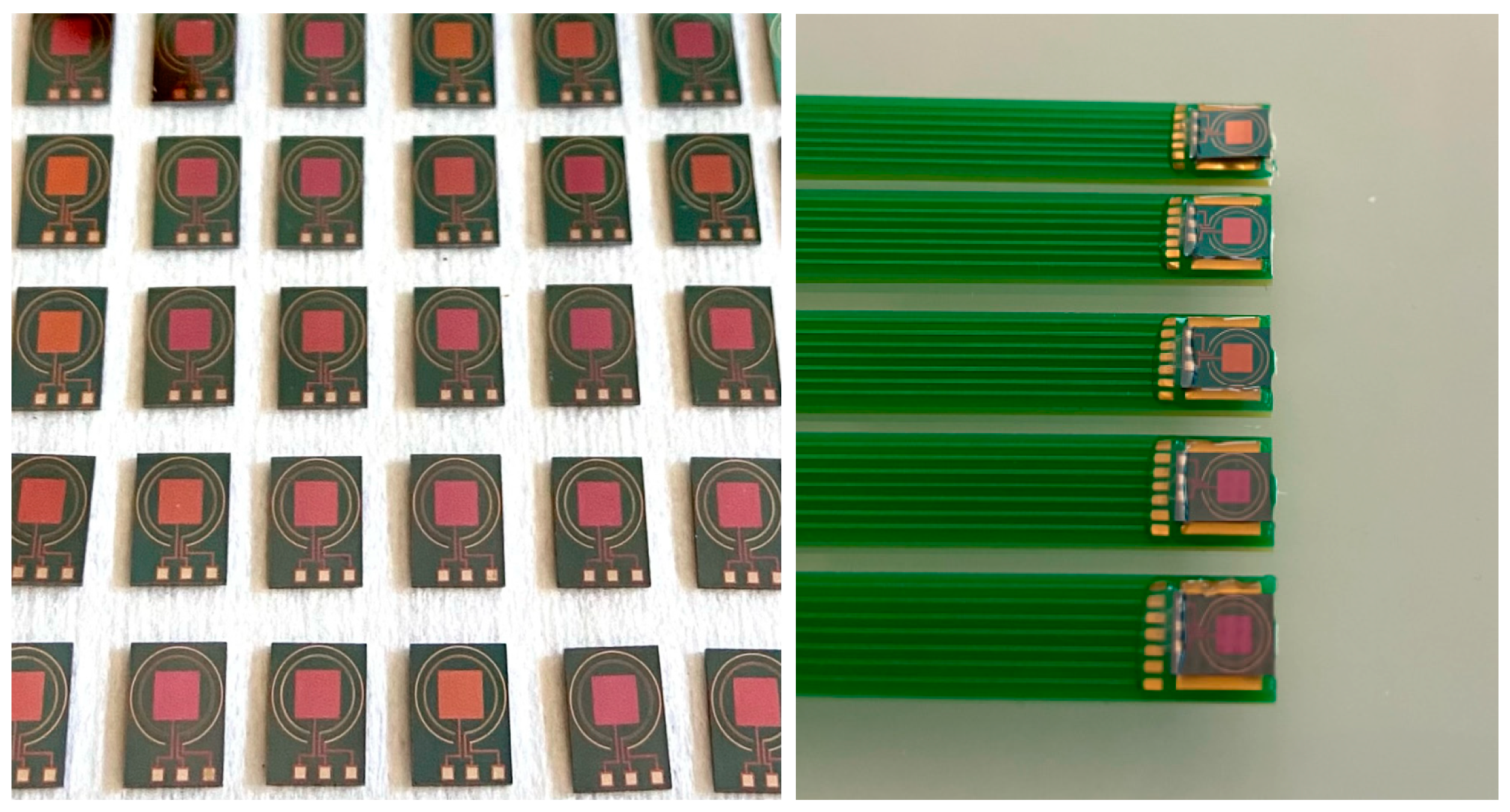
2.1. Microdevice Fabrication
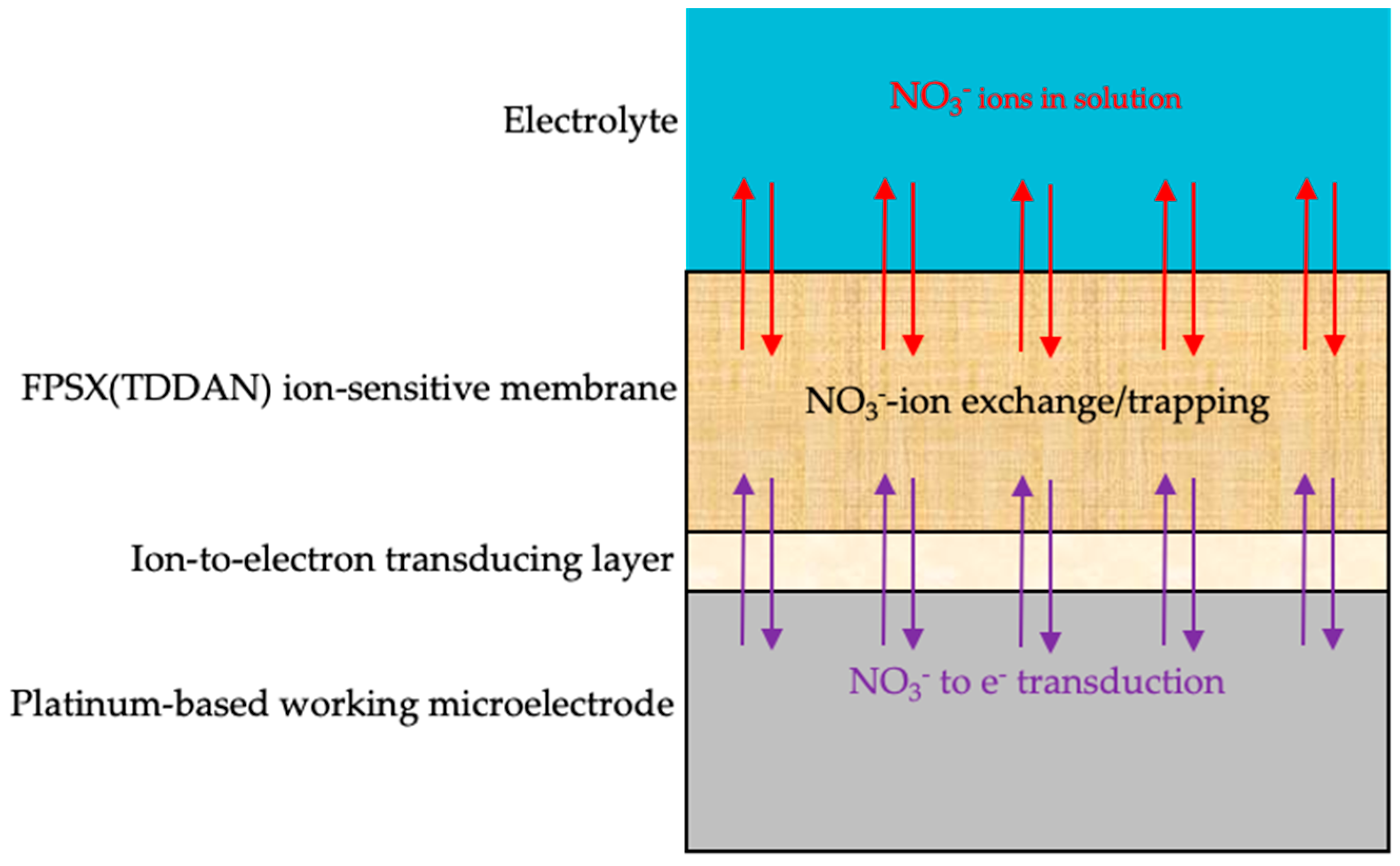
2.2. Integration of Ion-Sensitive Membranes in ElecCell Devices
- -
- Matrix #1: poly (3,4-ethylenedioxythiophene) (PEDOT) doped with sodium polystyrene sulfonate (NaPSS);
- -
- Matrix #2: poly (3,4-ethylenedioxythiophene) (PEDOT) doped with double-walled carbon nanotubes (DWCNTs);
- -
- Matrix #3: polypyrrole (PPy) doped with double-walled carbon nanotubes (DWCNTs).
2.3. Physical Characterization of Ion-Sensitive Membranes
2.4. Electrochemical Characterization in Liquid Phase
3. Results and Discussion
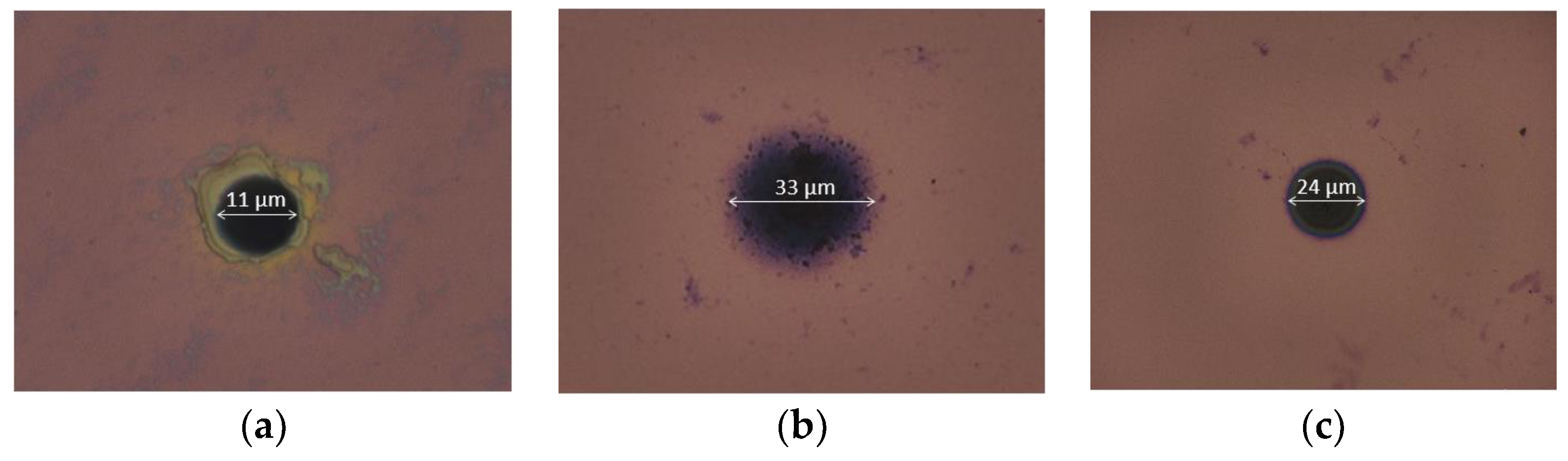
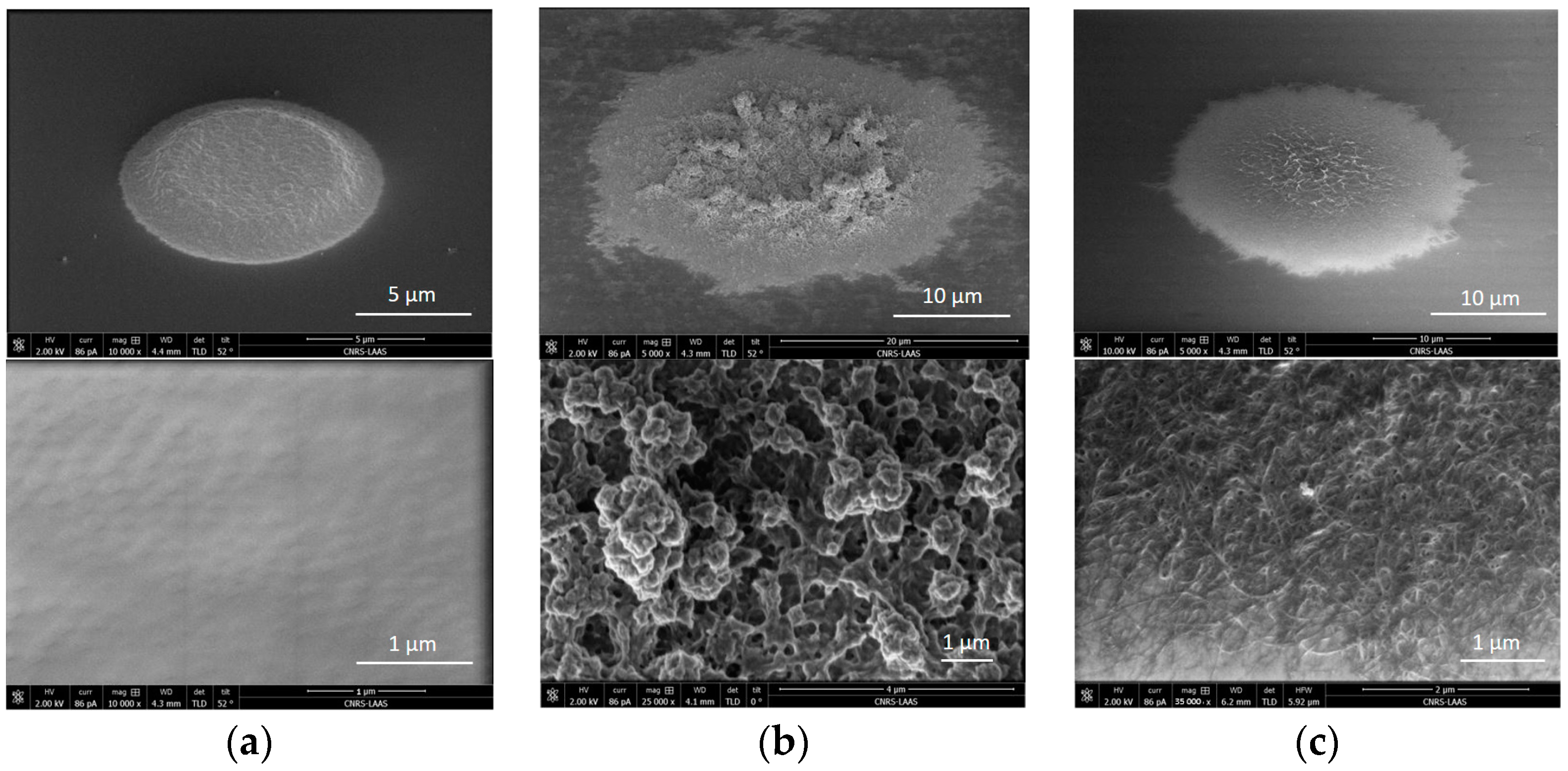
3.1. Physical Analysis of the Different Polymer-Based Membranes
3.2. pNO3-ELecCell Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrews, M.; Raven, J.A.; Lea, P.J. Do plants need nitrate? The mechanisms by which nitrogen forms affect plants. Ann. Appl. Biol. 2013, 163, 174–199. [Google Scholar] [CrossRef]
- Owen, A.G.; Jones, D.L. Competition for amino acids between roots and rhizosphere microorganisms and the role of amino acids in plant N acquisition. Soil Biol. Biochem. 2001, 33, 651–657. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. Nitrate leaching in temperate agroecosystems: Sources, factors and mitigating strategies. Nutr. Cycle Agroecosystems 2002, 64, 237–256. [Google Scholar] [CrossRef]
- Billen, G.; Garnier, J.; Lassaletta, L. The nitrogen cascade from agricultural soils to the sea: Modelling nitrogen transfers at regional watershed and global scales. Philos. Trans. R. Soc. B Biol. Sci. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Billen, G.; Beusen, A.; Bouwman, L.; Garnier, J. Anthropogenic nitrogen autography and heterotrophy of the world’s watersheds: Past, present and future trends. Glob. Biogeochem. Cycles 2010, 24, GB0A11. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- World Health Organization. Nitrate and Nitrite in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Mahmud, M.A.P.; Ejeian, F.; Azadi, S.; Myers, M.; Pejcic, B.; Abassi, R.; Razmjou, A.; Asadnia, M. Recent progress in sensing nitrate, nitrite, phosphate and ammonium in aquatic environments. Chemosphere 2020, 259, 127492. [Google Scholar] [CrossRef]
- Singh, S.; Anil, A.G.; Kumar, V.; Kapoor, D.; Subramanian, S.; Singh, J.; Ramamurthy, P.C. Nitrates in the environment: A critical review of their distribution, sensing techniques, ecological effects and remediation. Chemosphere 2022, 287, 131996. [Google Scholar] [CrossRef]
- Campanella, L.; Colapicchioni, C.; Crescentini, G.; Sammartino, M.P.; Su, Y. Tomassetti. Sensitive membrane ISFETs for nitrate analysis in waters. Sens. Actuators B 1995, B27, 329–335. [Google Scholar] [CrossRef]
- Chudy, M.; Wroblewski, W.; Dybko, A.; Brzozska, Z. Multi-ion analysis on versatile sensor head. Sens. Actuators B 2001, B78, 320–335. [Google Scholar] [CrossRef]
- Artigas, J.; Beltran, A.; Jimenez, C.; Baldi, A.; Mas, R.; Dominguez, C.; Alonso, J. Application of ion field-effect-transistor-based sensors to soil analysis. Comput. Electron. Agric. 2001, 31, 281–293. [Google Scholar] [CrossRef]
- Temple-Boyer, P.; Launay, J.; Humenyuk, I.; Conto, T.D.; Martinez, A.; Bériet, C.; Grisel, A. Study of front-side connected chemical field effect transistor for water analysis. Microelectron. Reliab. 2004, 44, 443–447. [Google Scholar] [CrossRef]
- Tsujimura, Y.; Tsubota, S.; Sasaki, K.; Karatani, H. Novel ion-sensitive field-effect transistor with ion sensing polymerized silsesquioxane membrane on detachable metal disk. Electrochemistry 2007, 75, 472–474. [Google Scholar] [CrossRef]
- Myers, M.; Khir, F.L.M.; Podolska, A.; Umana-Membreno, G.A.; Nener, B.; Baker, M.; Parish, G. Nitrate ion detection using AlGaN/GaN heterostructure-based devices without a reference electrode. Sens. Actuators B 2013, B181, 301–305. [Google Scholar] [CrossRef]
- Chairsriratanakul, W.; Bunjongpru, W.; Jeamsaksiri, W.; Srisuwan, A.; Porntheeraphat, S.; Chaowicharat, E.; Hruanun, C.; Poyai, A.; Promyothin, D.; Nukeaw, J. Durable nitrate sensor by surface modification. Surf. Coat. Technol. 2016, 306, 58–62. [Google Scholar] [CrossRef]
- Joly, M.; Marlet, M.; Durieu, C.; Bene, C.; Launay, J.; Temple-Boyer, P. Study of chemical field effect transistors for the detection of ammonium and nitrate ions in liquid and soil samples. Sens. Actuators B 2022, B351, 130949. [Google Scholar] [CrossRef]
- Wardak, C.; Grabarczyk, M. Analytical application of solid contact ion-selective electrodes for determination of copper and nitrate in various food products and drinking water. J. Environ. Sci. Health 2016, 51, 519–524. [Google Scholar] [CrossRef]
- Fayose, T.; Mendecki, L.; Ullah, S.; Radu, A. Single strip solid contact ion selective electrodes on a pencil-drawn electrode surface. Anal. Methods 2017, 9, 1213–1220. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.; Cherubini, T.; Pankratova, N.; Confalonieri, F.; Massa, F.; Tercier-Waeber, M.L.; Abdou, M.; Schäfer, J.; Bakker, E. In situ detection of macronutrients and chloride in seawater by submersible electrochemical sensors. Analytical. Chem. 2018, 90, 4702–4710. [Google Scholar] [CrossRef]
- Garland, N.T.; Lamore, E.S.M.; Cavallaro, N.D.; Mendivelso-Perez, D.; Smith, E.A.; Jing, D.; Claussen, J.C. Flexible laser-induced graphene for nitrogen sensing in soil. ACS Appl. Mater. Interfaces 2018, 10, 39124–39133. [Google Scholar] [CrossRef]
- Schwarz, J.; Trommer, K.; Mertig-Hauser, M. Solid-contact ion-selective electrodes based on graphite paste for potentiometric nitrate and ammonium determinations. Am. J. Anal. Chem. 2018, 9, 591–601. [Google Scholar] [CrossRef]
- Hassan, S.; Eldin, A.G.; Amr, A.E.G.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Improved solid-contact nitrate ion selective electrodes based on multi-walled carbon nanotubes (MWCNTs) as an ion-to-electron transducer. Sensors 2019, 19, 3891. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Meng, Z.; Qin, Y.; Jiang, D.; Xi, K.; Wang, P. Thiol-functionalized reduced graphene oxide as self-assembled ion-to-electron transducer for durable solid-contact ion-selective electrodes. Talanta 2020, 208, 120374. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, K.; Wardak, C. Comparative study of nitrate all solid state ion-selective electrode based on multiwalled carbon nanotubes-ionic liquid nanocomposite. Sens. Actuators B 2021, B348, 130720. [Google Scholar] [CrossRef]
- Hjort, R.G.; Soares, R.R.A.; Li, J.; Jing, D.; Hartfiel, L.; Chen, B.; Van Belle, B.; Soupir, M.; Smith, E.; McLamore, E.; et al. Hydrophobic laser-induced graphene potentiometric ion-selective electrodes for nitrate sensing. Microchim. Acta 2022, 189, 122. [Google Scholar] [CrossRef]
- Isildak, O.; Yildiz, I. Highly selective potentiometric detection of nitrate ions using bisdiethyldithiocarbamate based membrane electrodes. Electrochim. Acta 2023, 459, 142587. [Google Scholar] [CrossRef]
- Christophe, C.; Belaïdi, F.S.; Launay, J.; Gros, P.; Questel, E.; Temple-Boyer, P. Elaboration of integrated microelectrodes for the detection of antioxidant species. Sens. Actuators B 2013, B177, 350–356. [Google Scholar] [CrossRef]
- Vanhove, E.; Tsopéla, A.; Bouscayrol, L.; Desmoulins, A.; Launay, J.; Temple-Boyer, P. Final capping passivation layers for long-life microsensors in real fluids. Sens. Actuators B 2013, B178, 350–358. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- Dumschat, C.; Alazard, S.; Adam, S.; Knoll, M.; Camman, K. Filled fluorosiloxane as matrix for ion-selective membranes. Analyst 1996, 121, 527–529. [Google Scholar] [CrossRef]
- Hogg, G.; Lutze, O.; Camman, K. Novel membrane material for ion-selective field effect transistors with extended lifetime and improved selectivity. Anal. Chim. Acta 1996, 335, 103–109. [Google Scholar] [CrossRef]
- Lota, K.; Khomenko, V.; Frackowiak, E. Capacitance properties of poly(3,4-ethylenedioxythiophene)/carbon nanotubes composites. J. Phys. Chem. Solids 2004, 65, 295–301. [Google Scholar] [CrossRef]
- Peng, C.; Jin, J.; Chen, G.Z. A comparative study on electrochemical co-deposition and capacitance of composite films of conducting polymers and carbon nanotubes. Electrochim. Acta 2007, 53, 525–537. [Google Scholar] [CrossRef]
- Guzinski, M.; Jarvis, J.M.; Perez, F.; Pendley, B.D.; Lindner, E.; De Marco, R.; Crespo, G.A.; Acres, R.G.; Walker, R.; Bishop, J. PEDOT(PSS) as solid contact for ion-selective electrodes: The influence of the PEDOT(PSS) film thickness on the equilibration times. Anal. Chem. 2017, 89, 3508–3516. [Google Scholar] [CrossRef] [PubMed]
- Saunier, V.; Flahaut, E.; Blatché, M.-C.; Bergaud, C.; Maziz, A. Carbon nanofiber-PEDOT composite films as novel microelectrode for neural interfaces and biosensing. Biosens. Bioelectron. 2020, 165, 111413. [Google Scholar] [CrossRef]
- Umezawa, Y.; Umezawa, K.; Sato, H. Selectivity coefficients for ion-selective electrodes: Recommended methods for reporting KABpot values. Pure Appl. Chem. 1995, 67, 507–518. [Google Scholar] [CrossRef]
- Ouyang, J.; Chu, C.-W.; Chen, F.-C.; Xu, Q.; Yang, Y. High-conductivity poly(3,4-ethylenedioxythiophene): Poly(styrene sulfonate) film and its application in polymer optoelectronic oevices. Adv. Funct. Mater. 2005, 15, 203–208. [Google Scholar] [CrossRef]
- Belaidi, F.S.; Civélas, A.; Castagnola, V.; Tsopela, A.; Mazenq, L.; Gros, P.; Launay, J.; Temple-Boyer, P. PEDOT-modified integrated microelectrodes for the detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B 2015, B214, 1–9. [Google Scholar] [CrossRef]
- Crespo, G.A.; Macho, S.; Bobacka, J.; Rius, F.X. Transduction mechanism of carbon nanotubes in solid-contact ion-selective electrodes. Anal. Chem. 2009, 81, 676–681. [Google Scholar] [CrossRef]
- Bortolamiol, T.; Lukanov, P.; Galibert, A.-M.; Soula, B.; Lonchambon, P.; Datas, L.; Flahaut, E. Double-walled carbon nanotubes: Quantitative purification assessment, balance between purification and degradation and solution filling as an evidence of opening. Carbon 2014, 78, 79–90. [Google Scholar] [CrossRef]
- Pei, Q.; Qian, R. Protonation and deprotonation of polypyrrole chain in aqueous solutions. Synth. Met. 1991, 45, 35–48. [Google Scholar] [CrossRef]
- Moussavi, Z.; Bobacka, J.; Ivaska, A. Potentiometric Ag+ sensors based on conducting polymers: A comparison between poly(3,4-ethylenedioxythiophene) and polypyrrole doped with sulfonated calixarenes. Electroanalysis 2005, 17, 1609–1615. [Google Scholar] [CrossRef]
- Vasquez, M.; Bobacka, J.; Ivaska, A.; Lewenstam, A. Influence of oxygen and carbon dioxide on the electrochemical stability of poly(3,4-ethylenedioxythiophene) used as ion-to-electron transducer in all-solid-state ion-selective electrodes. Sens. Actuators B 2002, B82, 7–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Cremer, P.S. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef]



| Reference | Insulator Material | Polymeric Membrane | Ion-Sensitive Molecule | Sensitivity (mV/pNO3) | pNO3 Linear Range |
|---|---|---|---|---|---|
| [10] | Si3N4 | PVC-PVA | TDDAN | 53 ± 1 | from 2 to 5 |
| [11] | Si3N4 | PVA | TDDAN | 53 ± 1 | from 1 to 5 |
| [12] | Si3N4 | HDDA | TOAN | 59 ± 1 | from 1 to 5 |
| [13] | Si3N4 | PSX | TDDAN | 51 ± 1 | from 1 to 4 |
| [14] | Ti | SQ | QAS | 59 ± 1 | from 1 to 6 |
| [15] | AlGaN/GaN | PVC | TDDAB | 40 ± 1 | from 1 to 6 |
| [16] | Si3N4 | PVC-PVA | TDDAN | 56 ± 1 | not detailed |
| [17] | Si3N4 | FPSX | TDDAN | 56 ± 1 | from 1.5 to 5.5 |
| Reference | Electrode Material | Ion–Electron Transducer | Polymeric Membrane | Ion-Sensitive Molecule | Sensitivity (mV/pNO3) | pNO3 Linear Range |
|---|---|---|---|---|---|---|
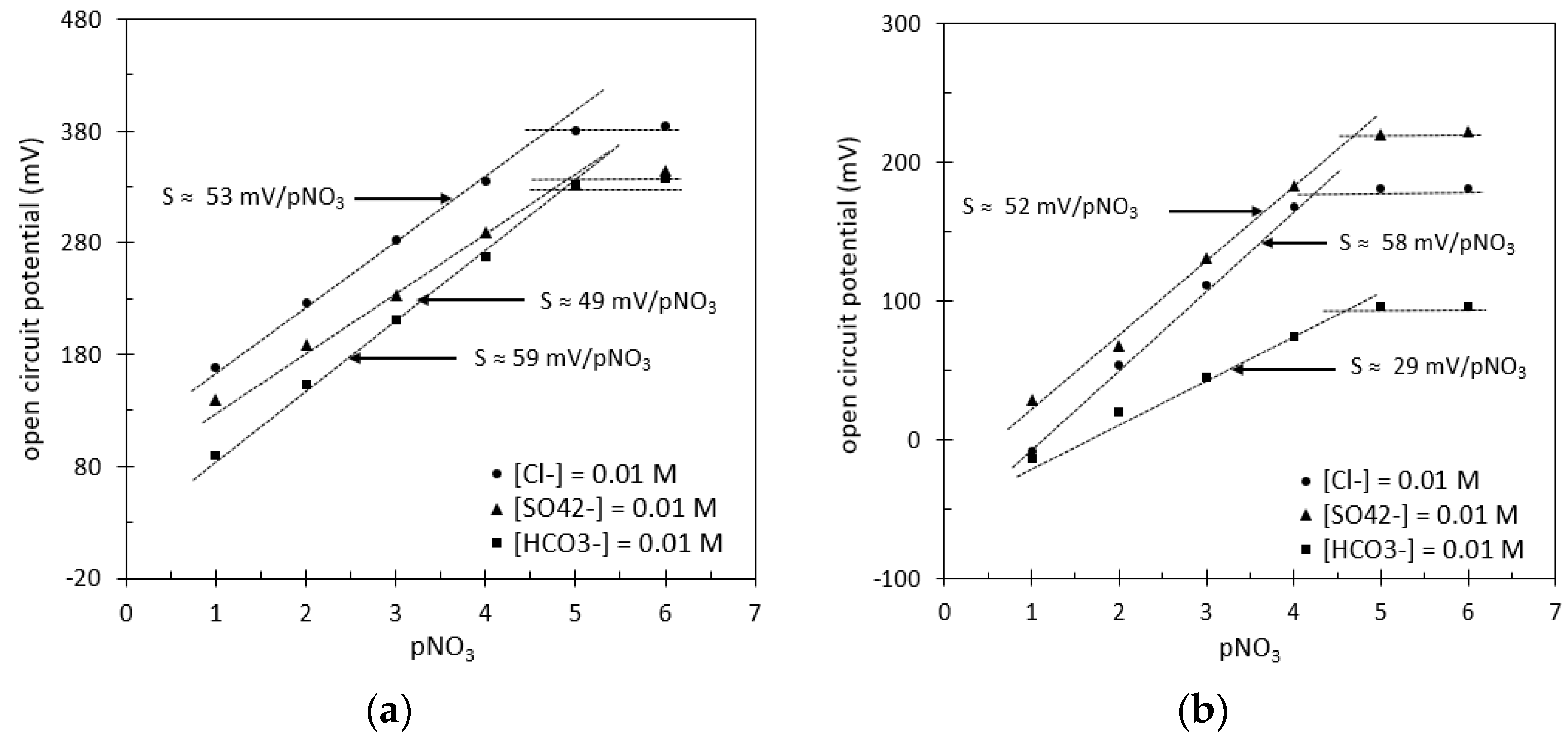
| [18] | Ag | Ag/AgCl | PVC | THTDPCl | 59 ± 1 | from 1 to 5 |
| [19] | Graphite | Cellulose acetate | PVC | TDDACl | 54 ± 1 | from 1 to 5 |
| [20] | Glassy carbon | Graphene | PMMA | TDMAN | 55 ± 1 | from 2 to 4.5 |
| [21] | Graphene | Graphene | PVC | TDMAN | 55 ± 1 | from 1 to 4.5 |
| [22] | Glassy carbon | PPy | PVC | TDMAN | 52 ± 1 | from 1 to 5 |
| [23] | Glassy carbon | MWCNT | PVC | Ni+NO3− | 55 ± 1 | from 1 to 5.5 |
| [24] | Au | TRGO | PVC | NI V | 59 ± 1 | from 1 to 5 |
| [25] | Glassy carbon | MWCNT composite | PVC | Co(Bphen)2 (NO3)2 | 57 ± 1 | from 1 to 6 |
| [26] | Graphene | - | PVC | TDMAN | 59 ± 1 | from 1 to 3.5 |
| [27] | Graphite | - | PVC | Ag(dtc)2 | 56 ± 1 | from 1 to 5 |
| Electrode Material | Ion-to-Electron Transducing Layer | Ion-Sensitive Membrane | Sensitivity (mV/pNO3) | pNO3 Linear Range | Time Constant (s) |
|---|---|---|---|---|---|
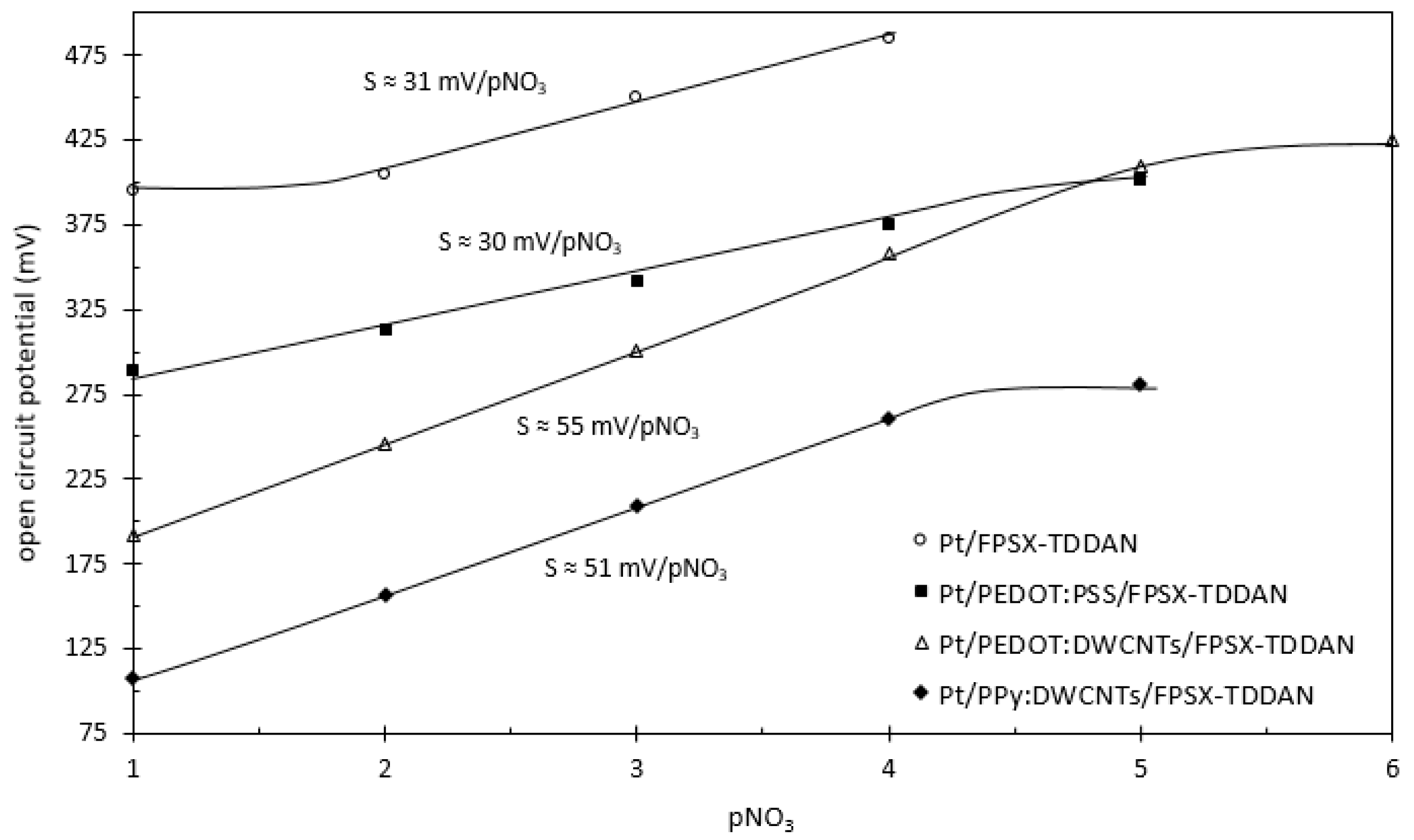
| Pt | - | FPSX(TDDAN) | 31 ± 2 | from 2 to 5 | ≈600 |
| Pt | PEDOT:PSS | FPSX(TDDAN) | 30 ± 1 | from 1 to 4 | ≈120 |
| Pt | PEDOT:DWCNTs | FPSX(TDDAN) | 55 ± 1 | from 1 to 5 | <1 |
| Pt | PPy:DWCNTs | FPSX(TDDAN) | 51 ± 1 | from 1 to 4 | <1 |
| ISE Ion-Sensitive Structure | Day 1 | Day 7 | Day 30 | |||
|---|---|---|---|---|---|---|
| Sensitivity (mV/pNO3) | Time Constant (s) | Sensitivity (mV/pNO3) | Time Constant (s) | Sensitivity (mV/pNO3) | Time Constant (s) | |
| Platinum PEDOT:DWCNT FPSX(TDDAN) | 55 ± 1 | <1 | 53 ± 2 | <1 | 42 ± 6 | ≈60 |
| Platinum PPy:DWCNT FPSX(TDDAN) | 51 ± 1 | <1 | 49 ± 2 | <1 | 40 ± 20 | ≈60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bene, C.; Laborde, A.; Légnani, M.; Flahaut, E.; Launay, J.; Temple-Boyer, P. Study of Ion-to-Electron Transducing Layers for the Detection of Nitrate Ions Using FPSX(TDDAN)-Based Ion-Sensitive Electrodes. Sensors 2024, 24, 5994. https://doi.org/10.3390/s24185994
Bene C, Laborde A, Légnani M, Flahaut E, Launay J, Temple-Boyer P. Study of Ion-to-Electron Transducing Layers for the Detection of Nitrate Ions Using FPSX(TDDAN)-Based Ion-Sensitive Electrodes. Sensors. 2024; 24(18):5994. https://doi.org/10.3390/s24185994
Chicago/Turabian StyleBene, Camille, Adrian Laborde, Morgan Légnani, Emmanuel Flahaut, Jérôme Launay, and Pierre Temple-Boyer. 2024. "Study of Ion-to-Electron Transducing Layers for the Detection of Nitrate Ions Using FPSX(TDDAN)-Based Ion-Sensitive Electrodes" Sensors 24, no. 18: 5994. https://doi.org/10.3390/s24185994








