Spectroscopic Characteristics of Highly Selective Manganese Catalysis in Acqueous Polyurethane Systems
Abstract
:Introduction
Experimental
Instrumental measurements
Synthesis of the complexes
Results and Discussion
Infrared spectra
Electronic spectra
Magnetic properties
Decomposition of the complex with the mixed ligand
Conclusions
Acknowledgments
References
- Noble, K.L. Waterborne polyurethanes. Progress in Organic Coatings 1997, 32, 131–136. [Google Scholar]
- Collong, W.; Gobel, A.; Kleuser, B.; Lenhard, W.; Sootag, M. 2K waterborne clearcoat-a competition between crosslinking and side reactions. Progress in Organic Coatings 2002, 45, 205–209. [Google Scholar]
- He, Z.A.; Blank, W.J.; Picci, M.E. A selective catalyst for two-component waterborne polyurethane coatings. Journal of Coatings Technology 2002, 74, 31–36. [Google Scholar]
- Blank, W.J.; He, Z.A.; Hessell, E.T. Catalysis of the isocyanate-hydroxyl reaction by non-tin catalysts. Progress in Organic Coatings 1999, 35, 19–29. [Google Scholar]
- Stamenkovic, J.; Cakic, S.; Nikolic, G. Study of the catalytic selectivity of an aqueous two-component polyurethane system by FTIR spectroscopy. Chem. Industry 2003, 57, 559–562. [Google Scholar]
- Nikolaev, A.F.; Belogorodskaya, K.V.; Shibalovich, V.G.; Andreeva, E.D. Poluchenie i svoistva bisacetilacetonatokarboksilatov marganca III. Journal of Applied Chemistry 1973, 12, 2718–2725. [Google Scholar]
- Diaz-Acosta, J.; Baker, J.F.; Pulay, P. Calculated and experimental geometries and infrared spectra of metal tris-acetylacetonates: vibrational spectroscopy as a probe of molecular structure for ionic complexes. Part II. Spectrochimica Acta Part A 2003, 59, 363–377. [Google Scholar]
- Bruenner, R.; Obirth, A. On the mechanism of metal chelate catalysis in the reaction between alcohols and isocyanates. Journal Chem. Society 1965, 31, 887–891. [Google Scholar]
- Shibalovich, V.G.; Belogorodskaya, K.V.; Karkozov, V.G. Novye kataliticheskie sistemy na osnove khelatnih komplesov marganca(III) i ikh primenenie v processakh polimerizacii i otverzhdeniya. Plast. massy 1989, 11, 18–25. [Google Scholar]
- Ligabue, R.; Monteiro, A.; Souza, R.; Souza, M. Catalytic propertis of Fe(acac)3 and Cu(acac)2 in the formation of urethane from a diisocyanate derivative and EtOH. Journal of Molecular Catalysis A: Chemical 1998, 130, 101–105. [Google Scholar]
- Stamenkovic, J.; Cakic, S.; Konstantinovic, S.; Stoilkovic, S. Catalysis of the isocyanate-hydroxyl reaction by non-tin catalysts in waterborne two-component polyurethane coatings. Facta Universitatis 2004, 2, 243–250. [Google Scholar]
- Stamenkovic, J.; Cakic, S.; Stoilkovic, S. The influence of catalysts selectivity at physical-mechanical properties of two-component polyurethane coatings. World of Polymers 2003, 6, 257–292. [Google Scholar]
- Nakamoto, K.; McCarthy, P.J.; Ruby, A.; Martell, A.E. Infrared Spectra of Metal Chelate Compounds. II. Infrared Spectra of Acetylacetonates of Trivalent Metals. Journal of American Chemical Society 1961, 83, 1066–1069. [Google Scholar]
- Stults, B.R.; Marianelli, R.S.; Day, V.W. Distortions of the coordination polyhedron in high-spin manganese(III) complexes. I. Synthesis and characterization of a series of five- and six-coordinate bis(acetylacetonato)manganese(III) complexes. Crystal structure of azidobis(acetylacetonato)-manganese(III). Inorganic Chemistry 1975, 14, 722–730. [Google Scholar]
- Perina, G.N.; Shibalovich, V.G.; Nikolaev, A.F. Smeshchanno-ligandnye kompleksy marganca (III) na osnove trisacetilacetonata marganca i neorganicheskih kislot. Journal of Applied Chemistry 1982, 1, 30–37. [Google Scholar]
- Josheska, N. The Study of acrilonitrile polymerization with water solution of sodium-rhodanide, iniciated by Mn(III) complex with mixed ligands. PhD Thesis, Faculty of Technology, University of Nis, Serbia, 1992. [Google Scholar]
- DeVos, D.E.; Weckhuysen, B.M.; Bein, T. ESR Fine Structure of Manganese Ions in Zeolite A Detects Strong Variations of the Coordination Environment. Journal of American Chemical Society 1996, 118(40), 9615–9622. [Google Scholar]
- Cakic, S.; Lacnjevac, C.; Rajkovic, M.B.; Raskovic, Lj.; Stamenkovic, J. Reticulation of Agueous Polyurethane Systems Controlled by DSC Method. Sensors 2006, 6, 536–545. [Google Scholar]
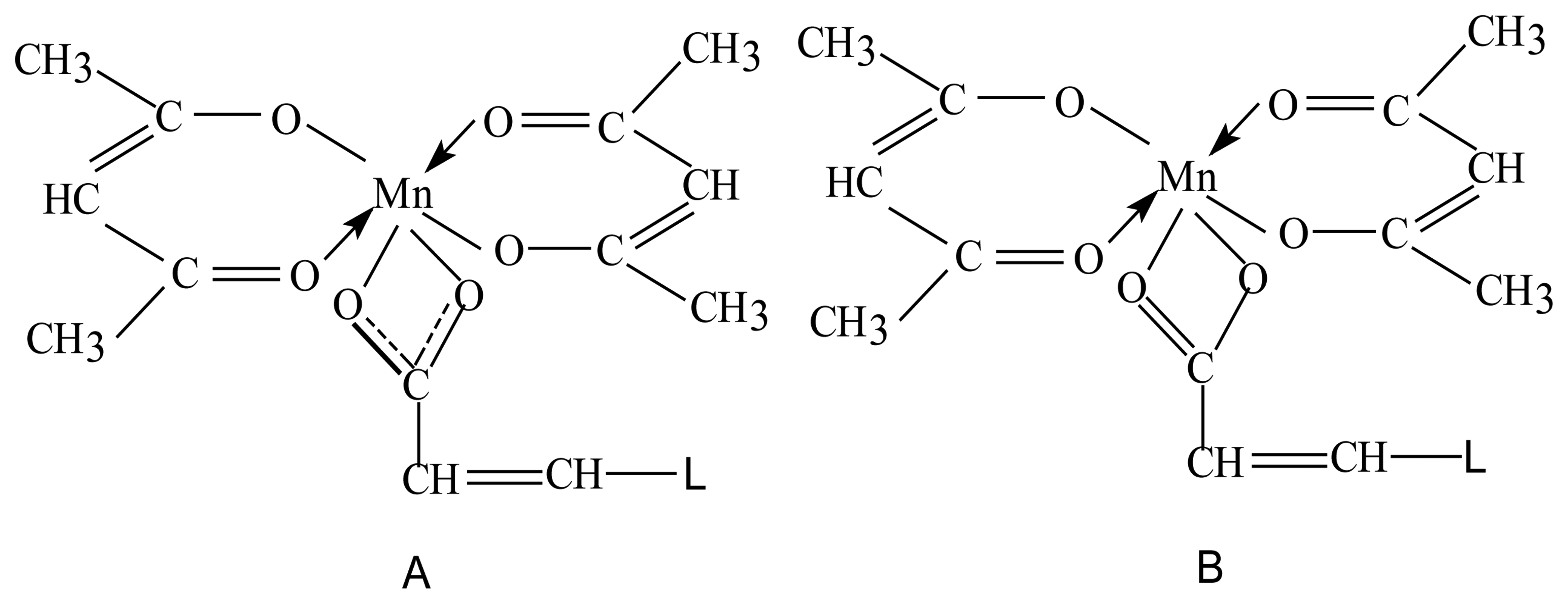
- A)
- the complex of Mn(III)-acetylacetone,
- B)
- the complex of Mn(III)-diacetylacetonemaleate.
- A)
- the complex of Mn(III)-acetylacetone,
- B)
- the complex of Mn(III)-diacetylacetonemaleate.


- A)
- the complex of Mn(III)-acetylacetone,
- B)
- the complex of Mn(III)-diacetylacetonehydroxylaminmaleate.
- A)
- the complex of Mn(III)-acetylacetone,
- B)
- the complex of Mn(III)-diacetylacetonehydroxylaminmaleate.


- a)
- of Mn(III)-acetylacetone and
- b)
- of Mn(III)-diacetylacetonemaleate in ethanol with respect to time.
- a)
- of Mn(III)-acetylacetone and
- b)
- of Mn(III)-diacetylacetonemaleate in ethanol with respect to time.


- a)
- of Mn(III)-diacetylacetonemaleate, and
- b)
- of Mn(III)-diacetylacetonehydroxylaminmaleate.
- a)
- of Mn(III)-diacetylacetonemaleate, and
- b)
- of Mn(III)-diacetylacetonehydroxylaminmaleate.

- a)
- of Mn(III)-acetylacetone,
- b)
- of Mn(III)-diacetylacetonemaleate and
- c)
- of Mn(III)-diacetylacetonehydroxylaminmaleate.
- a)
- of Mn(III)-acetylacetone,
- b)
- of Mn(III)-diacetylacetonemaleate and
- c)
- of Mn(III)-diacetylacetonehydroxylaminmaleate.

| Compounds | Molecular Formula | Yield(in %) | Colour | Carbon (in %) | Hydrogen (in %) | Nitrogen (in %) | Manganese (in %) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| found | calcd | found | calcd | found | calcd | found | calcd | ||||
| I | [Mn(C5H7O2)2L1] | 90 | dark-green | 44.33 | 45.61 | 4.57 | 4.62 | - | - | 15.3 | 14.9 |
| II | [Mn(C5H7O2)2L2] | 80 | green | 42.51 | 43.82 | 4.59 | 4.70 | 3.51 | 3.65 | 13.6 | 14.3 |
| [Mn(C5H7O2)3] | [Mn(C5H7O2)2L1] | [Mn(C5H7O2)2L2] | Assigment of bands a |
|---|---|---|---|
| 430 m | 417 m | 425 m | ν(Mn-O), δ(Mn-O=C) |
| 460 m | 482 m | 487 m | ν(Mn-O), ν(C-CH3), ν(C=C) |
| 550 m | 613 m | 575 mb | πring+ ν(Mn-O) |
| 588 s | 654 s | 633 m | ν(Mn-O), δ(Mn-O=C, CH3-C=O) |
| 669 ms | 689 m | 689 m | ν(C-CH3), ν(Mn-O), δ(O=C-CH3) |
| 771 m | γ(N-H). | ||
| 778 s | 782 s | 778 w c | π(C-H) |
| 875 m | 876 m | δ(COO) | |
| 928 s | 928 m | 927 m | ν(C-CH3) |
| 1020 s | 1020 s | 1023 s | γ(CH3) |
| 1185 w | 1173 w | 1180 w | δ(C=CH) |
| 1253 s | 1285 s | 1281 m | ν(C=C), ν(C-CH3) |
| 1312 m | ν(C-N), δ(N-H) | ||
| 1338 s | νsCOOH) | ||
| 1350 s | 1361 s | δ(CH3) | |
| 1388 s | 1388 w | δ(CH3) | |
| 1458 m | 1407 1462 m | νs(COO) δ(=CH) | |
| 1512 s | 1523 s | 1514 s | ν(C=C), δ(C=CH) |
| 1567 s | 1552 s | 1551 s | ν(C=C), δ(C=CH) |
| 1595 s | 1615 s | 1595 s | νas(COO) |
| 1635 s | ν(C=C) | ||
| 1651 m | ν(C=O) | ||
| 1708 m | νas(COOH) | ||
| 2920 w | 2915 w | 2920 w | ν(C-H) |
| 3229 m | 3213 m | ν(=CH) | |
| 3559 m | ν(N-H) | ||
| 3564 s | 3400 s | ν(O-H) |
| Complex | μeff/μB | ν(104cm-1)/ ε | ||||
|---|---|---|---|---|---|---|
| π→π* | π→π* | π*→d | d→d* | d→d* | ||
| [Mn(C5H7O2)3] | 4.90 | 40.8/17 500 | 36.4/24 000 | 30.7/9 500 | 24.8/950 | 17.5/100 |
| [Mn(C5H7O2)2L1] | 4.76 | 41.6/31 000 | 35.2/12 000 | 30.2/17 500 | 24.3/1 000 | 17.6/70 |
| [Mn(C5H7O2)2L2] | 4.81 | 42.0/22 500 | 35.9/14 500 | 30.3/11 000 | 24.8/1 500 | 17.5/170 |
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Cakic, S.; Lacnjevac, C.; Nikolic, G.; Stamenkovic, J.; Rajkovic, M.B.; Gligoric, M.; Barac, M. Spectroscopic Characteristics of Highly Selective Manganese Catalysis in Acqueous Polyurethane Systems. Sensors 2006, 6, 1708-1720. https://doi.org/10.3390/s6111708
Cakic S, Lacnjevac C, Nikolic G, Stamenkovic J, Rajkovic MB, Gligoric M, Barac M. Spectroscopic Characteristics of Highly Selective Manganese Catalysis in Acqueous Polyurethane Systems. Sensors. 2006; 6(11):1708-1720. https://doi.org/10.3390/s6111708
Chicago/Turabian StyleCakic, Suzana, Caslav Lacnjevac, Goran Nikolic, Jakov Stamenkovic, Milos B. Rajkovic, Miladin Gligoric, and Miroljub Barac. 2006. "Spectroscopic Characteristics of Highly Selective Manganese Catalysis in Acqueous Polyurethane Systems" Sensors 6, no. 11: 1708-1720. https://doi.org/10.3390/s6111708
APA StyleCakic, S., Lacnjevac, C., Nikolic, G., Stamenkovic, J., Rajkovic, M. B., Gligoric, M., & Barac, M. (2006). Spectroscopic Characteristics of Highly Selective Manganese Catalysis in Acqueous Polyurethane Systems. Sensors, 6(11), 1708-1720. https://doi.org/10.3390/s6111708





