Non-Nucleoside Reverse Transcriptase Inhibitors Join Forces with Integrase Inhibitors to Combat HIV
Abstract
:1. Introduction
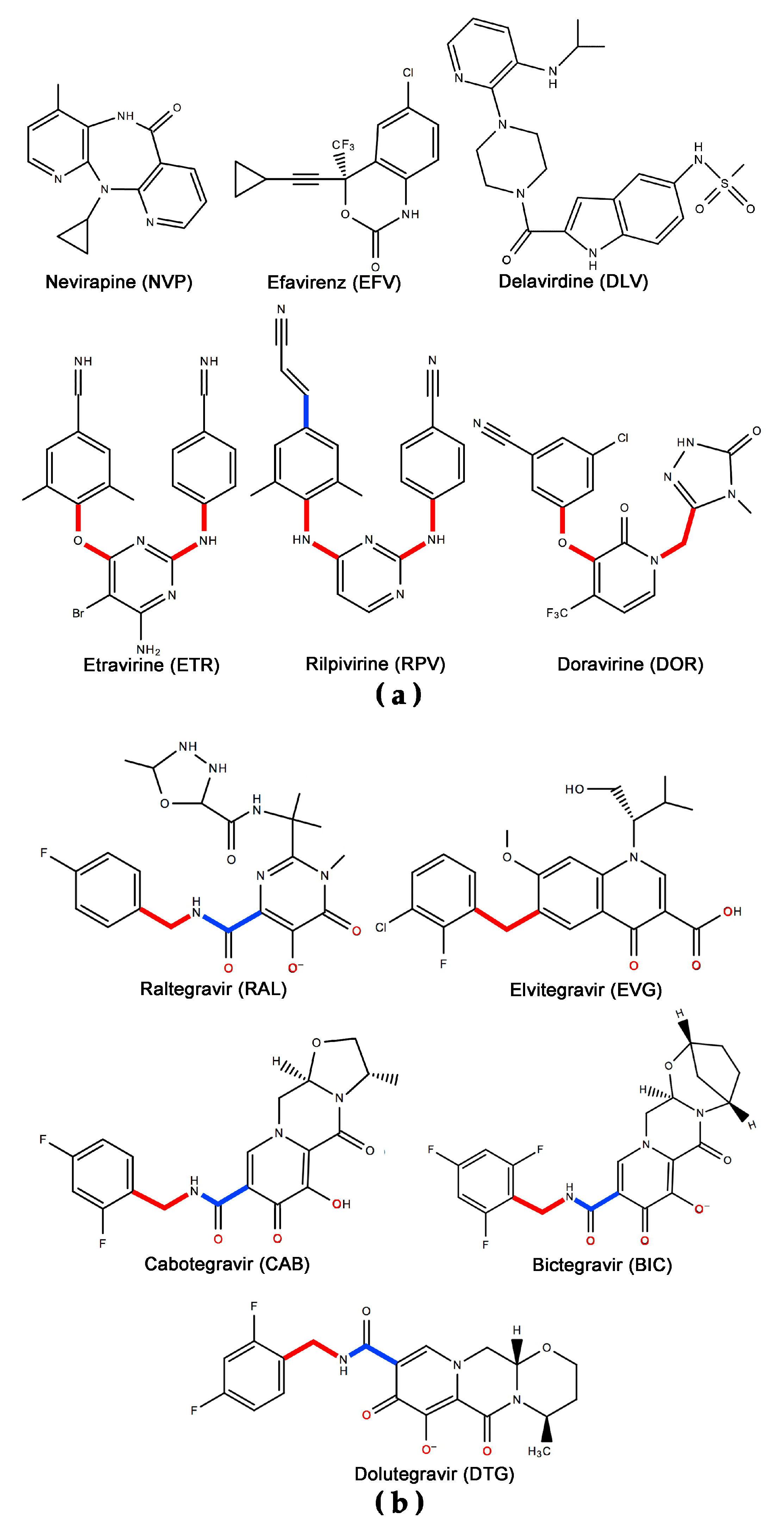
2. Efficacy of ETR and RPV
3. DAPY/INSTI Dual Therapies
3.1. ETR/RAL
3.2. RPV/CAB
3.3. RPV/DTG
4. Safety
4.1. Neuropsychiatric Comorbidities
4.2. ETR and RAL
4.3. RPV and DTG
5. Adherence
6. Drug Resistance
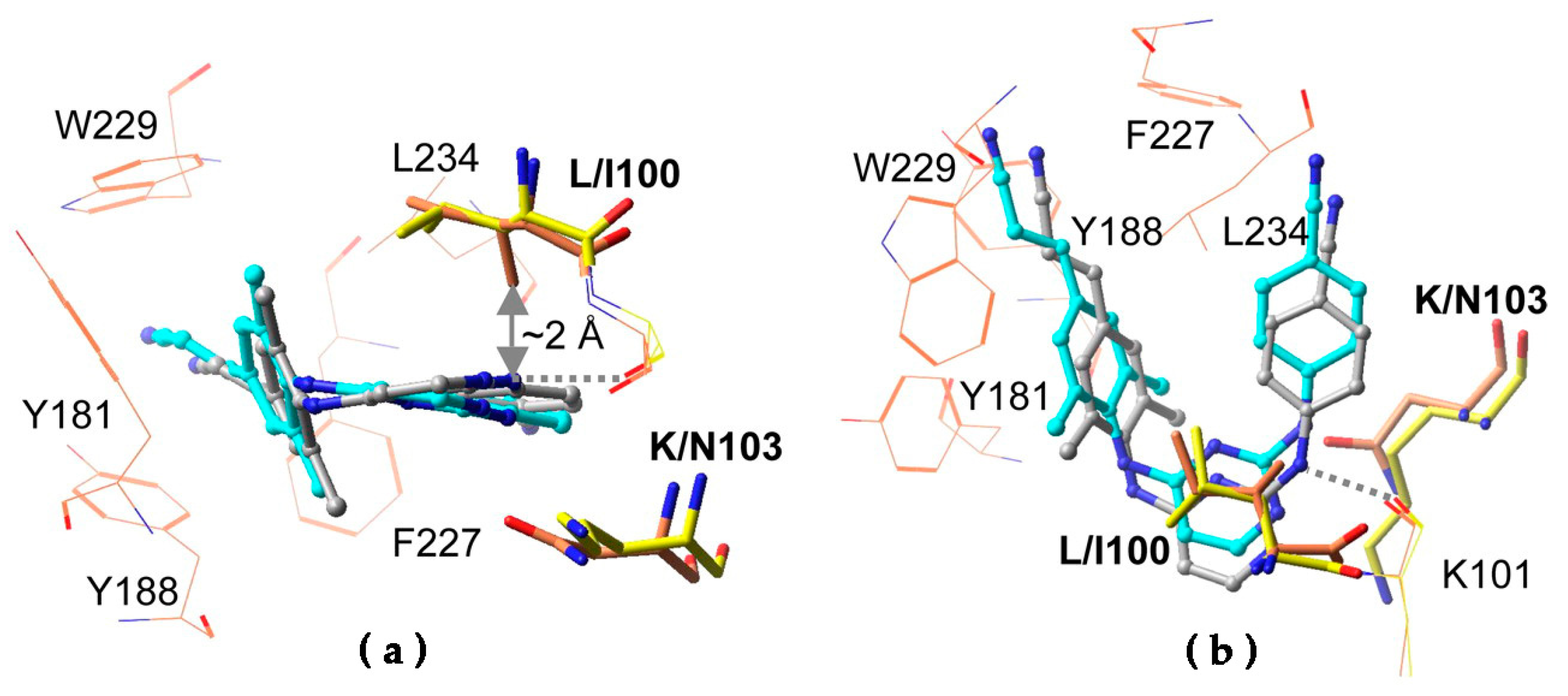
6.1. Resistance to DAPYs
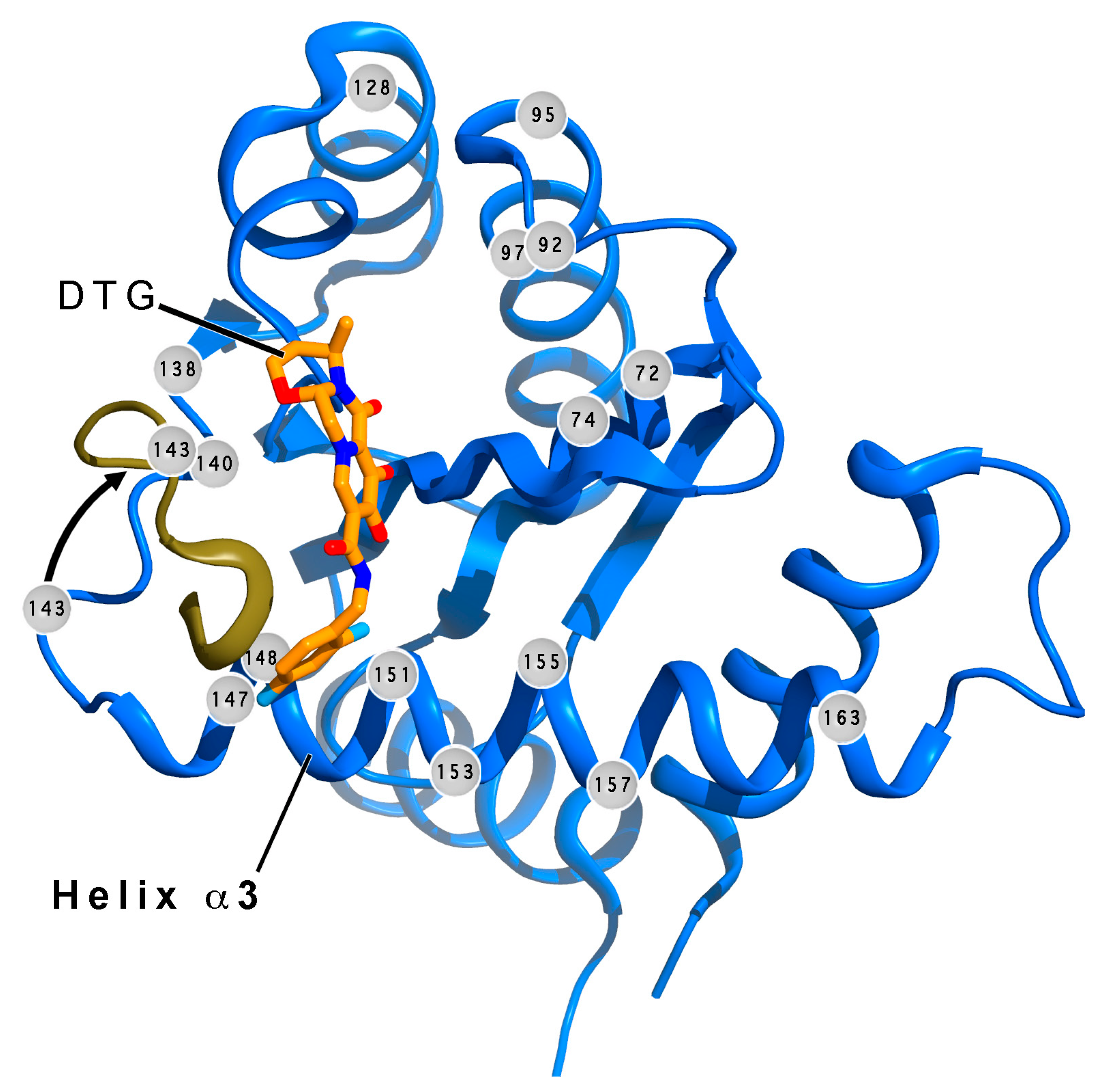
6.2. Resistance to INSTIs
7. Mechanism of Inhibitor Response to Drug-Resistance Mutations
8. Exciting Prospects for Long-Acting Treatments
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Clercq, E. The history of antiretrovirals: Key discoveries over the past 25 years. Rev. Med. Virol. 2009, 19, 287–299. [Google Scholar] [CrossRef]
- Cousteau, C.; Bigot-Massoni, D.; Vittecoq, D.; D’Agay, M.F.; Brocheriou, C.; Laufer, J. [Stomatological lesions seen in HIV infection. 1. Clinical study. Apropos 111 cases]. Rev. Stomatol. Chir. Maxillofac. 1987, 88, 85–92. [Google Scholar]
- Gluckman, J.C.; Klatzmann, D. [From HIV infection to AIDS]. Nouv. Rev. Fr. Hematol. 1987, 29, 9–12. [Google Scholar]
- Becker, J.L.; Hazan, U.; Nugeyre, M.T.; Rey, F.; Spire, B.; Barre-Sinoussi, F.; Georges, A.; Teulieres, L.; Chermann, J.C. [Infection of insect cell lines by the HIV virus, an agent of AIDS, and a demonstration of insects of African origin infected by this virus]. Comptes Rendus Acad. Sci. III 1986, 303, 303–306. [Google Scholar] [PubMed]
- Epstein, L.G.; Sharer, L.R.; Cho, E.S.; Myenhofer, M.; Navia, B.; Price, R.W. HTLV-III/LAV-like retrovirus particles in the brains of patients with AIDS encephalopathy. AIDS Res. 1984, 1, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Cheingsong-Popov, R.; Weiss, R.A.; Dalgleish, A.; Tedder, R.S.; Shanson, D.C.; Jeffries, D.J.; Ferns, R.B.; Briggs, E.M.; Weller, I.V.; Mitton, S.; et al. Prevalence of antibody to human T-lymphotropic virus type III in AIDS and AIDS-risk patients in Britain. Lancet 1984, 2, 477–480. [Google Scholar] [CrossRef]
- Wernicke, D.; van den Helm, K.; Abb, J.; Eberle, J.; Zoulek, G.; Pleyl, G.; Deinhardt, F.; Riethmuller, G.; Ziegler-Heitbrock, H.W.; Rieber, E.P.; et al. [Antibodies against human T-cell leukemia virus type III in acquired immunodeficiency syndrome and persistent lymphadenopathy]. Dtsch. Med. Wochenschr. 1984, 109, 1709–1711. [Google Scholar] [CrossRef]
- Barre-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Bin, C.; Vezinet-Brun, F.; Rouzioux, C.; et al. Isolation of T-lymphotropic Retrovirus from a Patient at Risk for Acquired Immune Deficiency Syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef] [Green Version]
- Gallo, R.C.; Sarin, P.S.; Gelmann, E.P.; Robert-Guroff, M.; Richardson, E.; Kalyanaraman, V.S.; Mann, D.; Sidhu, G.D.; Stahl, R.E.; Zolla-Pazner, S.; et al. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science 1983, 220, 865–867. [Google Scholar] [CrossRef]
- Popovic, M.; Sarin, P.S.; Robert-Gurroff, M.; Kalyanaraman, V.S.; Mann, D.; Minowada, J.; Gallo, R.C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science 1983, 219, 856–859. [Google Scholar] [CrossRef]
- Cihlar, T.; Fordyce, M. Current status and prospects of HIV treatment. Curr. Opin. Virol. 2016, 18, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffin, J.M. HIV population dynamics in vivo: Implications for genetic variation, pathogenesis, and therapy. Science 1995, 267, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Baral, S.; Rao, A.; Sullivan, P.; Phaswana-Mafuya, N.; Diouf, D.; Millett, G.; Musyoki, H.; Geng, E.; Mishra, S. The disconnect between individual-level and population-level HIV prevention benefits of antiretroviral treatment. Lancet HIV 2019, 6, e632–e638. [Google Scholar] [CrossRef] [Green Version]
- Eisinger, R.W.; Dieffenbach, C.W.; Fauci, A.S. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA 2019, 321, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Usach, I.; Melis, V.; Peris, J.E. Non-nucleoside reverse transcriptase inhibitors: A review on pharmacokinetics, pharmacodynamics, safety and tolerability. J. Int. AIDS Soc. 2013, 16, 1–14. [Google Scholar] [CrossRef]
- Menéndez-Arias, L.; Betancor, G.; Matamoros, T. HIV-1 Reverse Transcriptase Connection Subdomain Mutations Involved in Resistance to Approved Non-Nucleoside Inhibitors. Antivir. Res. 2011, 92, 139–149. [Google Scholar]
- De Clercq, E. The Role of Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) in the Therapy of HIV-1 Infection. Antivir. Res. 1998, 38, 153–179. [Google Scholar] [CrossRef]
- Telesnitsky, A.; Goff, S.P. Reverse Transcriptase and the Generation of Retroviral DNA. In Retroviruses; Coffin, J.M., Hughes, S.H., Varmus, H.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997. [Google Scholar]
- Hindmarsh, P.; Leis, J. Retroviral DNA integration. Microbiol. Mol. Biol. Rev. 1999, 63, 836–843. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.O. Integration; Coffin, J.M., Hughes, S.H., Varmus, H.E., Eds.; Cold Spring Harbor Laboratory Press: Plainview, NY, USA, 1997; pp. 161–203. [Google Scholar]
- Asante-Appiah, E.; Skalka, A.M. Molecular mechanisms in retrovirus DNA integration. Antivir. Res. 1997, 36, 139–156. [Google Scholar] [CrossRef]
- Vogt, V.M. Retroviral Virions and Genomes. In Retroviruses; Coffin, J.M., Hughes, S.H., Varmus, H.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997; pp. 27–69. [Google Scholar]
- Frankel, A.D.; Young, J.A. HIV-1: Fifteen proteins and an RNA. Annu. Rev. Biochem. 1998, 67, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Debouck, C.; Gorniak, J.G.; Strickler, J.E.; Meek, T.D.; Metcalf, B.W.; Rosenberg, M. Human immunodeficiency virus protease expressed in Escherichia coli exhibits autoprocessing and specific maturation of the gag precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 8903–8906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mervis, R.J.; Ahmad, N.; Lillehoj, E.P.; Raum, M.G.; Salazar, F.H.; Chan, H.W.; Venkatesan, S. The gag gene products of human immunodeficiency virus type 1: Alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J. Virol. 1988, 62, 3993–4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettit, S.C.; Moody, M.D.; Wehbie, R.S.; Kaplan, A.H.; Nantermet, P.V.; Klein, C.A.; Swanstrom, R. The p2 domain of human immunodeficiency virus type 1 Gag regulates sequential proteolytic processing and is required to produce fully infectious virions. J. Virol. 1994, 68, 8017–8027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacks, T.; Power, M.D.; Masiarz, F.R.; Luciw, P.A.; Barr, P.J.; Varmus, H.E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature 1988, 331, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernan, M.A.; Ledergerber, B.; Tilling, K.; Weber, R.; Sendi, P.; Rickenbach, M.; Robins, J.M.; Egger, M.; Swiss, H.I.V.C.S. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: A prospective cohort study. Lancet 2005, 366, 378–384. [Google Scholar] [CrossRef]
- Janssen, P.A.J.; Lewi, P.J.; Arnold, E.; Daeyaert, F.; de Jonge, M.; Heeres, J.; Koymans, L.; Vinkers, M.; Guillemont, J.; Pasquier, E.; et al. In search of a novel anti-HIV drug: Multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoetenyl]-2-6-dimethylphenyl]amino]-2-pyrimidinyl]amino]-benzonitrile (R278474-rilpivirine). J. Med. Chem. 2005, 48, 1901–1909. [Google Scholar] [CrossRef]
- Margolis, A.M.; Heverling, H.; Pham, P.A.; Stolbach, A. A review of the toxicity of HIV medications. J. Med. Toxicol. 2014, 10, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Morlat, P.; Vivot, A.; Vandenhende, M.A.; Dauchy, F.A.; Asselineau, J.; Deti, E.; Gerard, Y.; Lazaro, E.; Duffau, P.; Neau, D.; et al. Role of traditional risk factors and antiretroviral drugs in the incidence of chronic kidney disease, ANRS CO3 Aquitaine cohort, France, 2004–2012. PLoS ONE 2013, 8, e66223. [Google Scholar] [CrossRef] [Green Version]
- Ryom, L.; Mocroft, A.; Kirk, O.; Worm, S.W.; Kamara, D.A.; Reiss, P.; Ross, M.; Fux, C.A.; Morlat, P.; Moranne, O.; et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: The D:A:D study. J. Infect. Dis. 2013, 207, 1359–1369. [Google Scholar] [CrossRef]
- Kozal, M.J.; Lupo, S.; DeJesus, E.; Molina, J.M.; McDonald, C.; Raffi, F.; Benetucci, J.; Mancini, M.; Yang, R.; Wirtz, V.; et al. A nucleoside- and ritonavir-sparing regimen containing atazanavir plus raltegravir in antiretroviral treatment-naive HIV-infected patients: SPARTAN study results. HIV Clin. Trials 2012, 13, 119–130. [Google Scholar] [CrossRef]
- Choi, A.I.; Vittinghoff, E.; Deeks, S.G.; Weekley, C.C.; Li, Y.; Shlipak, M.G. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS 2011, 25, 1289–1298. [Google Scholar] [CrossRef] [Green Version]
- McComsey, G.A.; Kitch, D.; Daar, E.S.; Tierney, C.; Jahed, N.C.; Tebas, P.; Myers, L.; Melbourne, K.; Ha, B.; Sax, P.E. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J. Infect. Dis. 2011, 203, 1791–1801. [Google Scholar] [CrossRef]
- Mocroft, A.; Kirk, O.; Reiss, P.; De Wit, S.; Sedlacek, D.; Beniowski, M.; Gatell, J.; Phillips, A.N.; Ledergerber, B.; Lundgren, J.D.; et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS 2010, 24, 1667–1678. [Google Scholar] [CrossRef] [Green Version]
- Obel, N.; Farkas, D.K.; Kronborg, G.; Larsen, C.S.; Pedersen, G.; Riis, A.; Pedersen, C.; Gerstoft, J.; Sorensen, H.T. Abacavir and risk of myocardial infarction in HIV-infected patients on highly active antiretroviral therapy: A population-based nationwide cohort study. HIV Med. 2010, 11, 130–136. [Google Scholar] [CrossRef]
- Sabin, C.A.; Worm, S.W.; Weber, R.; Reiss, P.; El-Sadr, W.; Dabis, F.; De Wit, S.; Law, M.; D’Arminio Monforte, A.; Friis-Moller, N.; et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: A multi-cohort collaboration. Lancet 2008, 371, 1417–1426. [Google Scholar] [CrossRef] [Green Version]
- Arenas-Pinto, A.; Bhaskaran, K.; Dunn, D.; Weller, I.V. The risk of developing peripheral neuropathy induced by nucleoside reverse transcriptase inhibitors decreases over time: Evidence from the Delta trial. Antivir. Ther. 2008, 13, 289–295. [Google Scholar]
- Pinoges, L.; Schramm, B.; Poulet, E.; Balkan, S.; Szumilin, E.; Ferreyra, C.; Pujades-Rodriguez, M. Risk factors and mortality associated with resistance to first-line antiretroviral therapy: Multicentric cross-sectional and longitudinal analyses. J. Acquir. Immune Defic. Syndr. 2015, 68, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Maggiolo, F.; Ravasio, L.; Ripamonti, D.; Gregis, G.; Quinzan, G.; Arici, C.; Airoldi, M.; Suter, F. Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin. Infect. Dis. 2005, 40, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Bauman, J.D.; Clark, A.D., Jr.; Frenkel, Y.V.; Lewi, P.J.; Shatkin, A.J.; Hughes, S.H.; Arnold, E. High-Resolution Structures of HIV-1 Reverse Transcriptase/TMC278 Complexes: Strategic Flexibility Explains Potency Against Resistance Mutations. Proc. Natl. Acad.Sci. USA 2008, 105, 1466–1471. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Lewi, P.J.; Hughes, S.H.; Arnold, E. Crystallography and the Design of Anti-AIDS Drugs: Conformational Flexibility and Positional Adaptability are Important in the Design of Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitors. Prog. Biophys. Mol. Biol. 2005, 88, 209–231. [Google Scholar] [CrossRef]
- Das, K.; Clark, A.D., Jr.; Lewi, P.J.; Heeres, J.; de Jonge, M.R.; Koymans, L.M.H.; Vinkers, H.M.; Daeyaert, F.; Ludovici, D.W.; Kukla, M.J.; et al. Roles of Conformational and Positional Adaptability in Structure-Based Design of TMC125-R165335 (Etravirine) and Related Non-Nucleoside Reverse Transcriptase Inhibitors that are Highly Potent and Effective Against Wild-Type and Drug-Resistant HIV-1 Variants. J. Med. Chem. 2004, 47, 2550–2560. [Google Scholar]
- Sax, P.E. FDA approval: Etravirine. AIDS Clin. Care 2008, 20, 17–18. [Google Scholar]
- O’Neal, R. Rilpivirine and complera: New first-line treatment options. BETA 2011, 23, 14–18. [Google Scholar]
- Molina, J.M.; Clumeck, N.; Orkin, C.; Rimsky, L.T.; Vanveggel, S.; Stevens, M.; ECHO and THRIVE Study Groups. Week 96 analysis of rilpivirine or efavirenz in HIV-1-infected patients with baseline viral load ≤ 100,000 copies/mL in the pooled ECHO and THRIVE phase 3, randomized, double-blind trials. HIV Med. 2014, 15, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Echeverria, P.; Bonjoch, A.; Puig, J.; Molto, J.; Paredes, R.; Sirera, G.; Ornelas, A.; Perez-Alvarez, N.; Clotet, B.; Negredo, E. Randomised study to assess the efficacy and safety of once-daily etravirine-based regimen as a switching strategy in HIV-infected patients receiving a protease inhibitor-containing regimen. Etraswitch study. PLoS ONE 2014, 9, e84676. [Google Scholar] [CrossRef] [Green Version]
- van Lunzen, J.; Antinori, A.; Cohen, C.J.; Arribas, J.R.; Wohl, D.A.; Rieger, A.; Rachlis, A.; Bloch, M.; Segal-Maurer, S.; Garner, W.; et al. Rilpivirine vs. efavirenz-based single-tablet regimens in treatment-naive adults: Week 96 efficacy and safety from a randomized phase 3b study. AIDS 2016, 30, 251–259. [Google Scholar] [CrossRef]
- Cohen, C.J.; Molina, J.M.; Cahn, P.; Clotet, B.; Fourie, J.; Grinsztejn, B.; Wu, H.; Johnson, M.A.; Saag, M.; Supparatpinyo, K.; et al. Efficacy and safety of rilpivirine (TMC278) versus efavirenz at 48 weeks in treatment-naive HIV-1-infected patients: Pooled results from the phase 3 double-blind randomized ECHO and THRIVE Trials. J. Acquir. Immune Defic. Syndr. 2012, 60, 33–42. [Google Scholar] [CrossRef]
- Cohen, C.J.; Andrade-Villanueva, J.; Clotet, B.; Fourie, J.; Johnson, M.A.; Ruxrungtham, K.; Wu, H.; Zorrilla, C.; Crauwels, H.; Rimsky, L.T.; et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): A phase 3, randomised, non-inferiority trial. Lancet 2011, 378, 229–237. [Google Scholar] [CrossRef]
- Molina, J.M.; Cahn, P.; Grinsztejn, B.; Lazzarin, A.; Mills, A.; Saag, M.; Supparatpinyo, K.; Walmsley, S.; Crauwels, H.; Rimsky, L.T.; et al. Rilpivirine Versus Efavirenz with Tenofovir and Emtricitabine in Treatment-Naive Adults Infected with HIV-1 (ECHO): A Phase 3 Randomised Double-Blind Active-Controlled Trial. Lancet 2011, 378, 238–246. [Google Scholar] [CrossRef]
- Gazzard, B.; Duvivier, C.; Zagler, C.; Castagna, A.; Hill, A.; van Delft, Y.; Marks, S. Phase 2 double-blind, randomized trial of etravirine versus efavirenz in treatment-naive patients: 48-week results. AIDS 2011, 25, 2249–2258. [Google Scholar] [CrossRef] [Green Version]
- Ford, N.; Lee, J.; Andrieux-Meyer, I.; Calmy, A. Safety, efficacy, and pharmacokinetics of rilpivirine: Systematic review with an emphasis on resource-limited settings. HIV AIDS (Auckl) 2011, 3, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Katlama, C.; Clotet, B.; Mills, A.; Trottier, B.; Molina, J.M.; Grinsztejn, B.; Towner, W.; Haubrich, R.; Nijs, S.; Vingerhoets, J.; et al. Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antivir. Ther. 2010, 15, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Katlama, C.; Haubrich, R.; Lalezari, J.; Lazzarin, A.; Madruga, J.V.; Molina, J.M.; Schechter, M.; Peeters, M.; Picchio, G.; Vingerhoets, J.; et al. Efficacy and safety of etravirine in treatment-experienced, HIV-1 patients: Pooled 48 week analysis of two randomized, controlled trials. AIDS 2009, 23, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Lazzarin, A.; Campbell, T.; Clotet, B.; Johnson, M.; Katlama, C.; Moll, A.; Towner, W.; Trottier, B.; Peeters, M.; Vingerhoets, J.; et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007, 370, 39–48. [Google Scholar] [CrossRef]
- Madruga, J.V.; Cahn, P.; Grinsztejn, B.; Haubrich, R.; Lalezari, J.; Mills, A.; Pialoux, G.; Wilkin, T.; Peeters, M.; Vingerhoets, J.; et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007, 370, 29–38. [Google Scholar] [CrossRef]
- Azijn, H.; Tirry, I.; Vingerhoets, J.; de Bethune, M.P.; Kraus, G.; Boven, K.; Jochmans, D.; Van Craenenbroeck, E.; Picchio, G.; Rimsky, L.T. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob. Agents Chemother. 2010, 54, 718–727. [Google Scholar] [CrossRef] [Green Version]
- Behrens, G.; Rijnders, B.; Nelson, M.; Orkin, C.; Cohen, C.; Mills, A.; Elion, R.A.; Vanveggel, S.; Stevens, M.; Rimsky, L.; et al. Rilpivirine versus efavirenz with emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV-1-infected patients with HIV-1 RNA ≤ 100,000 copies/mL: Week 96 pooled ECHO/THRIVE subanalysis. AIDS Patient Care STDS, 2014; 28, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Rimsky, L.; Vingerhoets, J.; Van Eygen, V.; Eron, J.; Clotet, B.; Hoogstoel, A.; Boven, K.; Picchio, G. Genotypic and phenotypic characterization of HIV-1 isolates obtained from patients on rilpivirine therapy experiencing virologic failure in the phase 3 ECHO and THRIVE studies: 48-week analysis. J. Acquir. Immune Defic. Syndr. 2012, 59, 39–46. [Google Scholar] [CrossRef]
- Dowers, E.; Zamora, F.; Barakat, L.A.; Ogbuagu, O. Dolutegravir/rilpivirine for the treatment of HIV-1 infection. HIV AIDS (Auckl) 2018, 10, 215–224. [Google Scholar] [CrossRef] [Green Version]
- FDA. EDURANT (Rilpivirine) Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202022s011lbl.pdf (accessed on 26 April 2020).
- Tudor-Williams, G.; Cahn, P.; Chokephaibulkit, K.; Fourie, J.; Karatzios, C.; Dincq, S.; Opsomer, M.; Kakuda, T.N.; Nijs, S.; Tambuyzer, L.; et al. Etravirine in treatment-experienced, HIV-1-infected children and adolescents: 48-week safety, efficacy and resistance analysis of the phase II PIANO study. HIV Med. 2014, 15, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Kitahata, M.M.; Reed, S.D.; Dillingham, P.W.; Van Rompaey, S.E.; Young, A.A.; Harrington, R.D.; Holmes, K.K. Pharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and death. Int. J. STD AIDS 2004, 15, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.T.; Feng, M.; Falgueyret, J.P.; Tawa, P.; Witmer, M.; DiStefano, D.; Li, Y.; Burch, J.; Sachs, N.; Lu, M.; et al. In vitro characterization of MK-1439, a novel HIV-1 nonnucleoside reverse transcriptase inhibitor. Antimicrob. Agents Chemother. 2014, 58, 1652–1663. [Google Scholar] [CrossRef] [Green Version]
- Johns, B.A.; Kawasuji, T.; Weatherhead, J.G.; Taishi, T.; Temelkoff, D.P.; Yoshida, H.; Akiyama, T.; Taoda, Y.; Murai, H.; Kiyama, R.; et al. Carbamoyl pyridone HIV-1 integrase inhibitors 3. A diastereomeric approach to chiral nonracemic tricyclic ring systems and the discovery of dolutegravir (S/GSK1349572) and (S/GSK1265744). J. Med. Chem. 2013, 56, 5901–5916. [Google Scholar] [CrossRef]
- Ziegler, R.E.; Desai, B.K.; Jee, J.A.; Gupton, B.F.; Roper, T.D.; Jamison, T.F. 7-Step Flow Synthesis of the HIV Integrase Inhibitor Dolutegravir. Angew. Chem. Int. Ed. Engl. 2018, 57, 7181–7185. [Google Scholar] [CrossRef]
- Hare, S.; Vos, A.M.; Clayton, R.F.; Thuring, J.W.; Cummings, M.D.; Cherepanov, P. Molecular mechanisms of retroviral integrase inhibition and the evolution of viral resistance. Proc. Natl. Acad. Sci. USA 2010, 107, 20057–20062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hare, S.; Gupta, S.S.; Valkov, E.; Engelman, A.; Cherepanov, P. Retroviral intasome assembly and inhibition of DNA strand transfer. Nature 2010, 464, 232–236. [Google Scholar] [CrossRef]
- Hare, S.; Smith, S.J.; Metifiot, M.; Jaxa-Chamiec, A.; Pommier, Y.; Hughes, S.H.; Cherepanov, P. Structural and functional analyses of the second-generation integrase strand transfer inhibitor dolutegravir (S/GSK1349572). Mol. Pharmacol. 2011, 80, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.C.; Metifiot, M.; Pommier, Y.; Hughes, S.H. Molecular dynamics approaches estimate the binding energy of HIV-1 integrase inhibitors and correlate with in vitro activity. Antimicrob. Agents Chemother. 2012, 56, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Calza, L.; Magistrelli, E.; Colangeli, V.; Manfredi, R.; Borderi, M.; Rossi, N.; Conti, M.; Mancini, R.; Viale, P. Dual Raltegravir-Etravirine Combination as Maintenance Regimen in Virologically Suppressed HIV-1-Infected Patients. AIDS Res. Hum. Retrovir. 2017, 33, 632–638. [Google Scholar] [CrossRef]
- Monteiro, P.; Perez, I.; Laguno, M.; Martinez-Rebollar, M.; Gonzalez-Cordon, A.; Lonca, M.; Mallolas, J.; Blanco, J.L.; Gatell, J.M.; Martinez, E. Dual therapy with etravirine plus raltegravir for virologically suppressed HIV-infected patients: A pilot study. J. Antimicrob. Chemother. 2014, 69, 742–748. [Google Scholar] [CrossRef] [Green Version]
- Margolis, D.A.; Brinson, C.C.; Smith, G.H.R.; de Vente, J.; Hagins, D.P.; Eron, J.J.; Griffith, S.K.; Clair, M.H.S.; Stevens, M.C.; Williams, P.E.; et al. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): A randomised, phase 2b, dose-ranging trial. Lancet Infect. Dis. 2015, 15, 1145–1155. [Google Scholar] [CrossRef]
- Calin, R.; Paris, L.; Simon, A.; Peytavin, G.; Wirden, M.; Schneider, L.; Valantin, M.A.; Tubiana, R.; Agher, R.; Katlama, C. Dual raltegravir/etravirine combination in virologically suppressed HIV-1-infected patients on antiretroviral therapy. Antivir. Ther. 2012, 17, 1601–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casado, J.L.; Banon, S.; Rodriguez, M.A.; Moreno, A.; Moreno, S. Efficacy and pharmacokinetics of the combination of etravirine plus raltegravir as novel dual antiretroviral maintenance regimen in HIV-infected patients. Antivir. Res. 2015, 113, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Llibre, J.M.; Hung, C.C.; Brinson, C.; Castelli, F.; Girard, P.M.; Kahl, L.P.; Blair, E.A.; Angelis, K.; Wynne, B.; Vandermeulen, K.; et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: Phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018, 391, 839–849. [Google Scholar] [CrossRef]
- Revuelta-Herrero, J.L.; Chamorro-de-Vega, E.; Rodriguez-Gonzalez, C.G.; Alonso, R.; Herranz-Alonso, A.; Sanjurjo-Saez, M. Effectiveness, Safety, and Costs of a Treatment Switch to Dolutegravir Plus Rilpivirine Dual Therapy in Treatment-Experienced HIV Patients. Ann. Pharmacother. 2018, 52, 11–18. [Google Scholar] [CrossRef]
- Capetti, A.F.; Cossu, M.V.; Sterrantino, G.; Barbarini, G.; Di Giambenedetto, S.; De Socio, G.V.; Orofino, G.; Di Biagio, A.; Celesia, B.M.; Rusconi, S.; et al. Dolutegravir Plus Rilpivirine as a Switch Option in cART-Experienced Patients: 96-Week Data. Ann. Pharmacother. 2018, 52, 740–746. [Google Scholar] [CrossRef]
- Gantner, P.; Cuzin, L.; Allavena, C.; Cabie, A.; Pugliese, P.; Valantin, M.A.; Bani-Sadr, F.; Joly, V.; Ferry, T.; Poizot-Martin, I.; et al. Efficacy and safety of dolutegravir and rilpivirine dual therapy as a simplification strategy: A cohort study. HIV Med. 2017, 18, 704–708. [Google Scholar] [CrossRef] [Green Version]
- Cahn, P.; Pozniak, A.L.; Mingrone, H.; Shuldyakov, A.; Brites, C.; Andrade-Villanueva, J.F.; Richmond, G.; Buendia, C.B.; Fourie, J.; Ramgopal, M.; et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: Week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013, 382, 700–708. [Google Scholar] [CrossRef]
- Raffi, F.; Jaeger, H.; Quiros-Roldan, E.; Albrecht, H.; Belonosova, E.; Gatell, J.M.; Baril, J.G.; Domingo, P.; Brennan, C.; Almond, S.; et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect. Dis. 2013, 13, 927–935. [Google Scholar] [CrossRef]
- Raffi, F.; Rachlis, A.; Stellbrink, H.J.; Hardy, W.D.; Torti, C.; Orkin, C.; Bloch, M.; Podzamczer, D.; Pokrovsky, V.; Pulido, F.; et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013, 381, 735–743. [Google Scholar] [CrossRef]
- Walmsley, S.L.; Antela, A.; Clumeck, N.; Duiculescu, D.; Eberhard, A.; Gutierrez, F.; Hocqueloux, L.; Maggiolo, F.; Sandkovsky, U.; Granier, C.; et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N. Engl. J. Med. 2013, 369, 1807–1818. [Google Scholar] [CrossRef] [Green Version]
- Clotet, B.; Feinberg, J.; van Lunzen, J.; Khuong-Josses, M.A.; Antinori, A.; Dumitru, I.; Pokrovskiy, V.; Fehr, J.; Ortiz, R.; Saag, M.; et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014, 383, 2222–2231. [Google Scholar] [CrossRef]
- Raffi, F.; Rachlis, A.; Brinson, C.; Arasteh, K.; Gorgolas, M.; Brennan, C.; Pappa, K.; Almond, S.; Granier, C.; Nichols, W.G.; et al. Dolutegravir efficacy at 48 weeks in key subgroups of treatment-naive HIV-infected individuals in three randomized trials. AIDS 2015, 29, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccardi, N.; Del Puente, F.; Taramasso, L.; Di Biagio, A. Maintenance of Viral Suppression after Optimization Therapy from Etravirine Plus Raltegravir to Rilpivirine Plus Dolutegravir in HIV-1-Infected Patients. J. Int. Assoc. Provid. AIDS Care 2019, 18, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, A.; Wang, C.; Patton, C.; Murray, M.; Punekar, Y.; de Ruiter, A.; Steinhart, C. A Review of Long-Term Toxicity of Antiretroviral Treatment Regimens and Implications for an Aging Population. Infect. Dis. Ther. 2018, 7, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Milburn, J.; Jones, R.; Levy, J.B. Renal effects of novel antiretroviral drugs. Nephrol. Dial. Transpl. 2017, 32, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Lactic Acidosis International Study Group. Risk factors for lactic acidosis and severe hyperlactataemia in HIV-1-infected adults exposed to antiretroviral therapy. AIDS 2007, 21, 2455–2464. [Google Scholar] [CrossRef]
- Mollan, K.R.; Smurzynski, M.; Eron, J.J.; Daar, E.S.; Campbell, T.B.; Sax, P.E.; Gulick, R.M.; Na, L.; O’Keefe, L.; Robertson, K.R.; et al. Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: An analysis of trial data. Ann. Intern Med. 2014, 161, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Napoli, A.A.; Wood, J.J.; Coumbis, J.J.; Soitkar, A.M.; Seekins, D.W.; Tilson, H.H. No evident association between efavirenz use and suicidality was identified from a disproportionality analysis using the FAERS database. J. Int. AIDS Soc. 2014, 17, 19214–19217. [Google Scholar] [CrossRef]
- Mills, A.M.; Antinori, A.; Clotet, B.; Fourie, J.; Herrera, G.; Hicks, C.; Madruga, J.V.; Vanveggel, S.; Stevens, M.; Boven, K.; et al. Neurological and psychiatric tolerability of rilpivirine (TMC278) vs. efavirenz in treatment-naive, HIV-1-infected patients at 48 weeks. HIV Med. 2013, 14, 391–400. [Google Scholar] [CrossRef]
- Cazanave, C.; Reigadas, S.; Mazubert, C.; Bellecave, P.; Hessamfar, M.; Le Marec, F.; Lazaro, E.; Peytavin, G.; Bruyand, M.; Fleury, H.; et al. Switch to Rilpivirine/Emtricitabine/Tenofovir Single-Tablet Regimen of Human Immunodeficiency Virus-1 RNA-Suppressed Patients, Agence Nationale de Recherches sur le SIDA et les Hepatites Virales CO3 Aquitaine Cohort, 2012–2014. Open Forum Infect. Dis. 2015, 2, ofv018. [Google Scholar] [CrossRef] [Green Version]
- de Boer, M.G.; van den Berk, G.E.; van Holten, N.; Oryszcyn, J.E.; Dorama, W.; Moha, D.A.; Brinkman, K. Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS 2016, 30, 2831–2834. [Google Scholar] [CrossRef]
- Hoffmann, C.; Welz, T.; Sabranski, M.; Kolb, M.; Wolf, E.; Stellbrink, H.J.; Wyen, C. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. 2017, 18, 56–63. [Google Scholar] [CrossRef]
- Fettiplace, A.; Stainsby, C.; Winston, A.; Givens, N.; Puccini, S.; Vannappagari, V.; Hsu, R.; Fusco, J.; Quercia, R.; Aboud, M.; et al. Psychiatric Symptoms in Patients Receiving Dolutegravir. J. Acquir. Immune Defic. Syndr. 2017, 74, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Casado, J.L. Liver toxicity in HIV-infected patients receiving novel second-generation nonnucleoside reverse transcriptase inhibitors etravirine and rilpivirine. AIDS Rev. 2013, 15, 139–145. [Google Scholar]
- Fatkenheuer, G.; Duvivier, C.; Rieger, A.; Durant, J.; Rey, D.; Schmidt, W.; Hill, A.; van Delft, Y.; Marks, S.; Team, S.S. Lipid profiles for etravirine versus efavirenz in treatment-naive patients in the randomized, double-blind SENSE trial. J. Antimicrob. Chemother. 2012, 67, 685–690. [Google Scholar] [CrossRef] [Green Version]
- Tebas, P.; Sension, M.; Arribas, J.; Duiculescu, D.; Florence, E.; Hung, C.C.; Wilkin, T.; Vanveggel, S.; Stevens, M.; Deckx, H.; et al. Lipid levels and changes in body fat distribution in treatment-naive, HIV-1-Infected adults treated with rilpivirine or Efavirenz for 96 weeks in the ECHO and THRIVE trials. Clin. Infect. Dis. 2014, 59, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Di Biagio, A.; Riccardi, N.; Taramasso, L.; Capetti, A.; Cenderello, G.; Signori, A.; Vitiello, P.; Guerra, M.; de Socio, G.V.; Cassola, G.; et al. Switch from unboosted protease inhibitor to a single-tablet regimen containing rilpivirine improves cholesterol and triglycerides. Int. J. Antimicrob. Agents 2016, 48, 551–554. [Google Scholar] [CrossRef]
- Rokx, C.; Verbon, A.; Rijnders, B.J. Short communication: Lipids and cardiovascular risk after switching HIV-1 patients on nevirapine and emtricitabine/tenofovir-DF to rilpivirine/emtricitabine/tenofovir-DF. AIDS Res. Hum. Retrovir. 2015, 31, 363–367. [Google Scholar] [CrossRef]
- Koteff, J.; Borland, J.; Chen, S.; Song, I.; Peppercorn, A.; Koshiba, T.; Cannon, C.; Muster, H.; Piscitelli, S.C. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in healthy subjects. Br. J. Clin. Pharmacol. 2012, 75, 990–996. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Warren, M.S.; Zhang, X.; Diamond, S.; Williams, B.; Punwani, N.; Huang, J.; Huang, Y.; Yeleswaram, S. Impact on creatinine renal clearance by the interplay of multiple renal transporters: A case study with INCB039110. Drug Metab. Dispos. 2015, 43, 485–489. [Google Scholar] [CrossRef]
- McComsey, G.A.; Lupo, S.; Parks, D.; Poggio, M.C.; De Wet, J.; Kahl, L.P.; Angelis, K.; Wynne, B.; Vandermeulen, K.; Gartland, M.; et al. Switch from tenofovir disoproxil fumarate combination to dolutegravir with rilpivirine improves parameters of bone health. AIDS 2018, 32, 477–485. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Mancilla, J.R.; Haberer, J.E. Adherence Measurements in HIV: New Advancements in Pharmacologic Methods and Real-Time Monitoring. Curr. HIV/AIDS Rep. 2018, 15, 49–59. [Google Scholar] [CrossRef]
- Vandewalle, B.; Llibre, J.M.; Parienti, J.J.; Ustianowski, A.; Camacho, R.; Smith, C.; Miners, A.; Ferreira, D.; Felix, J. EPICE-HIV: An Epidemiologic Cost-Effectiveness Model for HIV Treatment. PLoS ONE 2016, 11, e0149007. [Google Scholar] [CrossRef] [Green Version]
- Calvo-Cidoncha, E.; Gonzalez-Bueno, J.; Almeida-Gonzalez, C.V.; Morillo-Verdugo, R. Influence of adding etravirine on complexity index and patients’ perceived complexity. J. Clin. Pharm. Ther. 2014, 39, 154–157. [Google Scholar] [CrossRef]
- Nachega, J.B.; Parienti, J.J.; Uthman, O.A.; Gross, R.; Dowdy, D.W.; Sax, P.E.; Gallant, J.E.; Mugavero, M.J.; Mills, E.J.; Giordano, T.P. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clin. Infect. Dis. 2014, 58, 1297–1307. [Google Scholar] [CrossRef] [Green Version]
- Buscher, A.; Hartman, C.; Kallen, M.A.; Giordano, T.P. Impact of antiretroviral dosing frequency and pill burden on adherence among newly diagnosed, antiretroviral-naive HIV patients. Int. J. STD AIDS 2012, 23, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Airoldi, M.; Zaccarelli, M.; Bisi, L.; Bini, T.; Antinori, A.; Mussini, C.; Bai, F.; Orofino, G.; Sighinolfi, L.; Gori, A.; et al. One-pill once-a-day HAART: A simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Prefer Adherence 2010, 4, 115–125. [Google Scholar]
- Haggblom, A.; Lindback, S.; Gisslen, M.; Flamholc, L.; Hejdeman, B.; Palmborg, A.; Leval, A.; Herweijer, E.; Valgardsson, S.; Svedhem, V. HIV drug therapy duration; a Swedish real world nationwide cohort study on InfCareHIV 2009–2014. PLoS ONE 2017, 12, e0171227. [Google Scholar] [CrossRef]
- Yunquera-Romero, L.; Asensi-Diez, R.; Gajardo-Alvarez, M.; Munoz-Castillo, I. [Dual therapy as an alternative treatment in HIV pretreated patients: Experience in a tertiary hospital]. Rev. Esp. Quimioter. 2016, 29, 25–31. [Google Scholar]
- Vingerhoets, J.; Azijn, H.; Fransen, E.; De Baere, I.; Smeulders, L.; Jochmans, D.; Andries, K.; Pauwels, R.; de Bethune, M.P. TMC125 displays a high genetic barrier to the development of resistance: Evidence from in vitro selection experiments. J. Virol. 2005, 79, 12773–12782. [Google Scholar] [CrossRef] [Green Version]
- Ghosn, J.; Chaix, M.L.; Delaugerre, C. HIV-1 Resistance to First- and Second-Generation Non-Nucleoside Reverse Transcriptase Inhibitors. AIDS Rev. 2009, 11, 165–173. [Google Scholar]
- Porter, D.P.; Kulkarni, R.; Fralich, T.; Miller, M.D.; White, K.L. Characterization of HIV-1 drug resistance development through week 48 in antiretroviral naive subjects on rilpivirine/emtricitabine/tenofovir DF or efavirenz/emtricitabine/tenofovir DF in the STaR study (GS-US-264-0110). J. Acquir. Immune Defic. Syndr. 2014, 65, 318–326. [Google Scholar] [CrossRef]
- Gallien, S.; Charreau, I.; Nere, M.L.; Mahjoub, N.; Simon, F.; de Castro, N.; Aboulker, J.P.; Molina, J.M.; Delaugerre, C. Archived HIV-1 DNA resistance mutations to rilpivirine and etravirine in successfully treated HIV-1-infected individuals pre-exposed to efavirenz or nevirapine. J. Antimicrob. Chemother. 2015, 70, 562–565. [Google Scholar] [CrossRef] [Green Version]
- Bunupuradah, T.; Ananworanich, J.; Chetchotisakd, P.; Kantipong, P.; Jirajariyavej, S.; Sirivichayakul, S.; Munsakul, W.; Prasithsirikul, W.; Sungkanuparph, S.; Bowonwattanuwong, C.; et al. Etravirine and rilpivirine resistance in HIV-1 subtype CRF01_AE-infected adults failing non-nucleoside reverse transcriptase inhibitor-based regimens. Antivir. Ther. 2011, 16, 1113–1121. [Google Scholar] [CrossRef] [Green Version]
- Anta, L.; Llibre, J.M.; Poveda, E.; Blanco, J.L.; Alvarez, M.; Perez-Elias, M.J.; Aguilera, A.; Caballero, E.; Soriano, V.; de Mendoza, C.; et al. Rilpivirine resistance mutations in HIV patients failing non-nucleoside reverse transcriptase inhibitor-based therapies. AIDS 2013, 27, 81–85. [Google Scholar] [CrossRef]
- Calvez, V.; Marcelin, A.G.; Vingerhoets, J.; Hill, A.; Hadacek, B.; Moecklinghoff, C. Systematic review to determine the prevalence of transmitted drug resistance mutations to rilpivirine in HIV-infected treatment-naive persons. Antivir. Ther. 2016, 21, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Fransen, S.; Paxinos, E.E.; Stawiski, E.; Huang, W.; Petropoulos, C.J. Combinations of mutations in the connection domain of human immunodeficiency virus type 1 reverse transcriptase: Assessing the impact on nucleoside and nonnucleoside reverse transcriptase inhibitor resistance. Antimicrob. Agents Chemother. 2010, 54, 1973–1980. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Vingerhoets, J.; Fransen, S.; Tambuyzer, L.; Azijn, H.; Frantzell, A.; Paredes, R.; Coakley, E.; Nijs, S.; Clotet, B.; et al. Connection domain mutations in HIV-1 reverse transcriptase do not impact etravirine susceptibility and virologic responses to etravirine-containing regimens. Antimicrob. Agents Chemother. 2011, 55, 2872–2879. [Google Scholar] [CrossRef] [Green Version]
- Sili, U.; Aksu, B.; Tekin, A.; Hasdemir, U.; Soyletir, G.; Korten, V. Assessment of Transmitted HIV-1 Drug Resistance Mutations Using Ultra- Deep Pyrosequencing in a Turkish Cohort. Curr. HIV Res. 2018, 16, 216–221. [Google Scholar] [CrossRef]
- SahBandar, I.N.; Samonte, G.; Telan, E.; Siripong, N.; Belcaid, M.; Schanzenbach, D.; Leano, S.; Chagan-Yasutan, H.; Hattori, T.; Shikuma, C.M.; et al. Ultra-Deep Sequencing Analysis on HIV Drug-Resistance-Associated Mutations Among HIV-Infected Individuals: First Report from the Philippines. AIDS Res. Hum. Retrovir. 2017, 33, 1099–1106. [Google Scholar] [CrossRef]
- Rojas Sanchez, P.; de Mulder, M.; Fernandez-Cooke, E.; Prieto, L.; Rojo, P.; Jimenez de Ory, S.; Jose Mellado, M.; Navarro, M.; Tomas Ramos, J.; Holguin, A.; et al. Clinical and virologic follow-up in perinatally HIV-1-infected children and adolescents in Madrid with triple-class antiretroviral drug-resistant viruses. Clin. Microbiol. Infect. 2015, 21, 605.e1–605.e9. [Google Scholar] [CrossRef] [Green Version]
- Penrose, K.J.; Brumme, C.J.; Scoulos-Hanson, M.; Hamanishi, K.; Gordon, K.; Viana, R.V.; Wallis, C.L.; Harrigan, P.R.; Mellors, J.W.; Parikh, U.M. Frequent cross-resistance to rilpivirine among subtype C HIV-1 from first-line antiretroviral therapy failures in South Africa. Antivir. Chem. Chemother. 2018, 26, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Picchio, G.R.; Rimsky, L.T.; Van Eygen, V.; Haddad, M.; Napolitano, L.A.; Vingerhoets, J. Prevalence in the USA of rilpivirine resistance-associated mutations in clinical samples and effects on phenotypic susceptibility to rilpivirine and etravirine. Antivir. Ther. 2014, 19, 819–823. [Google Scholar] [CrossRef] [Green Version]
- Ciccullo, A.; Baldin, G.; Capetti, A.; Rusconi, S.; Sterrantino, G.; d’Ettorre, G.; Colafigli, M.; Modica, S.; Lagi, F.; Giacomelli, A.; et al. A comparison between two dolutegravir-based two-drug regimens as switch strategies in a multicentre cohort of HIV-1-infected patients. Antivir. Ther. 2019, 24, 63–67. [Google Scholar] [CrossRef]
- Hauser, A.; Hofmann, A.; Meixenberger, K.; Altmann, B.; Hanke, K.; Bremer, V.; Bartmeyer, B.; Bannert, N. Increasing proportions of HIV-1 non-B subtypes and of NNRTI resistance between 2013 and 2016 in Germany: Results from the national molecular surveillance of new HIV-diagnoses. PLoS ONE 2018, 13, e0206234. [Google Scholar] [CrossRef]
- Charpentier, C.; Lee, G.Q.; Rodriguez, C.; Visseaux, B.; Storto, A.; Fagard, C.; Molina, J.M.; Katlama, C.; Yazdanpanah, Y.; Harrigan, P.R.; et al. Highly frequent HIV-1 minority resistant variants at baseline of the ANRS 139 TRIO trial had a limited impact on virological response. J. Antimicrob. Chemother. 2015, 70, 2090–2096. [Google Scholar] [CrossRef] [Green Version]
- Charpentier, C.; Roquebert, B.; Colin, C.; Taburet, A.M.; Fagard, C.; Katlama, C.; Molina, J.M.; Jacomet, C.; Brun-Vezinet, F.; Chene, G.; et al. Resistance analyses in highly experienced patients failing raltegravir, etravirine and darunavir/ritonavir regimen. AIDS 2010, 24, 2651–2656. [Google Scholar] [CrossRef]
- Marcelin, A.G.; Flandre, P.; Descamps, D.; Morand-Joubert, L.; Charpentier, C.; Izopet, J.; Trabaud, M.A.; Saoudin, H.; Delaugerre, C.; Tamalet, C.; et al. Factors associated with virological response to etravirine in nonnucleoside reverse transcriptase inhibitor-experienced HIV-1-infected patients. Antimicrob. Agents Chemother. 2010, 54, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, D.G.; Bauman, J.D.; Challa, J.R.; Patel, D.; Troxler, T.; Das, K.; Arnold, E.; Hochstrasser, R.M. Snapshot of the equilibrium dynamics of a drug bound to HIV-1 reverse transcriptase. Nat. Chem. 2013, 5, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Deeks, E.D. Doravirine: First Global Approval. Drugs 2018, 78, 1643–1650. [Google Scholar] [CrossRef]
- Feng, M.; Sachs, N.A.; Xu, M.; Grobler, J.; Blair, W.; Hazuda, D.J.; Miller, M.D.; Lai, M.T. Doravirine Suppresses Common Nonnucleoside Reverse Transcriptase Inhibitor-Associated Mutants at Clinically Relevant Concentrations. Antimicrob. Agents Chemother. 2016, 60, 2241–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.J.; Pauly, G.T.; Akram, A.; Melody, K.; Ambrose, Z.; Schneider, J.P.; Hughes, S.H. Rilpivirine and Doravirine Have Complementary Efficacies Against NNRTI-Resistant HIV-1 Mutants. J. Acquir. Immune Defic. Syndr. 2016, 72, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, M.; Ibanescu, R.I.; Anstett, K.; Mesplede, T.; Routy, J.P.; Robbins, M.A.; Brenner, B.G.; Montreal Primary, H.I.V.C.S.G. Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir. Retrovirology 2018, 15, 56. [Google Scholar] [CrossRef] [Green Version]
- Castagna, A.; Ferrara, M.; Galli, L.; Comi, L.; Sterrantino, G.; Cenderello, G.; Zaccarelli, M.; Foca, E.; Roncadori, A.; Lazzarin, A.; et al. Long-term efficacy of dolutegravir in treatment-experienced subjects failing therapy with HIV-1 integrase strand inhibitor-resistant virus. J. Antimicrob. Chemother. 2018, 73, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Castagna, A.; Maggiolo, F.; Penco, G.; Wright, D.; Mills, A.; Grossberg, R.; Molina, J.M.; Chas, J.; Durant, J.; Moreno, S.; et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J. Infect. Dis. 2014, 210, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Canducci, F.; Ceresola, E.R.; Saita, D.; Castagna, A.; Gianotti, N.; Underwood, M.; Burioni, R.; Lazzarin, A.; Clementi, M. In vitro phenotypes to elvitegravir and dolutegravir in primary macrophages and lymphocytes of clonal recombinant viral variants selected in patients failing raltegravir. J. Antimicrob. Chemother. 2013, 68, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Metifiot, M.; Maddali, K.; Naumova, A.; Zhang, X.; Marchand, C.; Pommier, Y. Biochemical and pharmacological analyses of HIV-1 integrase flexible loop mutants resistant to raltegravir. Biochemistry 2010, 49, 3715–3722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charpentier, C.; Karmochkine, M.; Laureillard, D.; Tisserand, P.; Belec, L.; Weiss, L.; Si-Mohamed, A.; Piketty, C. Drug resistance profiles for the HIV integrase gene in patients failing raltegravir salvage therapy. HIV Med. 2008, 9, 765–770. [Google Scholar] [CrossRef]
- Cooper, D.A.; Steigbigel, R.T.; Gatell, J.M.; Rockstroh, J.K.; Katlama, C.; Yeni, P.; Lazzarin, A.; Clotet, B.; Kumar, P.N.; Eron, J.E.; et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N. Engl. J. Med. 2008, 359, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Malet, I.; Delelis, O.; Valantin, M.A.; Montes, B.; Soulie, C.; Wirden, M.; Tchertanov, L.; Peytavin, G.; Reynes, J.; Mouscadet, J.F.; et al. Mutations associated with failure of raltegravir treatment affect integrase sensitivity to the inhibitor in vitro. Antimicrob. Agents Chemother. 2008, 52, 1351–1358. [Google Scholar] [CrossRef] [Green Version]
- Anstett, K.; Brenner, B.; Mesplede, T.; Wainberg, M.A. HIV drug resistance against strand transfer integrase inhibitors. Retrovirology 2017, 14, 36–51. [Google Scholar] [CrossRef]
- Tsiang, M.; Jones, G.S.; Goldsmith, J.; Mulato, A.; Hansen, D.; Kan, E.; Tsai, L.; Bam, R.A.; Stepan, G.; Stray, K.M.; et al. Antiviral Activity of Bictegravir (GS-9883), a Novel Potent HIV-1 Integrase Strand Transfer Inhibitor with an Improved Resistance Profile. Antimicrob. Agents Chemother. 2016, 60, 7086–7097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, B.G.; Thomas, R.; Blanco, J.L.; Ibanescu, R.I.; Oliveira, M.; Mesplede, T.; Golubkov, O.; Roger, M.; Garcia, F.; Martinez, E.; et al. Development of a G118R mutation in HIV-1 integrase following a switch to dolutegravir monotherapy leading to cross-resistance to integrase inhibitors. J. Antimicrob. Chemother. 2016, 71, 1948–1953. [Google Scholar] [CrossRef] [PubMed]
- Hassounah, S.A.; Alikhani, A.; Oliveira, M.; Bharaj, S.; Ibanescu, R.I.; Osman, N.; Xu, H.T.; Brenner, B.G.; Mesplede, T.; Wainberg, M.A. Antiviral Activity of Bictegravir and Cabotegravir against Integrase Inhibitor-Resistant SIVmac239 and HIV-1. Antimicrob. Agents Chemother. 2017, 61, e01695-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshinaga, T.; Kobayashi, M.; Seki, T.; Miki, S.; Wakasa-Morimoto, C.; Suyama-Kagitani, A.; Kawauchi-Miki, S.; Taishi, T.; Kawasuji, T.; Johns, B.A.; et al. Antiviral characteristics of GSK1265744, an HIV integrase inhibitor dosed orally or by long-acting injection. Antimicrob. Agents Chemother. 2015, 59, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Yun, J.H.; Shi, Y.; Han, J.; Li, X.; Jin, Z.; Kim, T.; Park, J.; Park, S.; Liu, H.; et al. Non-Cryogenic Structure and Dynamics of HIV-1 Integrase Catalytic Core Domain by X-ray Free-Electron Lasers. Int. J. Mol. Sci. 2019, 20, 1943. [Google Scholar] [CrossRef] [Green Version]
- Charpentier, C.; Descamps, D. Resistance to HIV Integrase Inhibitors: About R263K and E157Q Mutations. Viruses 2018, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Wright, D.W.; Sadiq, S.K.; De Fabritiis, G.; Coveney, P.V. Thumbs down for HIV: Domain level rearrangements do occur in the NNRTI-bound HIV-1 reverse transcriptase. J. Am. Chem. Soc. 2012, 134, 12885–12888. [Google Scholar] [CrossRef]
- Sluis-Cremer, N.; Temiz, N.A.; Bahar, I. Conformational Changes in HIV-1 Reverse Transcriptase Induced by Nonnucleoside Reverse Transcriptase Inhibitor Binding. Curr. HIV Res. 2004, 2, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.; Marchand, B.; Rai, D.K.; Sharma, B.; Michailidis, E.; Ryan, E.M.; Matzek, K.B.; Leslie, M.D.; Hagedorn, A.N.; Li, Z.; et al. Biochemical Mechanism of HIV-1 Resistance to Rilpivirine. J. Biol. Chem. 2012, 287, 38110–38123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.T.; Colby-Germinario, S.P.; Huang, W.; Oliveira, M.; Han, Y.; Quan, Y.; Petropoulos, C.J.; Wainberg, M.A. Role of the K101E substitution in HIV-1 reverse transcriptase in resistance to rilpivirine and other nonnucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 2013, 57, 5649–5657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarafianos, S.G.; Das, K.; Hughes, S.H.; Arnold, E. Taking Aim at a Moving Target: Designing Drugs to Inhibit Drug-Resistant HIV-1 Reverse Transcriptases. Curr. Opin. Struct. Biol. 2004, 14, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Marchand, B.; Kirby, K.A.; Michailidis, E.; Sarafianos, S.G. Structural Aspects of Drug Resistance and Inhibition of HIV-1 Reverse Transcriptase. Viruses 2010, 2, 606–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansdon, E.B.; Brendza, K.M.; Hung, M.; Wang, R.; Mukund, S.; Jin, D.; Birkus, G.; Kutty, N.; Liu, X. Crystal structures of HIV-1 reverse transcriptase with etravirine (TMC125) and rilpivirine (TMC278): Implications for drug design. J. Med. Chem. 2010, 53, 4295–4299. [Google Scholar] [CrossRef]
- Himmel, D.M.; Das, K.; Clark, A.D., Jr.; Hughes, S.H.; Benjahad, A.; Oumouch, S.; Guillemont, J.; Coupa, S.; Poncelet, A.; Csoka, I.; et al. Crystal Structures for HIV-1 Reverse Transcriptase in Complexes with Three Pyridinone Derivatives: A New Class of Non-nucleoside Inhibitors Effective Against a Broad Range of Drug-Resistant Strains. J. Med. Chem. 2005, 48, 7582–7591. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, D.; Nguyen, L.A.; Smithline, Z.B.; Pannecouque, C.; Zhan, P.; Liu, X.; Steitz, T.A. Structural basis for potent and broad inhibition of HIV-1 RT by thiophene[3,2-d]pyrimidine non-nucleoside inhibitors. Elife 2018, 7. [Google Scholar] [CrossRef]
- Hsiou, Y.; Ding, J.; Das, K.; Clark, A.D., Jr.; Boyer, P.L.; Lewi, P.; Janssen, P.A.; Kleim, J.P.; Rosner, M.; Hughes, S.H.; et al. The Lys103Asn Mutation of HIV-1 RT: A Novel Mechanism of Drug Resistance. J. Mol. Biol. 2001, 309, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.; Sarafianos, S.G.; Sonnerborg, A. Long-Acting Anti-HIV Drugs Targeting HIV-1 Reverse Transcriptase and Integrase. Pharmaceuticals (Basel) 2019, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Margolis, D.A.; Boffito, M. Long-acting antiviral agents for HIV treatment. Curr. Opin. HIV AIDS 2015, 10, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Kirtane, A.R.; Abouzid, O.; Minahan, D.; Bensel, T.; Hill, A.L.; Selinger, C.; Bershteyn, A.; Craig, M.; Mo, S.S.; Mazdiyasni, H.; et al. Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Margolis, D.A.; Gonzalez-Garcia, J.; Stellbrink, H.J.; Eron, J.J.; Yazdanpanah, Y.; Podzamczer, D.; Lutz, T.; Angel, J.B.; Richmond, G.J.; Clotet, B.; et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017, 390, 1499–1510. [Google Scholar] [CrossRef]



| Resistance Mutation | Compounds with Antiretroviral Activity (EC50, nM) § | ||||
|---|---|---|---|---|---|
| Rilpivirine (RPV, TMC278) | Etravirine (ETR, TMC125) | Efavirenz (EFV) | Nevirapine (NVP) | Delavirdine (DLV) | |
| Wild-type | 0.4 | 2 | 1.4 | 81–85 | 16 |
| L100I | 0.4–0.5 | 3 | 35–38 | 597–638 | 3467 |
| K103N | 0.3 | 1 | 28–39 | 2467–2879 | 1697 |
| Y181C | 0.1–1.3 | 6 | 2 | 5351–10,000 | 1336 |
| K103 + Y181C | 1.0 §–1.4 * | 5.0 §–8.2 * | 37.0 §–51.1 * | ≥10,000 | >10,000 |
| Y188L | 2.0 | 3.0 | 78–138 | ≥10,000 | 178 |
| Legend | <1.0 Highly active | 1.0–10 Active | 10.1–100 | High Resistance (>100) | |
| Fold Change § | ||||
|---|---|---|---|---|
| NNRTI-Resistance Mutations | Rilpivirine | Etravirine | Efavirenz | Nevirapine |
| Y181C | 2.7 | 4.0–5.1 | 2.1 | >43.0 |
| Y181C + V179F | 8.7 | 158.9 | 4.6 | >358.3 |
| Y181C + N348I + T369I | 51.2 ‡ | 17.0 | >400 ‡ | |
| K101P | 51.7 | 4.36 ‡–5.3 § | 72.3 | >166.1 |
| K103N | 0.9 | 0.9 §–1.28 ‡ | 21.3 ‡–32.5 § | >42.1 |
| K103N + Y181I | 94.9 | 16.1 | 6.4 | >71.5 |
| L100I + K103N + V179L | 46.1 | 13.4 | 5,660.60 | >71.5 |
| L100I + K103N + Y181C | 80.8 | 58.1 | 1,812.00 | 468.1 |
| K101P + K103N + V108I | >162.1 | 18.4 | 12,931.10 | >51.6 |
| V179 + Y181C + F227C | 553.8 | 638.6 | 25.7 | >71.5 |
| Legend | Low-Level Resistance (1.0 = Wild-Type Activity) | Resistance (10–50) | High Resistance (>50) | Data Unavailable |
| Emergent INSTI-Resistant Mutations | Fold Change § | ||||
|---|---|---|---|---|---|
| Dolutegravir (DTG) | Bictegravir (BIC) | Cabotegravir (CAB) | Elvitegravir (EVG) | Raltegravir (RAL) | |
| T66I + Q95K + E157Q | 0.8 | 0.6 | 0.9 | 89 | 4.7 |
| T66I + Q95K + E157Q + S230R | 0.1 | 0.01 | 0.1 | 156 | 3.2 |
| T97A + A128T + E157Q + V151I | 0.6 | 0.3 | 0.5 | 52 | 21 |
| T97A + Y143R | 1.0–1.2 | 1.9–2.1 | 11.6–60.4 | ||
| T97A + Y143C | 1.0–1.5 | 4.3–4.8 | 44.7– >100 | ||
| N155H | 1.0–1.8 | 1.6–1.8 | 1.0–2.1 | 7.0–8.5 ab | 20–47.8 b |
| G140S | 0.86–1.3 | 0.81 | 2.7–9.7 | 1.1–1.8 | |
| Q148H | 0.97–1.3 | 0.86 | 7.3–9.3 | 13–16 | |
| G140S + Q148H | 2.6–11.0 | 4.8 | 6.1–16.8 | 38.9–>100 | >100 |
| T92A + G140S + Q148H | 5.5–18.3 | >100 | >100 | ||
| G140S + Q148H + G163R | 10.0–18.4 | >100 | >100 | ||
| Q148K | 1.1–2.0 | 2.2 | 3.1–5.6 | 11.8 c | 3.2 c |
| G140S + G147S + Q148K | 4.7 | 1.8 | 40 | 720 | 60 |
| E138K + Q148R + R263K | 14 | 8 | 8.3 | >100 | 7 |
| L74I + E138K + G140S + Q148R | 25 | 5.3 | 87 | 57 | >100 |
| L74M + E138K + Q148R + R263K | 17–24 | 12–17 | >100 | >1500 | 188–355 |
| L74M + G140S + S147G + Q148K | 162 | 120 | 700 | >1000 | 900 |
| Legend | Most Susceptible (<1.0) | Susceptible or Low-level Resistance (1.0 = Wild-type activity) | Resistance (10–50) | High Resistance (>50) | Data Unavailable |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Himmel, D.M.; Arnold, E. Non-Nucleoside Reverse Transcriptase Inhibitors Join Forces with Integrase Inhibitors to Combat HIV. Pharmaceuticals 2020, 13, 122. https://doi.org/10.3390/ph13060122
Himmel DM, Arnold E. Non-Nucleoside Reverse Transcriptase Inhibitors Join Forces with Integrase Inhibitors to Combat HIV. Pharmaceuticals. 2020; 13(6):122. https://doi.org/10.3390/ph13060122
Chicago/Turabian StyleHimmel, Daniel M., and Eddy Arnold. 2020. "Non-Nucleoside Reverse Transcriptase Inhibitors Join Forces with Integrase Inhibitors to Combat HIV" Pharmaceuticals 13, no. 6: 122. https://doi.org/10.3390/ph13060122