Protective Effect of Monoterpene Isoespintanol in a Rat Model of Prediabetes Induced by Fructose
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Food Intake, and Drink Intake
2.2. Serum Parameters
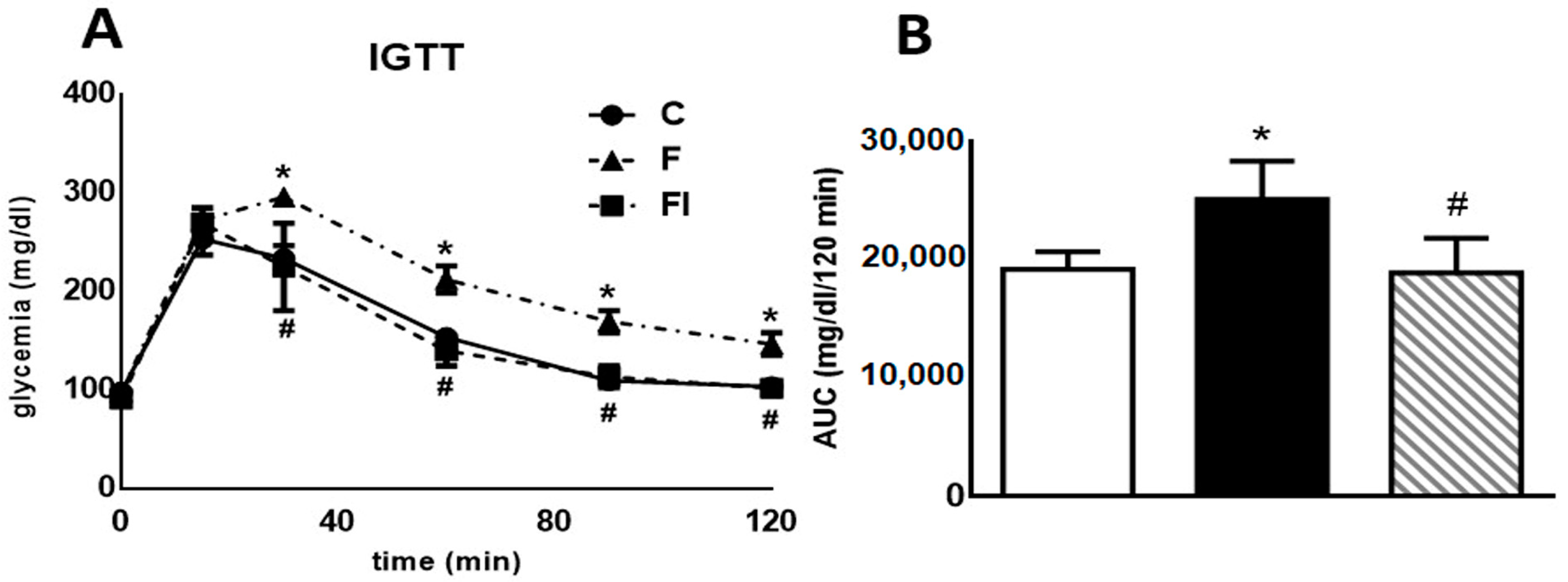
2.3. Intraperitoneal Glucose Tolerance Test (IGTT)
2.4. Liver Parameters
2.4.1. Liver Glycogen Content, p-GSK3β Protein Levels, and Lipogenic Gene Expression
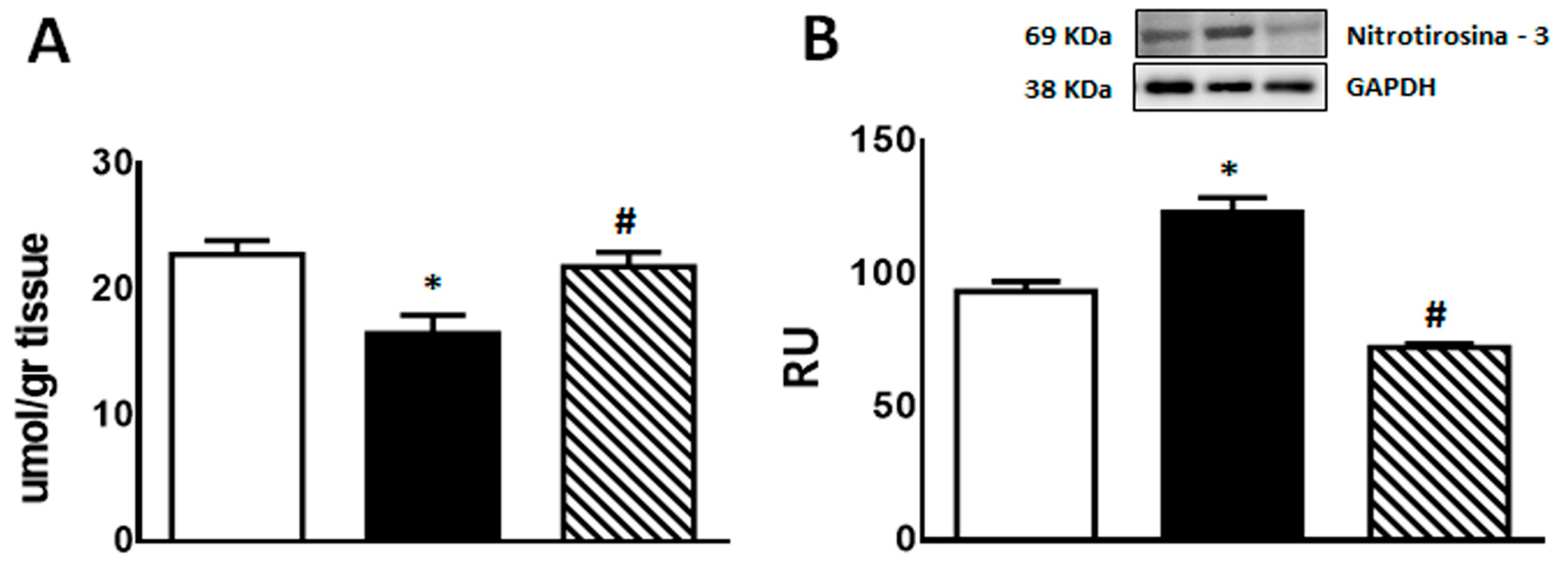
2.4.2. Oxidative Stress (GSH and 3′-Nitrotyrosine) and Inflammation Markers (TNF-α, PAI-1, and iNOS Gene Expression; COX-2 and iNOS Protein Expression)
2.4.3. Protein Levels of p-Akt and p-eNOS
3. Discussion
4. Materials and Methods
4.1. Chemicals and Drugs
4.2. Experimental Groups
4.3. Determination of Serum Parameters
4.4. Intraperitoneal Glucose Tolerance Test (IGTT)
4.5. Determination of Liver Parameters
4.5.1. Glycogen Estimation
4.5.2. Estimation of Reduced Glutathione (GSH) Content
4.5.3. Protein Expression by Western Blot
4.5.4. Total RNA Isolation and Analysis of Gene Expression by Real-Time PCR (qPCR)
4.6. Statistical Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lovic, D.; Piperidou, A.; Zografou, I.; Grassos, H.; Pittaras, A.; Manolis, A. The Growing Epidemic of Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 104–109. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, S1–S193. [Google Scholar] [CrossRef] [PubMed]
- Carvallo, P.; Carvallo, E.; Barbosa-Da-Silva, S.; Mandarim-De-Lacerda, C.; Hernández, A.; Del Sol, M. Metabolic Effects of Excessive Fructose Consumption Added. Int. J. Morphol. 2019, 37, 1058–1066. [Google Scholar] [CrossRef]
- Nier, A.; Brandt, A.; Conzelmann, I.B.; Özel, Y.; Bergheim, I. Non-alcoholic fatty liver disease in overweight children: Role of fructose intake and dietary pattern. Nutrients 2018, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Czerwonogrodzka-Senczyna, A.; Rumińska, M.; Majcher, A.; Credo, D.; Jeznach-Steinhagen, A.; Pyrżak, B. Fructose Consump-tion and Lipid Metabolism in Obese Children and Adolescents. In Advances in Experimental Medicine and Biology; Pokorski, M., Ed.; Springer: Cham, Switzerland, 2019; Volume 1153, pp. 91–100. [Google Scholar] [CrossRef]
- Olson, E.; Suh, J.H.; Schwarz, J.M.; Noworolski, S.M.; Jones, G.M.; Barber, J.R.; Erkin-Cakmak, A.; Mulligan, K.; Lustig, R.H.; Mietus-Snyder, M. Effects of Isocaloric Fructose Restriction on Ceramide Levels in Children with Obesity and Cardi-ometabolic Risk: Relation to Hepatic De Novo Lipogenesis and Insulin. Sensitivity. Nutrients 2022, 14, 1432. [Google Scholar] [CrossRef]
- Bence, K.K.; Birnbaum, M.J. Metabolic drivers of non-alcoholic fatty liver disease. Mol. Metab. 2021, 50, 101–143. [Google Scholar] [CrossRef]
- Castro, M.C.; Villagarcía, H.G.; Román, C.L.; Maiztegui, B.; Flores, L.E.; Schinella, G.R.; Massa, M.L.; Francini, F. Chronolog-ical appearance of endocrine and metabolic dysfunctions induced by an unhealthy diet in rats. Medicina 2022, 58, 8. [Google Scholar] [CrossRef]
- Castro, M.C.; Massa, M.L.; Arbeláez, L.G.; Schinella, G.; Gagliardino, J.J.; Francini, F. Fructose-induced inflammation, insulin resistance and oxidative stress: A liver pathological triad effectively disrupted by lipoic acid. Life Sci. 2015, 137, 1–6. [Google Scholar] [CrossRef]
- Francini, F.; Castro, M.C.; Gagliardino, J.J.; Massa, M.L. Regulation of liver glucokinase activity in rats with fructose-induced insulin resistance and impaired glucose and lipid metabolism. Can. J. Physiol. Pharmacol. 2009, 87, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Francini, F.; Castro, M.C.; Schinella, G.; García, M.E.; Maiztegui, B.; Raschia, M.A.; Gagliardino, J.J.; Massa, M.L. Changes in-duced by a fructose-rich diet on hepatic metabolism and the antioxidant system. Life Sci. 2010, 86, 965–971. [Google Scholar] [CrossRef]
- Maiztegui, B.; Borelli, M.I.; Raschia, M.A.; Del Zotto, H.; Gagliardino, J.J. Islet adaptive changes to fructose-induced insulin resistance: Beta-cell mass, glucokinase, glucose metabolism, and insulin secretion. J. Endocrinol. 2009, 200, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, A.; Bagherniya, M.; Mirenayat, M.S.; Atkin, S.L.; Sahebkar, A. Medicinal Plants and Phytochemicals Regulating Insulin Resistance and Glucose Homeostasis in Type 2 Diabetic Patients: A Clinical Review. Adv. Exp. Med. Biol. 2021, 1308, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Bessell, E.; Fuller, N.R.; Markovic, T.P.; Burk, J.; Picone, T.; Hendy, C.; CTan, M.M.; Caterson, I.D. Effects of alpha-cyclodextrin on cholesterol control and Compound K on glycaemic control in people with pre-diabetes: Protocol for a Phase III randomized controlled trial. Clin. Obes. 2019, 9, e12324. [Google Scholar] [CrossRef]
- Naseri, K.; Saadati, S.; Sadeghi, A.; Asbaghi, O.; Ghaemi, F.; Zafarani, F.; Li, H.-B.; Gan, R.-Y. The efficacy of Ginseng (Panax) on Human Prediabetes and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2401. [Google Scholar] [CrossRef]
- Bessell, E.; Fuller, N.R.; Markovic, T.P.; Lau, N.S.; Burk, J.; Hendy, C.; Picone, T.; Li, A.; Caterson, I.D. Effects of α-cyclodextrin on cholesterol control and hydrolyzed ginseng extract on glycemic control in people with prediabetes: A randomized clinical trial. JAMA Netw. Open 2020, 3, e2023491. [Google Scholar] [CrossRef]
- Rojano, B.; Pérez, E.; Figadère, B.; Martin, M.T.; Recio, M.C.; Giner, R.; Ríos, J.L.; Schinella, G.; Sáez, J. Constituents of Oxandra cf. xylopioides with anti-inflammatory activity. J. Nat. Prod. 2007, 70, 835–838. [Google Scholar] [CrossRef]
- Rojano, B.; Gaviria, C.; Gil, M.; Saez, J.; Guillermo, S.; Tournier, H. Antioxidant Activity of the Isoespintanol in Different Media. Vitae 2008, 15, 173–181. [Google Scholar]
- Gavilánez Buñay, T.C.; Colareda, G.A.; Ragone, M.I.; Bonilla, M.; Rojano, B.A.; Schinella, G.R.; Consolini, A.E. Intestinal, urinary and uterine antispasmodic effects of isoespintanol, metabolite from Oxandra xylopioides leaves. Phytomedicine 2018, 51, 20–28. [Google Scholar] [CrossRef]
- González Arbeláez, L.F.; Ciocci Pardo, A.; Fantinelli, J.C.; Rojano, B.; Schinella, G.R.; Mosca, S.M. Isoespintanol, a mono-terpene isolated from oxandra cf xylopioides, ameliorates the myocardial ischemia-reperfusion injury by AKT/PKCε/eNOS-dependent pathways. Naunyn-Schmiedeberg Arch. Pharmacol. 2020, 393, 629–638. [Google Scholar] [CrossRef]
- Rojano, B.; Saez, J.; Schinella, G.; Quijano, J.; Vélez, E.; Gil, A.; Notario, R. Experimental and theoretical determination of the antioxidant properties of isoespintanol (2-isopropyl-3,6-dimethoxy-5-methylphenol). J. Mol. Struct. 2008, 877, 1–6. [Google Scholar] [CrossRef]
- Rinaldi, G.J.; Rojano, B.; Schinella, G.; Mosca, S.M. Participation of NO in the vasodilatory action of Isoespintanol. Vitae 2019, 26, 78–83. [Google Scholar] [CrossRef]
- Borst, S.E. The role of TNF-α in insulin resistance. Endocrine 2004, 23, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H. Adipose Tissue-Derived Plasminogen Activator Inhibitor-1 Function and Regulation. Compr. Physiol. 2016, 6, 1873–1896. [Google Scholar] [CrossRef] [PubMed]
- Samad, F.; Uysal, K.T.; Wiesbrock, S.M.; Pandey, M.; Hotamisligil, G.S.; Loskutoff, D.J. Tumor necrosis factor α is a key component in the obesity-linked elevation of plasminogen activator inhibitor 1. Proc. Nat. Acad. Sci. USA 1999, 96, 6902–6907. [Google Scholar] [CrossRef]
- Beurel, E.; Michalek, S.M.; Jope, R.S. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 2010, 31, 24–31. [Google Scholar] [CrossRef]
- Cohen, P.; Goedert, M. GSK3 inhibitors: Development and therapeutic potential. Nat. Rev. Drug Discov. 2004, 3, 479–487. [Google Scholar] [CrossRef]
- McManus, E.J.; Sakamoto, K.; Armit, L.J.; Ronaldson, L.; Shpiro, N.; Marquez, R.; Alessi, D.R. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005, 24, 1571–1583. [Google Scholar] [CrossRef]
- Rayasam, G.V.; Tulasi, V.K.; Sodhi, R.; Davis, J.A.; Ray, A. Glycogen synthase kinase 3: More than a namesake. Br. J. Pharmacol. 2009, 156, 885–898. [Google Scholar] [CrossRef]
- Wang, H.; Brown, J.; Martin, M. Glycogen synthase kinase 3: A point of convergence for the host inflammatory response. Cytokine 2011, 53, 130–140. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Ni, M.; Yue, S.; Xia, Y.; Busuttil, R.W.; Kupiec-Weglinski, J.W.; Lu, L.; Wang, X.; Zhai, Y. Glycogen syn-thase kinase 3β promotes liver innate immune activation by restraining AMP-activated protein kinase activation. J. Hepatol. 2018, 69, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Vieyra, R.; Silva-García, O.; Gómez-García, A.; Gutiérrez-Castellanos, S.; Álvarez-Aguilar, C.; Baizabal-Aguirre, V.M. Glycogen Synthase Kinase 3β Modulates the Inflammatory Response Activated by Bacteria, Viruses, and Parasites. Front. Immunol. 2021, 12, 675751. [Google Scholar] [CrossRef] [PubMed]
- Jope, R.S.; Yuskaitis, C.J.; Beurel, E. Glycogen Synthase Kinase-3 (GSK3): Inflammation, Diseases, and Therapeutics. Neurochem. Res. 2007, 32, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.R.; Batista, T.M.; Victorio, J.A.; Clerici, S.P.; Delbin, M.A.; Carneiro, E.M.; Davel, A.P. Taurine supplementation reduces blood pressure and prevents endothelial dysfunction and oxidative stress in post-weaning protein-restricted rats. PLoS ONE 2014, 9, e105851. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Siamwala, J.H.; Chatterjee, S. eNOS phosphorylation in health and disease. Biochimie 2010, 92, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Atochin, D.N.; Wang, A.; Liu VW, T.; Critchlow, J.D.; Dantas AP, V.; Looft-Wilson, R.; Murata, T.; Salomone, S.; Shin, H.K.; Ayata, C.; et al. The phosphorylation state of eNOS modulates vascu-lar reactivity and outcome of cerebral ischemia in vivo. J. Clin. Investig. 2007, 117, 1961–1967. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. Modulation of Akt-p38-MAPK/Nrf2/SIRT1 and NF-κB pathways by wine pomace product in hyperglycemic endothelial cell line. J. Funct. Foods. 2019, 58, 255–265. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. The protective effects of wine pomace products on the vascular endothelial barrier function. Food. Funct. 2020, 11, 7878–7891. [Google Scholar] [CrossRef]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. MTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef]
- Han, J.W.; Zhan, X.R.; Li, X.Y.; Xia, B.; Wang, Y.Y.; Zhang, J.; Li, B.X. Impaired PI3K/Akt signal pathway and hepatocellu-lar injury in high-fat fed rats. World J. Gastroenterol. 2010, 16, 6111–6118. [Google Scholar] [CrossRef]
- Castro, M.C.; Villagarcía, H.; Nazar, A.; Arbeláez, L.G.; Massa, M.L.; Del Zotto, H.; Ríos, J.L.; Schinella, G.R.; Francini, F. Cacao extract enriched in polyphenols prevents endocrine-metabolic disturbances in a rat model of prediabetes triggered by a sucrose rich diet. J. Ethnopharmacol. 2020, 247, 112–263. [Google Scholar] [CrossRef] [PubMed]
- Bijl, N.; Sokolović, M.; Vrins, C.; Langeveld, M.; Moerland, P.D.; Ottenhoff, R.; Van Roomen, C.P.A.A.; Claessen, N.; Boot, R.G.; Aten, J.; et al. Modulation of glycosphingolipid metabolism significantly im-proves hepatic insulin sensitivity and reverses hepatic steatosis in mice. Hepatology 2009, 50, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hai, J.; Cao, M.; Zhang, Y.; Pei, S.; Wang, J.; Zhang, Q. Silibinin ameliorates steatosis and insulin resistance during non-alcoholic fatty liver disease development partly through targeting IRS-1/PI3K/Akt pathway. Int. Immunopharmacol. 2013, 17, 714–720. [Google Scholar] [CrossRef]
- Wang, C.; Chi, Y.; Li, J.; Miao, Y.; Li, S.; Su, W.; Jia, S.; Chen, Z.; Du, S.; Zhang, X.; et al. FAM3A activates PI3K p110α/Akt signaling to ameliorate hepatic gluconeogenesis and lipogenesis. Hepatology 2014, 59, 1779–1790. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, C.L.; Zhao, N.; Guan, M.J.; Xiao, M.; Yang, R.; Zhao, X.L.; Yu, L.H.; Zhu, Z.P.; Xie, K.Q. Impairment of Akt activity by CYP2E1 mediated oxidative stress is involved in chronic ethanol-induced fatty liver. Redox Biol. 2018, 14, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik MH, J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2022, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Laws, A.; Reaven, G.M. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J. Intern. Med. 1992, 231, 25–30. [Google Scholar] [CrossRef]
- De Vogel-Van Den Bosch, J.; Hoeks, J.; Timmers, S.; Houten, S.M.; Van Dijk, P.J.; Boon, W.; Van Beurden, D.; Schaart, G.; Kersten, S.; Voshol, P.J.; et al. The effects of long-or medium-chain fat diets on glucose tolerance and myocellular content of lipid intermediates in rats. Obesity 2011, 19, 792–799. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s rea-gent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2003–2007. [Google Scholar] [CrossRef]







| Plasma Parameters | C | F | FI | p Value |
|---|---|---|---|---|
| Glucose (mg/dL) | 134.8 ± 5.8 | 133.6 ± 6.7 | 133.1 ± 4.4 | NS |
| Triglyceride (mg/dL) | 96.3 ± 16.1 | 264.2 ± 21.2 * | 79.9 ± 9 # | *# p < 0.0001 |
| Chol-HDL (mg/dL) | 50.3 ± 5.7 | 43.4 ± 4.8 | 52.1 ± 2.8 | NS |
| IRX (TG/HDL) | 2.0 ± 0.3 | 6.4 ± 0.8 * | 1.6 ± 0.3 # | *# p < 0.001 |
| Cholesterol-Total (mg/dL) | 64.0 ± 5.4 | 78.0 ± 6.7 | 86.8 ± 6.4 | NS |
| Cholesterol-NoHDL (mg/dL) | 13.7 ± 2.0 | 34.6 ± 2.7 * | 34.7 ± 6.0 | * p < 0.0001 |
| Gene | GeneBank® | Sequences |
|---|---|---|
| SREBP-1c | XM_213329.6 | FW 5′-TTTCTTCGTGGATGGGGACT-3′ RV 5′-CTGTAGATATCCAAGAGCATC-3′ |
| FAS | NM_017332.1 | FW 5′-GTCTGCAGCTACCCACCCGTG-3′ RV 5′-CTTCTCCAGGGTGGGGACCAG-3′ |
| GPAT-1 | NM_017274.1 | FW 5′-GACGAAGCCTTCCGAAGGA-3′ RV 5′-GACGAAGCCTTCCGAAGGA-3′ |
| PAI-1 | NM_012620.1 | FW 5′-CCACGGTGAAGCAGGTGGACT-3′ RV 5′-TGCTGGCCTCTAAGAAGGGG-3′ |
| TNF-α | NM_012675.3 | FW 5′-GGCATGGATCTCAAAGACAACC-3′ RV 5′-CAAATCGGCTGACGGTGTG-3′ |
| CPT1 | NM_031559.2 | FW: 5′ GGGCGGTACTTCAAGGTCTGG 3′ RV: 5′ GTCTGCCGACACTTTGCCCA 3′ |
| eNOS | NM_021838.2 | FW: 5′ GCTGGGGGATCAGCAACGCT 3′ RV: 5′ GCGGGTCAAAGGACCAGGGC 3′ |
| iNOS | NM_012611.3 | FW: 5′ GAAGCTCAGCCGCACCACCC 3′ RV: 5′ CAGGGCCGTCTGGTTGCCTG 3′ |
| β-ACTIN | NM_031144.2 | FW 5′-AGAGGGAAATCGTGCGTGAC-3′ RV 5′-CGATAGTGATGACCTGACCGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Sarli Gutiérrez, L.; Castro, M.C.; Farromeque Vásquez, S.; Villagarcía, H.G.; González Arbeláez, L.; Rojano, B.; Schinella, G.; Maiztegui, B.; Francini, F. Protective Effect of Monoterpene Isoespintanol in a Rat Model of Prediabetes Induced by Fructose. Pharmaceuticals 2024, 17, 47. https://doi.org/10.3390/ph17010047
Di Sarli Gutiérrez L, Castro MC, Farromeque Vásquez S, Villagarcía HG, González Arbeláez L, Rojano B, Schinella G, Maiztegui B, Francini F. Protective Effect of Monoterpene Isoespintanol in a Rat Model of Prediabetes Induced by Fructose. Pharmaceuticals. 2024; 17(1):47. https://doi.org/10.3390/ph17010047
Chicago/Turabian StyleDi Sarli Gutiérrez, Luciana, María Cecilia Castro, Sherley Farromeque Vásquez, Hernán Gonzalo Villagarcía, Luisa González Arbeláez, Benjamín Rojano, Guillermo Schinella, Bárbara Maiztegui, and Flavio Francini. 2024. "Protective Effect of Monoterpene Isoespintanol in a Rat Model of Prediabetes Induced by Fructose" Pharmaceuticals 17, no. 1: 47. https://doi.org/10.3390/ph17010047








