Non-Invasive Characterization of Different Saccharomyces Suspensions with Ultrasound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Yeast Suspensions and Filtrates
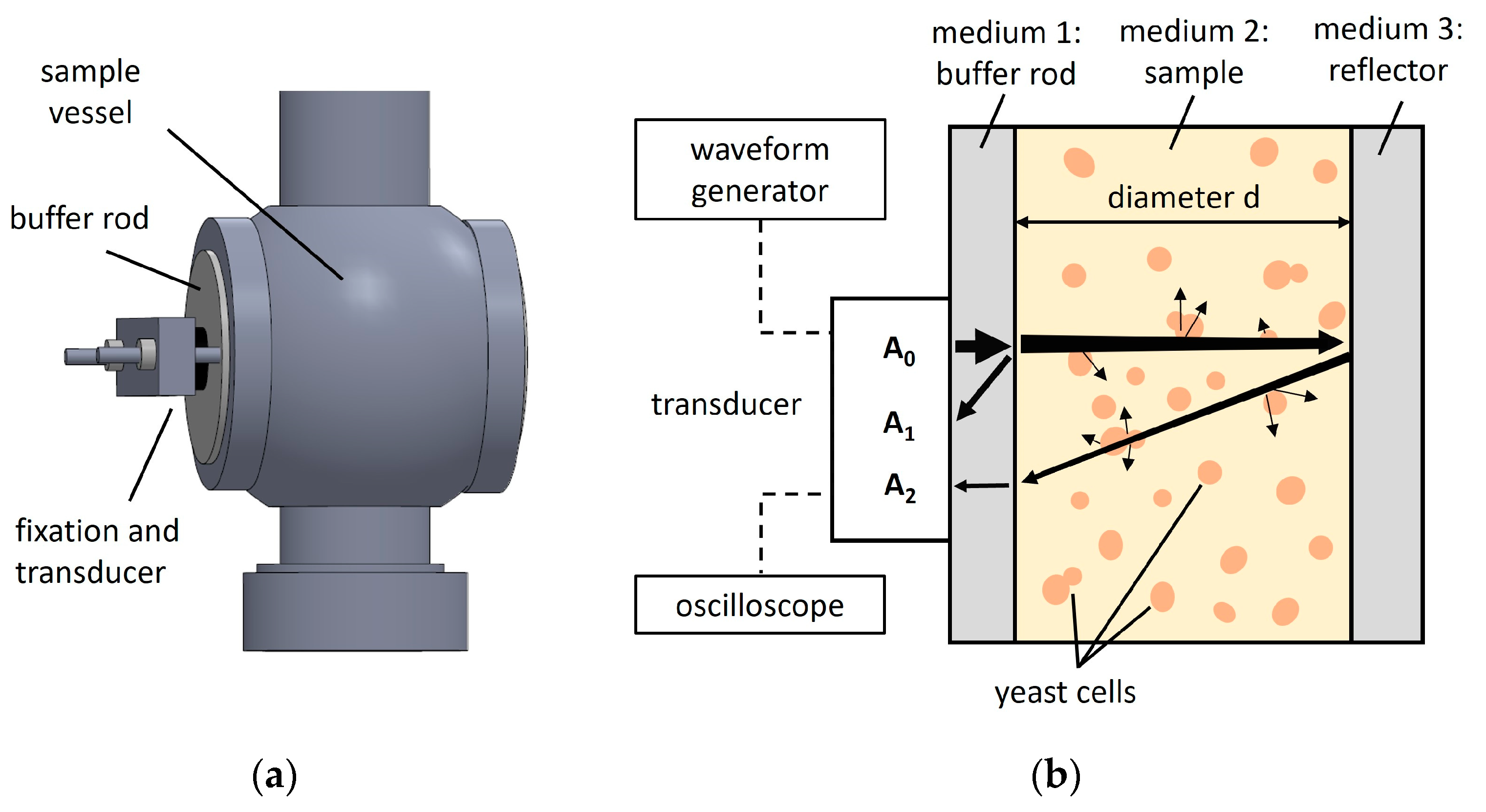
2.2. Experimental Ultrasound Setup and Operating Principle
2.3. Ultrasound Measurements of Yeast Suspensions and Filtrates
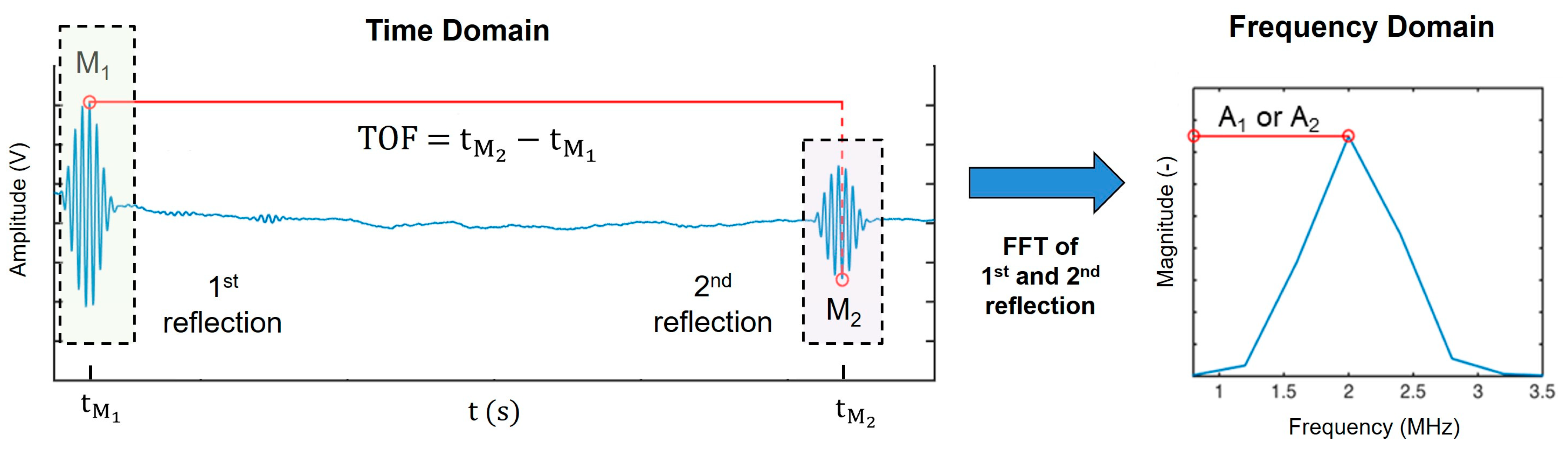
2.4. Ultrasound Signal Processing and Analysis
2.4.1. Determination of Speed of Sound
2.4.2. Determination of the Attenuation Coefficient
2.5. Analytical Methods for Sample Assessment
2.5.1. Particle Size Distribution
2.5.2. Extract, Density, and Alcohol
2.5.3. Osmolality
2.5.4. Free Amino Nitrogen (FAN)
2.6. Statistical Analysis
3. Results and Discussion
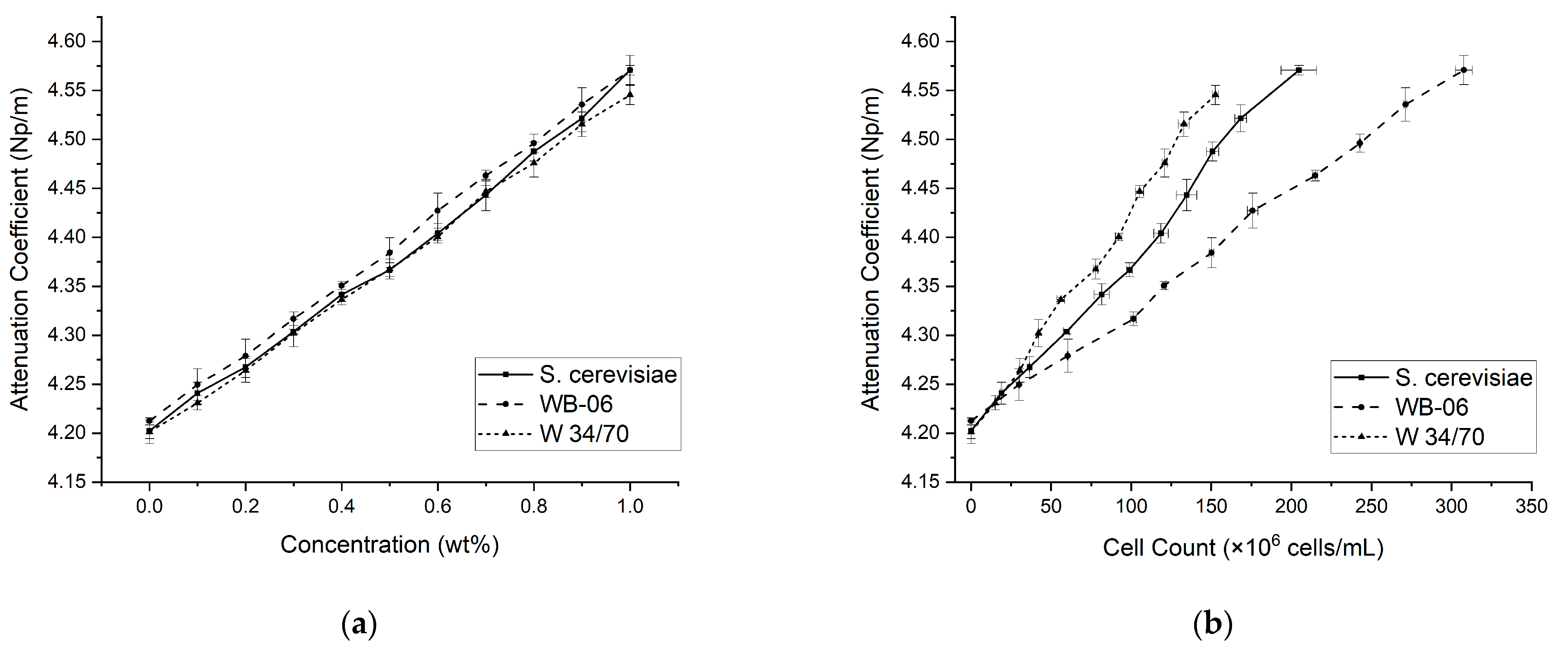
3.1. Yeast Suspension Concentration Gradient (0.0–1.0 wt%)—Attenuation Coefficients and Speed of Sound
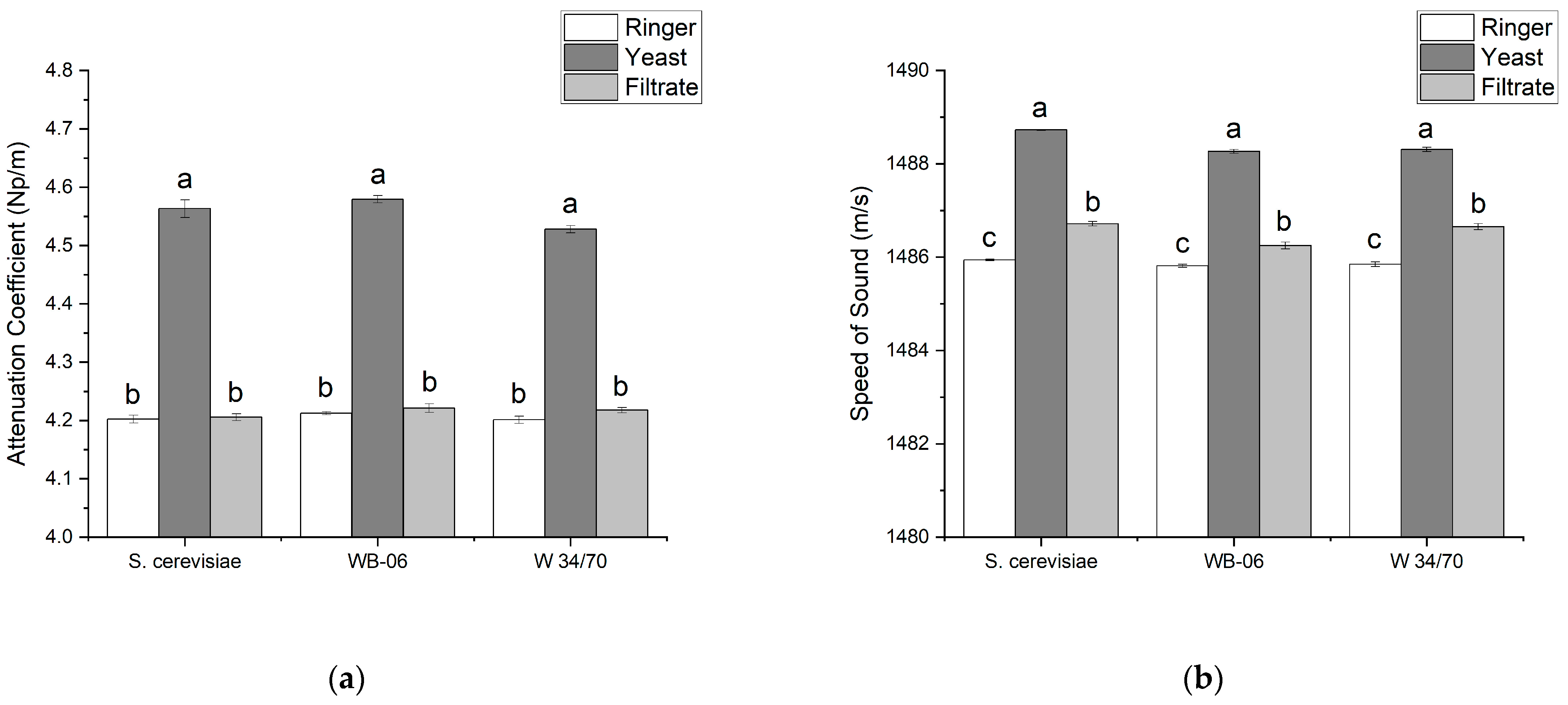
3.2. Comparison of Ringer Solution, Yeast Suspensions (1 wt%), and Corresponding Filtrates
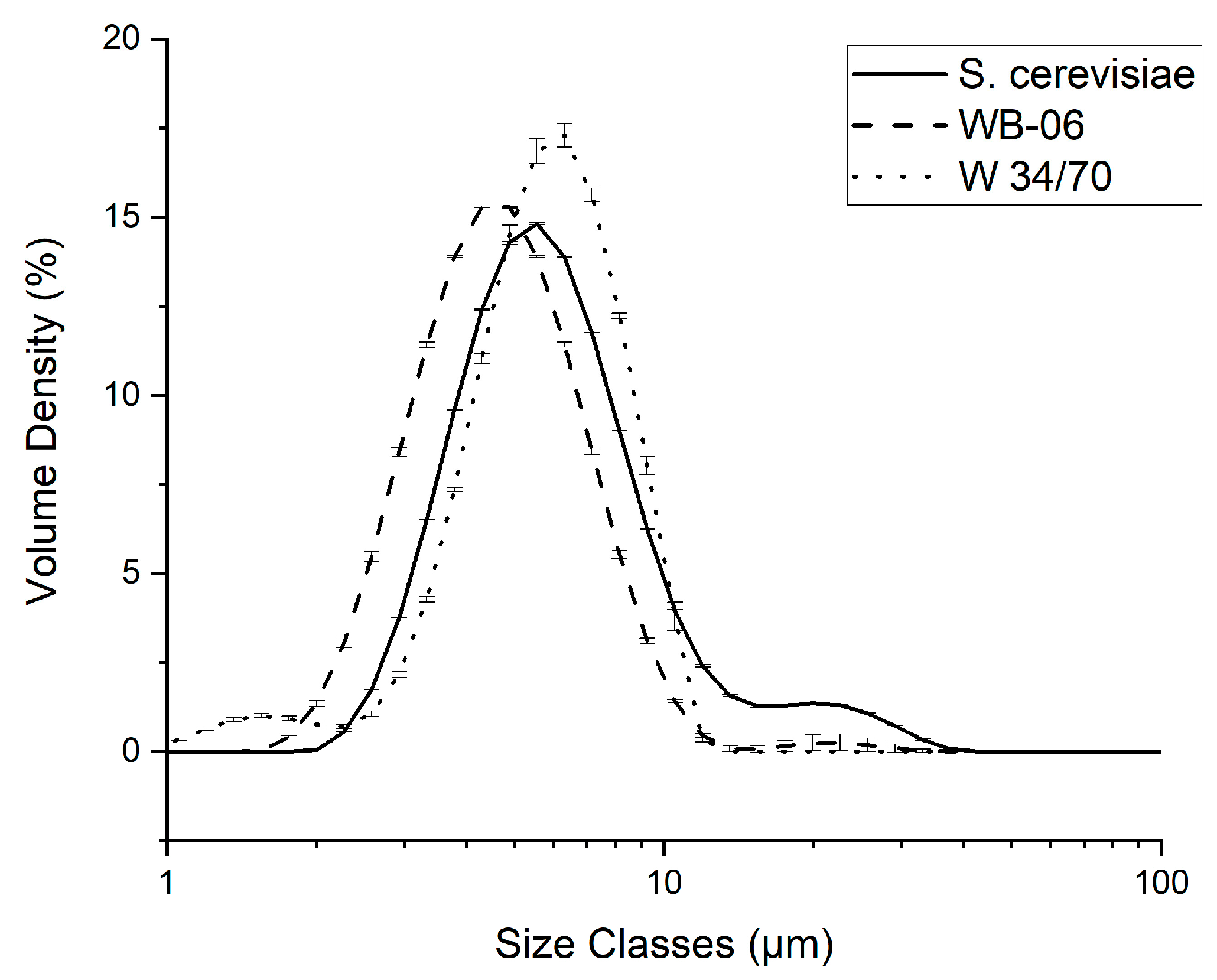
3.3. Yeast Cell Size Distribution—Dispersed Phase
3.4. Assessment of Filtrates—Liquid Phase and Dissolved Substances
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boekhout, T.; Amend, A.S.; El Baidouri, F.; Gabaldón, T.; Geml, J.; Mittelbach, M.; Robert, V.; Tan, C.S.; Turchetti, B.; Vu, D.; et al. Trends in yeast diversity discovery. Fungal Divers. 2022, 114, 491–537. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces cerevisiae and its Industrial Applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Loira, I. Yeast—Industrial Applications; IntechOpen: London, UK, 2017; ISBN 9789535135999. [Google Scholar]
- Rai, A.K.; Pandey, A.; Sahoo, D. Biotechnological potential of yeasts in functional food industry. Trends Food Sci. Technol. 2019, 83, 129–137. [Google Scholar] [CrossRef]
- Feldmann, H. Yeast: Molecular and Cell Biology, 2nd ed.; Wiley-Blackwell: Weinheim, Germany, 2012; ISBN 3527332529. [Google Scholar]
- Soares, E.V. Flocculation in Saccharomyces cerevisiae: A Review. J. Appl. Microbiol. 2011, 110, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Panchal, C.J. Yeast Strain Selection; Taylor & Francis Group: Boca Raton, FL, USA, 1990; ISBN 9781000147551. [Google Scholar]
- Mandenius, C.-F.; Titchener-Hooker, N.J. Measurement, Monitoring, Modelling and Control of Bioprocesses; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2014; ISBN 9783642368387. [Google Scholar]
- Batani, D.; Masini, A.; Pozzi, A.; Previdi, F.; Milani, M. Spectrophotometry for the characterisation of yeast cells suspensions. Eur. Phys. J. AP 2002, 19, 55–62. [Google Scholar] [CrossRef]
- Kontturi, V.; Turunen, P.; Uozumi, J.; Peiponen, K.-E. Robust sensor for turbidity measurement from light scattering and absorbing liquids. Opt. Lett. 2009, 34, 3743–3745. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Bao, C.; Qiu, W.; Lu, Z. Online turbidity measurement using light surface scattering. In Automated Optical Inspection for Industry: Theory, Technology, and Applications II: 16–19 September 1998 Beijing, China; Ye, S., Ed.; SPIE: Bellingham, WA, USA, 1998; pp. 28–30. [Google Scholar]
- Delgado, A.; Briciu-Burghina, C.; Regan, F. Antifouling Strategies for Sensors Used in Water Monitoring: Review and Future Perspectives. Sensors 2021, 21, 389. [Google Scholar] [CrossRef] [PubMed]
- Locher, G.; Sonnleitner, B.; Fiechter, A. On-line measurement in biotechnology: Techniques. J. Biotechnol. 1992, 25, 23–53. [Google Scholar] [CrossRef]
- Goetz, C. Flow Cytometry Basics for the Non-Expert; Springer: Cham, Switzerland, 2018; ISBN 9783319980713. [Google Scholar]
- Robinson, J.P. Single Cell Analysis: Contemporary Research and Clinical Applications; Springer Singapore Pte. Limited: Singapore, 2017; ISBN 9789811044991. [Google Scholar]
- Iqbal, S.M.A.; Butt, N.Z. Design and analysis of microfluidic cell counter using spice simulation. SN Appl. Sci. 2019, 1, 1290. [Google Scholar] [CrossRef]
- Dittami, G.M.; Sethi, M.; Rabbitt, R.D.; Ayliffe, H.E. Determination of mammalian cell counts, cell size and cell health using the Moxi Z mini automated cell counter. J. Vis. Exp. 2012, 64, 3842. [Google Scholar] [CrossRef]
- Vembadi, A.; Menachery, A.; Qasaimeh, M.A. Cell Cytometry: Review and Perspective on Biotechnological Advances. Front. Bioeng. Biotechnol. 2019, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Fagan, C. Process Analytical Technology for the Food Industry; Springer: New York, NY, USA, 2014; ISBN 9781493903115. [Google Scholar]
- Hirsch, E.; Pataki, H.; Domján, J.; Farkas, A.; Vass, P.; Fehér, C.; Barta, Z.; Nagy, Z.K.; Marosi, G.J.; Csontos, I. Inline noninvasive Raman monitoring and feedback control of glucose concentration during ethanol fermentation. Biotechnol. Prog. 2019, 35, e2848. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.H.; Börger, M.; Thien, J.; Koß, H.-J. Bioprocess in-line monitoring and control using Raman spectroscopy and Indirect Hard Modeling (IHM). Biotechnol. Bioeng. 2024, 121, 2225–2233. [Google Scholar] [CrossRef]
- Smulko, J.M.; Dingari, N.C.; Soares, J.S.; Barman, I. Anatomy of noise in quantitative biological Raman spectroscopy. Bioanalysis 2014, 6, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P. Practical Raman Spectroscopy: An Introduction, 1st ed.; John Wiley & Sons Incorporated: Hoboken, NJ, USA, 2013; ISBN 1119961904. [Google Scholar]
- Cadusch, P.J.; Hlaing, M.M.; Wade, S.A.; McArthur, S.L.; Stoddart, P.R. Improved methods for fluorescence background subtraction from Raman spectra. J Raman Spectrosc. 2013, 44, 1587–1595. [Google Scholar] [CrossRef]
- Esmonde-White, K.A.; Cuellar, M.; Lewis, I.R. The role of Raman spectroscopy in biopharmaceuticals from development to manufacturing. Anal. Bioanal. Chem. 2022, 414, 969–991. [Google Scholar] [CrossRef] [PubMed]
- Lewis, I.R. Handbook of Raman spectroscopy: From the Research Laboratory to the Process Line, 4. Print; CRC Press: New York, NY, USA, 2001; ISBN 0824705572. [Google Scholar]
- Marquard, D.; Enders, A.; Roth, G.; Rinas, U.; Scheper, T.; Lindner, P. In situ microscopy for online monitoring of cell concentration in Pichia pastoris cultivations. J. Biotechnol. 2016, 234, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Guez, J.S.; Cassar, J.P.; Wartelle, F.; Dhulster, P.; Suhr, H. Real time in situ microscopy for animal cell-concentration monitoring during high density culture in bioreactor. J. Biotechnol. 2004, 111, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Bittner, C.; Wehnert, G.; Scheper, T. In situ microscopy for on-line determination of biomass. Biotechnol. Bioeng. 1998, 60, 24–35. [Google Scholar] [CrossRef]
- Dukhin, A.S. Ultrasound for Characterizing Colloids: Particle Sizing, Zeta Potential, Rheology, 1st ed.; Elsevier: Boston, MA, USA, 2010; ISBN 0444511644. [Google Scholar]
- Allegra, J.R.; Hawley, S.A. Attenuation of Sound in Suspensions and Emulsions: Theory and Experiments. J. Acoust. Soc. Am. 1972, 51, 1545–1564. [Google Scholar] [CrossRef]
- Rossing, T.D. Springer Handbook of Acoustics: With CD-ROM, 962 Figures and 91 Tables; Springer: New York, NY, USA, 2007; ISBN 978-0-387-30446-5. [Google Scholar]
- Dukhin, A.S.; Goetz, P.J. Characterization of Liquids, Nano- and Microparticulates, and Porous Bodies using Ultrasound, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2002; ISBN 9780444602237. [Google Scholar]
- Goodenough, T.I.J.; Rajendram, V.S.; Meyer, S.; Prêtre, D. Detection and quantification of insoluble particles by ultrasound spectroscopy. Ultrasonics 2005, 43, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Lin, Y.-H.; Li, W.-T.; Wang, S.-H.; Huang, C.-C. Estimation of Cell Concentration Using High-Frequency Ultrasonic Backscattering. J. Med. Biol. Eng. 2012, 32, 157. [Google Scholar] [CrossRef]
- Elvira, L.; Vera, P.; Cañadas, F.J.; Shukla, S.K.; Montero, F. Concentration measurement of yeast suspensions using high frequency ultrasound backscattering. Ultrasonics 2016, 64, 151–161. [Google Scholar] [CrossRef]
- Rodriguez-Molares, A.; Howard, C.; Zander, A. Determination of biomass concentration by measurement of ultra-sonic attenuation. Appl. Acoust. 2014, 81, 26–30. [Google Scholar] [CrossRef]
- Zhan, X.; Jiang, S.; Yang, Y.; Liang, J.; Shi, T.; Li, X. Inline Measurement of Particle Concentrations in Multicomponent Suspensions using Ultrasonic Sensor and Least Squares Support Vector Machines. Sensors 2015, 15, 24109–24124. [Google Scholar] [CrossRef]
- Akbari, S.; Anderson, P.; Zang, H.; Ganjian, A.; Balke, R.; Kwon, T.; Pollard, D. Non-invasive real-time monitoring of cell concentration and viability using Doppler ultrasound. SLAS Technol. 2022, 27, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Schöck, T.; Becker, T. Sensor array for the combined analysis of water–sugar–ethanol mixtures in yeast fermentations by ultrasound. Food Control 2010, 21, 362–369. [Google Scholar] [CrossRef]
- Kuo, F.-J.; Sheng, C.-T.; Ting, C.-H. Evaluation of ultrasonic propagation to measure sugar content and viscosity of reconstituted orange juice. J. Food Eng. 2008, 86, 84–90. [Google Scholar] [CrossRef]
- Resa, P.; Elvira, L.; Montero de Espinosa, F. Concentration control in alcoholic fermentation processes from ultra-sonic velocity measurements. Food Res. Int. 2004, 37, 587–594. [Google Scholar] [CrossRef]
- Vatandas, M.; Koc, A.B.; Koc, C. Ultrasonic velocity measurements in ethanol–water and methanol–water mixtures. Eur Food Res. Technol. 2007, 225, 525–532. [Google Scholar] [CrossRef]
- Hoche, S.; Hussein, M.A.; Becker, T. Critical process parameter of alcoholic yeast fermentation: Speed of sound and density in the temperature range 5–30 °C. Int. J. Food Sci. Technol. 2014, 49, 2441–2448. [Google Scholar] [CrossRef]
- Çelik, D.A.; Amer, M.A.; Novoa-Díaz, D.F.; Chávez, J.A.; Turó, A.; García-Hernández, M.J.; Salazar, J. Design and implementation of an ultrasonic sensor for rapid monitoring of industrial malolactic fermentation of wines. Instrum. Sci. Technol. 2018, 46, 387–407. [Google Scholar] [CrossRef]
- Krause, D.; Hussein, W.B.; Hussein, M.A.; Becker, T. Ultrasonic sensor for predicting sugar concentration using multivariate calibration. Ultrasonics 2014, 54, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.; Heermann, K.; Hussein, M.; Becker, T. Effects of yeast and maltose concentration on ultrasonic velocity and attenuation coefficient and its application for process monitoring. Eng. Life Sci. 2014, 14, 433–441. [Google Scholar] [CrossRef]
- Zhan, X.; Jiang, S.; Yang, Y.; Liang, J.; Shi, T.; Li, X. Ultrasonic characterization of aqueous mixture comprising in-soluble and soluble substances with temperature compensation. Chemom. Intell. Lab. Syst. 2016, 159, 12–19. [Google Scholar] [CrossRef]
- Hafiz Amer Eissa, A. Food Production and Industry; IntechOpen: London, UK, 2015; ISBN 9789535121916. [Google Scholar]
- Narziß, L.; Back, W.; Gastl, M.; Zarnkow, M. Applied Malting and Brewing Science: A Weihenstephan Compendium; Wiley-VCH: Weinheim, Germany, 2024; ISBN 9783527347346. [Google Scholar]
- Jacob, F. Wort, Beer, Beer-Based Beverages: Collection of Brewing Analysis Methods of the Mitteleuropäische Brautechnische Analysenkommission; MEBAK: Freising-Weihenstephan, Germany, 2013; ISBN 9783980581479. [Google Scholar]
- Hoche, S.; Hussein, M.A.; Becker, T. Density, ultrasound velocity, acoustic impedance, reflection and absorption coefficient determination of liquids via multiple reflection method. Ultrasonics 2015, 57, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.E. Physical Properties of Polymers Handbook, 2nd ed.; Springer Science+Business Media LLC: New York, NY, USA, 2007; ISBN 978-0-387-69002-5. [Google Scholar]
- Królikowski, J.; Szczepek, J. Phase shift of the reflection coefficient of ultrasonic waves in the study of the contact interface. Wear 1992, 157, 51–64. [Google Scholar] [CrossRef]
- Butler, J.L.; Sherman, C.H. Transducers and Arrays for Underwater Sound; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Marczak, W. Water as a standard in the measurements of speed of sound in liquids. J. Acoust. Soc. Am. 1997, 102, 2776–2779. [Google Scholar] [CrossRef]
- Cheeke, J.D.N. Fundamentals and Applications of Ultrasonic Waves; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781138077201. [Google Scholar]
- Guo, N.; Lim, M.K.; Pialucha, T. Measurement of attenuation using a normalized amplitude spectrum. J. Nondestruct. Eval. 1995, 14, 9–19. [Google Scholar] [CrossRef]
- MEBAK online. Method B-590.06.900. Osmolality—Osmometer. Rev. 2020-10.; Mitteleuropäische Brautechnische Analysenkommission (MEBAK®) e.V.: Freising, Germany, 2020. [Google Scholar]
- MEBAK online. Method B-400.11.111. Free Amino Nitrogen (FAN)—Ninhydrin Method (Spectrophotometric). Rev. 2020-10; Mitteleuropäische Brautechnische Analysenkommission (MEBAK®) e.V.: Freising, Germany, 2020. [Google Scholar]
- Hahs-Vaughn, D.L.; Lomax, R.G. Statistical Concepts: A Second Course; Routledge: New York, NY, USA, 2019; ISBN 1-000-13471-7. [Google Scholar]
- Ensminger, D.; Bond, L.J. Ultrasonics: Fundamentals, Technologies, and Applications, 4th ed.; CRC Press: Boca Raton, FL, USA; Taylor et Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2024; ISBN 9780367252816. [Google Scholar]
- Novoa-Díaz, D.; García-Álvarez, J.; Chávez, J.A.; Turó, A.; García-Hernández, M.J.; Salazar, J. Comparison of methods for measuring ultrasonic velocity variations during ageing or fermentation of food materials. IET Sci. Meas. Technol. 2012, 6, 205. [Google Scholar] [CrossRef]
- Smith, J.O. Mathematics of the Discrete Fourier Transform (DFT): With Audio Applications, 2nd ed.; BookSurge: North Charleston, SC, USA, 2007; ISBN 097456074X. [Google Scholar]
- Savva, M. Pharmaceutical Calculations: A Conceptual Approach; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Gibson, B.R.; Lawrence, S.J.; Leclaire, J.P.R.; Powell, C.D.; Smart, K.A. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol. Rev. 2007, 31, 535–569. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.M.; Powell, C.D.; Fischborn, T.; Smart, K.A. Rehydration of Active Dry Brewing Yeast and its Effect on Cell Viability. J. Inst. Brew. 2011, 117, 377–382. [Google Scholar] [CrossRef]
- Bowler, A.L.; Bakalis, S.; Watson, N.J. Monitoring Mixing Processes Using Ultrasonic Sensors and Machine Learning. Sensors 2020, 20, 1813. [Google Scholar] [CrossRef] [PubMed]
- Mowbray, M.; Savage, T.; Wu, C.; Song, Z.; Cho, B.A.; Del Rio-Chanona, E.A.; Zhang, D. Machine learning for bio-chemical engineering: A review. Biochem. Eng. J. 2021, 172, 108054. [Google Scholar] [CrossRef]

| Code | Strain | Product and Manufacturer |
|---|---|---|
| S. cerevisiae | Saccharomyces cerevisiae | FERMIPAN® RED, Casteggio Lieviti srl, Casteggio, Italy |
| WB-06 | Saccharomyces cerevisiae var. diastaticus | SafAle™ WB-06, Fermentis, Division of S.I. Lesaffre, Marcq en Baroeul, France |
| W 34/70 | Saccharomyces pastorianus | SafLager™ W 34/70, Fermentis, Division of S.I. Lesaffre, Marcq en Baroeul, France |
| Extract [%mas] | Density [kg/m3] | Alcohol [%mas] | Osmolality [mOsm/kg] | FAN [mg/100 g] | pH | ||
|---|---|---|---|---|---|---|---|
| Ringer | 0.52 ± 0.00 c | 1000.23 ± 0.00 c | 0.00 ± 0.00 c | 105.33 ± 0.61 c | 0.00 ± 0.00 c | 6.90 ± 0.03 a | |
| Filtrates | S. cerevisiae | 0.64 ± 0.01 a | 1000.63 ± 0.03 a | 0.03 ± 0.00 a | 116.87 ± 0.15 b | 8.69 ± 0.18 a | 5.41 ± 0.02 c |
| WB-06 | 0.60 ± 0.01 b | 1000.50 ± 0.04 b | 0.02 ± 0.01 b | 116.47 ± 0.51 b | 7.79 ± 0.35 b | 5.51 ± 0.02 b | |
| W 34/70 | 0.66 ± 0.02 a | 1000.70 ± 0.09 a | 0.02 ± 0.00 b | 121.18 ± 0.16 a | 8.95 ± 0.51 a | 5.22 ± 0.04 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geier, D.; Mailänder, M.; Whitehead, I.; Becker, T. Non-Invasive Characterization of Different Saccharomyces Suspensions with Ultrasound. Sensors 2024, 24, 6271. https://doi.org/10.3390/s24196271
Geier D, Mailänder M, Whitehead I, Becker T. Non-Invasive Characterization of Different Saccharomyces Suspensions with Ultrasound. Sensors. 2024; 24(19):6271. https://doi.org/10.3390/s24196271
Chicago/Turabian StyleGeier, Dominik, Markus Mailänder, Iain Whitehead, and Thomas Becker. 2024. "Non-Invasive Characterization of Different Saccharomyces Suspensions with Ultrasound" Sensors 24, no. 19: 6271. https://doi.org/10.3390/s24196271






