Population Pharmacokinetics of Colistin Methanesulfonate Sodium and Colistin in Critically Ill Patients: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
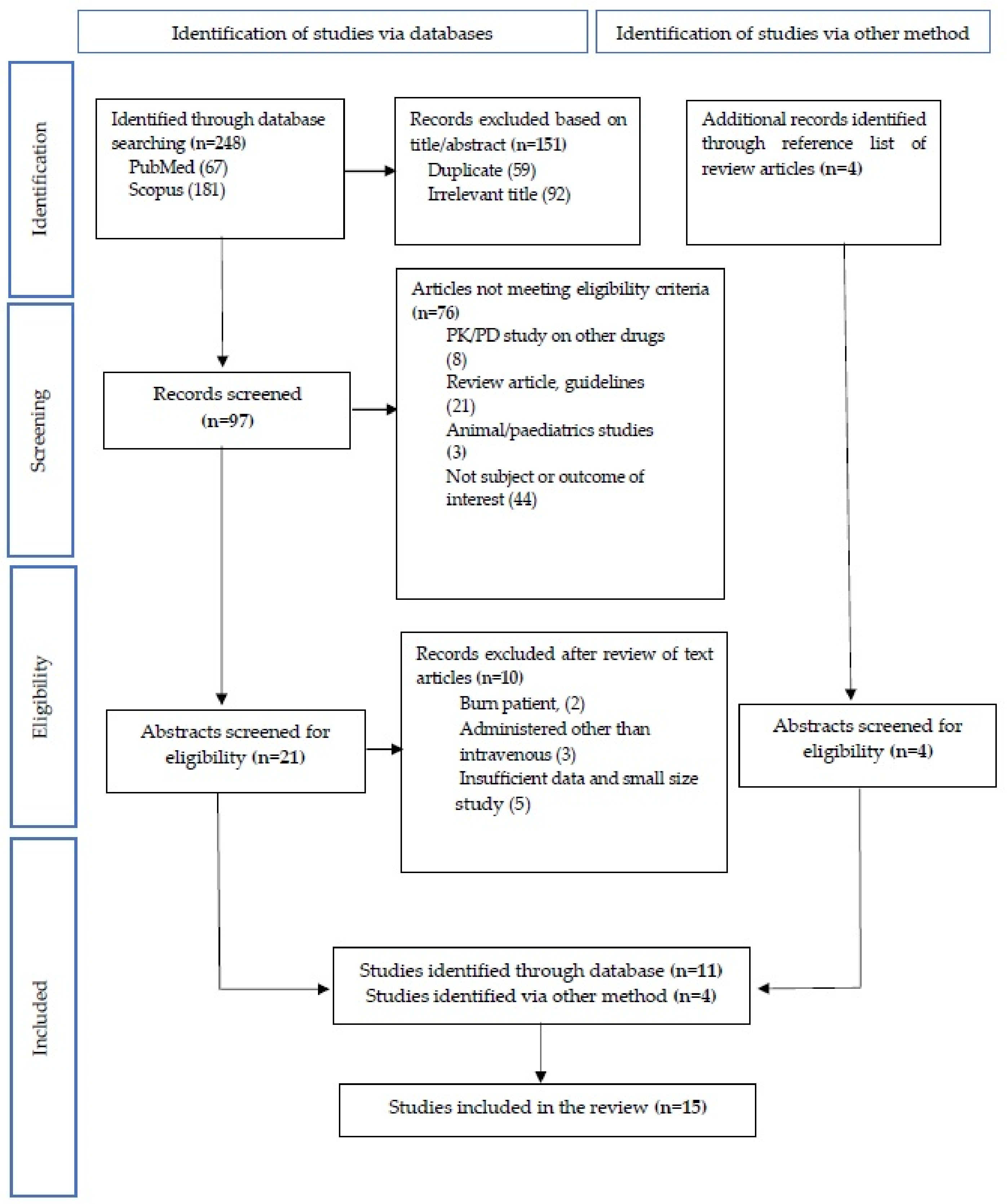
2.1. Search Strategy for Identification of Studies
2.1.1. Study Selection and Study Eligibility
2.1.2. Inclusion and Exclusion Criteria
2.2. Data Extraction and Quality Assessment
- Study characteristics: authors, year, study size, study population, drug manufacturer/brand, CMS preparation, and bioanalytical method;
- Patient characteristics: sample collection, age, body weight, creatinine clearance (CrCl), and number of renal impaired patients;
- Outcomes: program for pharmacokinetic analysis, pharmacokinetic modeling, CMS dose, maximum concentration (Cmax), steady-state concentration (Css), half-life (t1/2), clearance (Cl), and volume of distribution (Vd).
2.3. Data Synthesis and Analysis
3. Results
3.1. Demographic Study and Patient Characteristics
3.2. Concentration of CMS and Colistin in Plasma after CMS Administration
3.2.1. Concentration of CMS and Colistin in Plasma after the First Dose of CMS Administration without a Loading Dose
3.2.2. Concentration of Colistin in Plasma after Application of a Loading Dose
3.2.3. Concentration of Colistin in Plasma after Repeated Doses
3.2.4. Concentration of Colistin in Patients with Renal Impairment and Patients Receiving Renal Replacement Therapy
3.3. Pharmacokinetic Parameters of CMS and Colistin in Plasma at Steady State after CMS Administration
3.3.1. Volume of Distribution (Vd) of CMS and Colistin
3.3.2. Clearance (CL) of CMS and Colistin
3.3.3. Elimination Half-Life (t1/2) of CMS and Colistin
3.4. Correlation between Pharmacokinetics Parameters of CMS and Colistin with Various Covariate Factors
4. Discussion
4.1. Heterogeneity in the Population Pharmacokinetics of CMS and Colistin in Critically Ill Patients
4.2. Colistin Use in Critically Ill Patients
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergen, P.J.; Li, J.; Rayner, C.R.; Nation, R.L. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 1953–1958. [Google Scholar] [CrossRef] [Green Version]
- Kristoffersson, A.N.; Rognås, V.; Brill, M.J.E.; Dishon-Benattar, Y.; Durante-Mangoni, E.; Daitch, V.; Skiada, A.; Lellouche, J.; Nutman, A.; Kotsaki, A.; et al. Population pharmacokinetics of colistin and the relation to survival in critically ill patients infected with colistin susceptible and carbapenem-resistant bacteria. Clin. Microbiol. Infect. 2020, 26, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Karaiskos, I.; Friberg, L.E.; Pontikis, K.; Ioannidis, K.; Tsagkari, V.; Galani, L. Colistin Population Pharmacokinetics after Application of a Loading. Antimicrob. Agents Chemother. 2015, 59, 7240–7248. [Google Scholar] [CrossRef] [Green Version]
- Ram, K.; Sheikh, S.; Bhati, R.K.; Tripathi, C.D.; Suri, J.C.; Meshram, G.G. Steady-state pharmacokinetic and pharmacodynamic profiling of colistin in critically ill patients with multi-drug–resistant gram-negative bacterial infections, along with differences in clinical, microbiological and safety outcome. Basic Clin. Pharmacol. Toxicol. 2021, 128, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Moni, M.; Sudhir, A.S.; Dipu, T.S.; Mohamed, Z.; Prabhu, B.P.; Edathadathil, F.; Balachandran, S.; Singh, S.K.; Prasanna, P.; Menon, V.P.; et al. Clinical efficacy and pharmacokinetics of colistimethate sodium and colistin in critically ill patients in an Indian hospital with high endemic rates of multidrug-resistant Gram-negative bacterial infections: A prospective observational study. Int. J. Infect. Dis. 2020, 100, 497–506. [Google Scholar] [CrossRef]
- Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Paterson, D.L.; Shoham, S.; Jacob, J.; Silveira, F.P.; Forrest, A.; Nation, R.L. Population Pharmacokinetics of Colistin Methanesulfonate and Formed Colistin in Critically Ill Patients from a Multicenter Study Provide Dosing Suggestions for Various Categories of Patients. Antimicrob. Agents Chemother. 2011, 55, 3284–3294. [Google Scholar] [CrossRef] [Green Version]
- Nation, R.L.; Garonzik, S.M.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Forrest, A.; Paterson, D.L.; Li, J.; Silveira, F.P. Dosing guidance for intravenous colistin in critically ill patients. Clin. Infect. Dis. 2017, 64, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Cheah, S.E.; Li, J.; Tsuji, B.T.; Forrest, A.; Bulitta, J.B.; Nation, R.L. Colistin and polymyxin B dosage regimens against Acinetobacter baumannii: Differences in activity and the emergence of resistance. Antimicrob. Agents Chemother. 2016, 60, 3921–3933. [Google Scholar] [CrossRef] [Green Version]
- Plachouras, D.; Karvanen, M.; Friberg, L.E.; Papadomichelakis, E.; Antoniadou, A.; Tsangaris, I.; Karaiskos, I.; Poulakou, G.; Kontopidou, F.; Armaganidis, A.; et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob. Agents Chemother. 2009, 53, 3430–3436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, A.F.; Karaiskos, I.; Plachouras, D.; Karvanen, M.; Pontikis, K.; Jansson, B.; Papadomichelakis, E.; Antoniadou, A.; Giamarellou, H.; Armaganidis, A.; et al. Application of a Loading Dose of Colistin Methanesulfonate in Critically Ill Patients: Population Pharmacokinetics, Protein Binding, and Prediction of Bacterial Kill. Antimicrob. Agents Chemother. 2012, 56, 4241–4249. [Google Scholar] [CrossRef] [Green Version]
- Grégoire, N.; Mimoz, O.; Mégarbane, B.; Comets, E.; Chatelier, D.; Lasocki, S.; Gauzit, R.; Balayn, D.; Gobin, P.; Marchand, S.; et al. New Colistin Population Pharmacokinetic Data in Critically Ill Patients Suggesting an Alternative Loading Dose Rationale. Antimicrob. Agents Chemother. 2014, 58, 7324–7330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnik, N.D.; Sridharan, K.; Jadhav, S.P.; Kadam, P.P.; Naidu, R.K.; Namjoshi, R.D.; Gupta, V.; Gore, M.S.; Surase, P.V.; Mehta, P.R.; et al. Pharmacokinetics of colistin in critically ill patients with multidrug-resistant Gram-negative bacilli infection. Eur. J. Clin. Pharmacol. 2013, 69, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Oliota, A.F.; Penteado, S.T.; Tonin, F.S.; Fernandez-Llimos, F.; Sanches, A.C. Nephrotoxicity prevalence in patients treated with polymyxins: A systematic review with meta-analysis of observational studies. Diagn. Microbiol. Infect. Dis. 2019, 94, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Leuppi-Taegtmeyer, A.B.; Decosterd, L.; Osthoff, M.; Mueller, N.J.; Buclin, T.; Corti, N. Multicenter population pharmacokinetic study of colistimethate sodium and colistin dosed as in normal renal function in patients on continuous renal replacement therapy. Antimicrob. Agents Chemother. 2019, 63, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Matthieu, B.; Matthieu, J.; Nicolas, G.; Patrice, G.; Srine, M.; William, C.; Olivier, M. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob. Agents Chemother. 2014, 58, 7331–7339. [Google Scholar]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDS0. Pharmacotherapy 2019, 39, 10–39. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Sohn, Y.M.; Jeong, S.H.; Park, H.J.; Na, S.J.; Huh, K.; Suh, G.Y.; Jeon, K. Inhalation with intravenous loading dose of colistin in critically ill patients with pneumonia caused by carbapenem-resistant gram-negative bacteria. Ther. Adv. Respir. Dis. 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372. [Google Scholar]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- Markou, N.; Markantonis, S.L.; Dimitrakis, E.; Panidis, D.; Boutzouka, E.; Karatzas, S.; Rafailidis, P.; Apostolakos, H.; Baltopoulos, G. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: A prospective, open-label, uncontrolled study. Clin. Ther. 2008, 30, 143–151. [Google Scholar] [CrossRef]
- Karvanen, M.; Plachouras, D.; Friberg, L.E.; Paramythiotou, E.; Papadomichelakis, E.; Karaiskos, I.; Tsangaris, I.; Armaganidis, A.; Cars, O.; Giamarellou, H. Colistin methanesulfonate and colistin pharmacokinetics in critically ill patients receiving continuous venovenous hemodiafiltration. Antimicrob. Agents Chemother. 2012, 57, 668–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, M.; Gregoire, N.; Mégarbane, B.; Gobin, P.; Balayn, D.; Marchand, S.; Mimoz, O.; Couet, W. Population pharmacokinetics of colistin methanesulfonate and colistin in critically ill patients with acute renal failure requiring intermittent hemodialysis. Antimicrob. Agents Chemother. 2016, 60, 1788–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imberti, R.; Cusato, M.; Villani, P.; Carnevale, L.; Iotti, G.A.; Langer, M.; Regazzi, M. Steady-state pharmacokinetics and BAL concentration of colistin in critically Ill patients after IV colistin methanesulfonate administration. Chest 2010, 138, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, J.C.; Nation, R.L.; Jacob, J.; Chen, G.; Lee, H.J.; Tsuji, B.T.; Thompson, P.E.; Roberts, K.; Velkov, T.; et al. Pharmacokinetics of four different brands of colistimethate and formed colistin in rats. J. Antimicrob. Chemother. 2013, 68, 2311–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nation, R.L.; Velkov, T.; Li, J. Colistin and polymyxin B: Peas in a pod, or chalk and cheese? Clin. Infect. Dis. 2014, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nation, R.L.; Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Turnidge, J.D.; Forrest, A.; Silveira, F.P. Updated US and European Dose Recommendations for Intravenous Colistin: How Do They Perform? Clin. Infect. Dis. 2016, 62, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Cheah, S.E.; Wang, J.; Nguyen, V.T.T.; Turnidge, J.D.; Li, J.; Nation, R.L. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: Smaller response in lung infection. J. Antimicrob. Chemother. 2015, 70, 3291–3297. [Google Scholar]
- Sorlí, L.; Luque, S.; Grau, S.; Berenguer, N.; Segura, C.; Montero, M.M.; Álvarez-Lerma, F.; Knobel, H.; Benito, N.; Horcajada, J.P. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. BMC Infect. Dis. 2013, 13, 380. [Google Scholar] [CrossRef] [Green Version]
- Forrest, A.; Garonzik, S.M.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Li, J.; Silveira, F.P.; Nation, R.L. Pharmacokinetic/toxicodynamic analysis of colistin-associated acute kidney injury in critically Ill patients. Antimicrob. Agents Chemother. 2017, 61, e01367-17. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.F.; Cars, O.; Friberg, L.E. A pharmacokinetic/pharmacodynamic model developed for the effect of colistin on Pseudomonas aeruginosa in vitro with evaluation of population pharmacokinetic variability on simulated bacterial killing. J. Antimicrob. Chemother. 2014, 69, 1350–1361. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.A.; Beaumont, K.; Maurer, T.S.; Di, L. Volume of Distribution in Drug Design. J. Med. Chem. 2015, 58, 5691–5698. [Google Scholar] [CrossRef]
- Grégoire, N.; Aranzana-Climent, V.; Magréault, S.; Marchand, S.; Couet, W. Clinical Pharmacokinetics and Pharmacodynamics of Colistin. Clin. Pharmacokinet. 2017, 56, 1441–1460. [Google Scholar] [CrossRef] [PubMed]
- Couet, W.; Grégoire, N.; Gobin, P.; Saulnier, P.J.; Frasca, D.; Marchand, S.; Mimoz, O. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin. Pharmacol. Ther. 2011, 89, 875–879. [Google Scholar] [CrossRef]
- Boucher, B.A.; Wood, G.C.; Swanson, J.M. Pharmacokinetic Changes in Critical Illness. Crit. Care Clin. 2006, 22, 255–271. [Google Scholar] [CrossRef]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef] [Green Version]
- Blot, S.I.; Pea, F.; Lipman, J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—Concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 2014, 77, 3–11. [Google Scholar] [CrossRef]
- Mizuyachi, K.; Hara, K.; Wakamatsu, A.; Nohda, S.; Hirama, T. Safety and pharmacokinetic evaluation of intravenous colistin methanesulfonate sodium in Japanese healthy male subjects. Curr. Med. Res. Opin. 2011, 27, 2261–2270. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wu, X.J.; Fan, Y.X.; Zhang, Y.; Guo, B.N.; Yu, J.; Cao, G.; Chen, Y.; Wu, J.; Shi, Y.; et al. Pharmacokinetics of colistin methanesulfonate (CMS) in healthy Chinese subjects after single and multiple intravenous doses. Int. J. Antimicrob. Agents 2018, 51, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Samodelov, S.L.; Kullak-Ublick, G.A.; Visentin, M. Molecular Mechanisms of Colistin-Induced Nephrotoxicity. Molecules 2019, 24, 653. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Coulthard, K.; Milne, R.; Nation, R.L.; Conway, S.; Peckham, D.; Etherington, C.; Turnidge, J. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J. Antimicrob. Chemother. 2003, 52, 987–992. [Google Scholar] [CrossRef]
- Diao, L.; Meibohm, B. Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides. Clin. Pharmacokinet. 2013, 52, 855–868. [Google Scholar] [CrossRef]
- Sime, F.B.; Roberts, M.S.; Roberts, J.A. Optimization of dosing regimens and dosing in special populations. Clin. Microbiol. Infect. 2015, 21, 886–893. [Google Scholar] [CrossRef] [Green Version]
- Kunin, C.M.; Bugg, A. Binding of polymyxin antibiotics to tissues: The major determinant of distribution and persistence in the body. J. Infect. Dis. 1971, 124, 394–400. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Huang, J.X.; Cooper, M.A.; Roberts, K.D.; Thompson, P.E.; Nation, R.L.; Li, J.; Velkov, T. Structure-activity relationships for the binding of polymyxins with human α-1-acid glycoprotein. Biochem. Pharmacol. 2012, 84, 278–291. [Google Scholar] [CrossRef] [Green Version]
- Bonten, M.J.M. Colonization pressure: A critical parameter in the epidemiology of antibiotic-resistant bacteria. Crit. Care 2012, 16, 142. [Google Scholar] [CrossRef] [Green Version]
- Vardakas, K.Z.; Rellos, K.; Triarides, N.A.; Falagas, M.E. Colistin loading dose: Evaluation of the published pharmacokinetic and clinical data. Int. J. Antimicrob. Agents 2016, 48, 475–484. [Google Scholar] [CrossRef]
- Wallace, S.J.; Li, J.; Nation, R.L.; Prankerd, R.J.; Velkov, T.; Boyd, B.J. Self-assembly behavior of colistin and its prodrug colistin methanesulfonate: Implications for solution stability and solubilization. J. Phys. Chem. B 2010, 114, 4836–4840. [Google Scholar] [CrossRef] [Green Version]
- Wallace, S.J.; Li, J.; Rayner, C.R.; Coulthard, K.; Nation, R.L. Stability of colistin methanesulfonate in pharmaceutical products and solutions for administration to patients. Antimicrob. Agents Chemother. 2008, 52, 3047–3051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansson, B.; Karvanen, M.; Cars, O.; Plachouras, D.; Friberg, L.E. Quantitative analysis of colistin A and colistin B in plasma and culture medium using a simple precipitation step followed by LC/MS/MS. J. Pharm. Biomed. Anal. 2009, 49, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Aitullina, A.; Krūmina, A.; Svirskis, Š.; Purvina, S. Colistin use in patients with extreme renal function: From dialysis to augmented clearance. Medicina 2019, 55, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jitmuang, A.; Nation, R.L.; Koomanachai, P.; Chen, G.; Lee, H.J.; Wasuwattakul, S.; Sritippayawan, S.; Li, J.; Thamlikitkul, V.; Landersdorfer, C.B. Extracorporeal clearance of colistin methanesulphonate and formed colistin in end-stage renal disease patients receiving intermittent haemodialysis: Implications for dosing. J. Antimicrob. Chemother. 2014, 70, 1804–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiaccadori, E.; Antonucci, E.; Morabito, S.; D’Avolio, A.; Maggiore, U.; Regolisti, G. Colistin use in patients with reduced kidney function. Am. J. Kidney Dis. 2016, 68, 296–306. [Google Scholar] [CrossRef] [PubMed]
| Criterion | Scope of Review |
|---|---|
| Population | Critically ill patients with or without renal replacement therapy |
| Intervention | Patient receiving intravenously administered colistin methanesulphonate sodium (CMS) |
| Outcome | Adult patients receiving single or multiple doses of colistin methanesulphonate sodium (CMS), the following outcomes were evaluated: |
| Cmax, Cmin, Cave | |
| CL | |
| Vd | |
| t1/2 | |
| Study design | Studies which samples were collected from subjects representative of target population with pharmacokinetics evidence able to inform any of the above-mentioned outcomes. |
| Author year | Brand | First Dose | Reported Value | CMS | Colistin | ||
|---|---|---|---|---|---|---|---|
| Tmax (h) | Cmax (mg/L) | Tmax (h) | Cmax (mg/L) | ||||
| Plachouras et al. 2009 [9] | Colistin | 3 MIU | Predicted value | NR | ~3.5 | 7 | 0.6 |
| Karaiskos et al. 2015 [3] | Colistin | 9 MIU | Predicted value | 1 | 17 | 7 | 2.3 |
| Grégoire et al. 2014 [11] | Colimycine | 2 MIU | Predicted value | 1 | 6.5 | 3 | 2 |
| Jacob et al. 2015 [22] | Colimycine | 1–9 MIU | Predicted value | 1 | 9 | 6 | 3 |
| Mohamed et al. 2012 [10] | Colistin | 6 MIU | Mean ± SD | NR | NR | 8 | 1.4 ± 0.7 |
| Karvanen et al. 2012 [21] | Colistin | 2 MIU | Mean ± SD | 0.6 ± 0.1 | 6.9 ± 2 | 6 | 0.3 ± 0.1 |
| Kristoffersson et al. 2020 [2] | Colimycine | 9 MIU | Mean ± SD | 0.75 | 33.3 ± 9.3 | 0.75 | 0.8 ± 0.4 |
| Karnik et al. 2013 [12] | XylistinTM | 2 MIU | Mean ± SD | NR | NR | 1 | 7.8 ± 5 |
| Moni et al. 2020 [5] | Coly-monas® | 9 MIU | Mean ± SD | 0.5 ± 0.2 | 14.5 ± 136 | 2.75 ± 1.8 | 2.66 ± 1.2 |
| Author Year | Dose | Reported Value | Plasma Concentration | Pharmacokinetic Parameters | ||||
|---|---|---|---|---|---|---|---|---|
| Cmax (mg/L) | Cmin (mg/L) | Cave (mg/L) | t1/2 (h) | CL (ml/min) | Vd (L) | |||
| Plachouras et al. 2009 [9] | 3 MIU 8 hourly | Predicted value | 8 to 9 | NR | NR | 0.05, 2.3 | 228.3 | 13.5, 28.9 |
| Mohamed et al. 2012 [10] | 6 MIU then 1–3 MIU 8 hourly | Predicted value | NR | NR | NR | 0.03, 2.2 | 218.3 | 11.8, 28.4 |
| Matthieu et al. 2014 [15] | 2 MIU 8 hourly | Predicted value | NR | NR | NR | 2.7 | 64.6 | 15.3 |
| Grégoire et al. 2014 [11] | 6 MIU/day 8–12 hourly | Predicted value | 6.5 | NR | NR | 1.9 | 110.1 | 18.2 |
| Karaiskos et al. 2015 [3] | 9 MIU then 4.5 MIU 12 hourly | Predicted value | NR | NR | NR | 5.4 | 90 | 28 |
| Kristoffersson et al. 2020 [2] | 9 MIU then 4.5 MIU 12 hourly | Predicted value | NR | NR | NR | 5.6 | 27 | 13 |
| Markou et al. 2008 [20] | 3 MIU 8–12 hourly | Mean ± SD | NR | NR | NR | 2.9 ± 1.2 | 227.7 ± 96.7 | NR |
| Karvanen et al. 2012 [21] | 2 MIU 8 hourly | Mean ± SD | 6.9 ± 2.8 | 1.5 ± 0.6 | NR | 3.3 | 137.2 ± 51.2 | NR |
| Leuppi-Taegtmeyer et al. 2019 [14] | 6–9 MIU then 3 MIU 8 hourly | Mean ± SD | NR | 1.3 ± 0.7 | 5.0 ± 1.9 | 2.1 ± 0.5 | 70.6 ± 28.9 | 12.9 ± 5.8 |
| Moni et al., 2020 [5] | 9 MIU then 3 MIU 8 hourly | Mean ± SD | 3.8 ± 2.2 | 0.3 ± 0.3 | 1.7 ± 1.0 | NR | NR | 915 ± 69.7 |
| Author Year | Dose | Reported Value | Plasma Concentration | Pharmacokinetic Parameters | ||||
|---|---|---|---|---|---|---|---|---|
| Cmax (mg/L) | Cmin (mg/L) | Cave (mg/L) | t1/2 (h) | CL (ml/min) | Vd (L) | |||
| Plachouras et al. 2009 [9] | 3 MIU 8 hourly | Predicted value | 2.3 | NR | NR | 14.4 | 151.5 | 189 |
| Garonzik et al. 2011 [6] | 1–5 MIU 8–24 hourly | Predicted value | NR | NR | 2.4 | 4.6 | 45.3 | 45.1 |
| Mohamed et al. 2012 [10] | 6 MIU then 1–3 MIU 8 hourly | Predicted value | NR | NR | <2 | 18.5 | 136.7 | 218 |
| Grégoire et al. 2014 [11] | 6 MIU/day 8–12 hourly | Predicted value | 2 | NR | NR | 3.2 | 94.3 | 25.7 |
| Matthieu et al. 2014 [15] | 2 MIU 8 hourly | Predicted value | 4.7 | 0.15 | NR | 4.3 | 53.1 | 13.7 |
| Jacob et al. 2015 [22] | 1–9 MIU then 1.5 MIU 12 hourly | Predicted value | 3.6 | NR | NR | 9.8 | 33.3 | 28.3 |
| Karaiskos et al. 2015 [3] | 9 MIU then 4.5 MIU 12 hourly | Predicted value | 0.68–8.72 | NR | NR | 11.2 | 81.7 | 80.4 |
| Kristoffersson et al. 2020 [2] | 9 MIU then 4.5 MIU 12 hourly | Predicted value | NR | NR | >2 | 12, 17 and 25 * | 50.5 | 81.2 |
| Markou et al. 2008 [20] | 3 MIU 8–12 hourly | Mean ± SD | 2.9 ± 1.2 | 1.0 ± 0.4 | NR | 7.4 ± 1.7 | 226.7 ± 96.7 | 139.9 ± 60.3 |
| Imberti et al. 2010 [23] | 2 MIU 8 hourly | Mean ± SD | 2.21 ± 1.1 | 1.03 ± 0.7 | NR | 5.9 ± 2.6 | 346 ± 240 | 120 ± 88 |
| Karvanen et al. 2012 [21] | 2 MIU 8 hourly | Mean ± SD | NR | NR | 0.9 ± 0.5 | NR | 315.2 ± 99.2 | NR |
| Karnik et al. 2013 [12] | 2 MIU 8–12 hourly | Mean ± SD | 8.6 ± 5.7 | 0.8 ± 0.5 | 2 ± 0.8 | 8.6 ± 5.7 | 74.9 ± 16.6 | 40.5 ± 21.2 |
| Leuppi-Taegtmeyer et al. 2019 [14] | 6–9 MIU then 3 MIU 8 hourly | Mean ± SD | NR | 3.9 ± 0.8 | 4.7 ± 1.5 | 17.8 ± 7.5 | 50.9 ± 15.6 | 72.2 ± 24.6 |
| Moni et al. 2020 [5] | 9 MIU then 3 MIU 8 hourly | Mean ± SD | 2.4 ± 2.2 | 1.5 ± 0.9 | 2 ± 1.2 | NR | NR | 644.5 ± 32.2 |
| Ram, et al. 2020 [4] | 2 MIU 8 hourly | Mean ± SD | 10.6 ± 1.6 | 0.7 ± 0.2 | NR | 2.9 ± 0.43 | 130 ± 40 | 33 ± 6.6 |
| Author Year | Dose | Reported Value | CMS | Colistin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t1/2 (h) | CL (ml/min) | Vd (L) | t1/2 (h) | CL (ml/min) | Vd (L) | |||||
| Total | Dialysis | Total | Dialysis | |||||||
| Garonzik et al. 2011 [6] | 1–5 MIU 8–24 hourly | Predicted value | 4.6 | 103.3 | 94.8 (HD) 64.2 (CRRT) | 18.7 | 4.6 | 45.3 | 56.7 (HD) 34.3 (CRRT) | 45.1 |
| Jacob et al. 2015 [22] | 1–9 MIU then 1.5 MIU 12 hourly | Predicted value | 2.1 | 113 | 90 (HD) | 21 | 9.8 | 33.3 | 137 (HD) | 28.3 |
| Karvanen et al. 2012 [21] | 2 MIU 8 hourly | Mean ± SD | 3.3 | 137.2 ± 51.2 | 32.3 ± 13.3 (CRRT) | NR | NR | 315.2 ± 99.2 | 71.7 ± 21.7 (CRRT) | NR |
| Leuppi-Taegtmeyer et al. 2019 [14] | 6–9 MIU then 3 MIU 8 hourly | Mean ± SD | 2.1 ± 0.5 | 70.6 ± 28.9 | 26.3 ± 3.7 (CRRT) | 12.9 ± 5.8 | 17.8 ± 7.5 | 50.9 ± 15.6 | 13.3 ± 3.2 (CRRT) | 72.2 ± 24.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabidi, M.S.; Abu Bakar, R.; Musa, N.; Mustafa, S.; Wan Yusuf, W.N. Population Pharmacokinetics of Colistin Methanesulfonate Sodium and Colistin in Critically Ill Patients: A Systematic Review. Pharmaceuticals 2021, 14, 903. https://doi.org/10.3390/ph14090903
Zabidi MS, Abu Bakar R, Musa N, Mustafa S, Wan Yusuf WN. Population Pharmacokinetics of Colistin Methanesulfonate Sodium and Colistin in Critically Ill Patients: A Systematic Review. Pharmaceuticals. 2021; 14(9):903. https://doi.org/10.3390/ph14090903
Chicago/Turabian StyleZabidi, Mohd Shafie, Ruzilawati Abu Bakar, Nurfadhlina Musa, Suzana Mustafa, and Wan Nazirah Wan Yusuf. 2021. "Population Pharmacokinetics of Colistin Methanesulfonate Sodium and Colistin in Critically Ill Patients: A Systematic Review" Pharmaceuticals 14, no. 9: 903. https://doi.org/10.3390/ph14090903






