Protective Role of Astaxanthin in Regulating Lipopolysaccharide-Induced Inflammation and Apoptosis in Human Neutrophils
Abstract
:1. Introduction
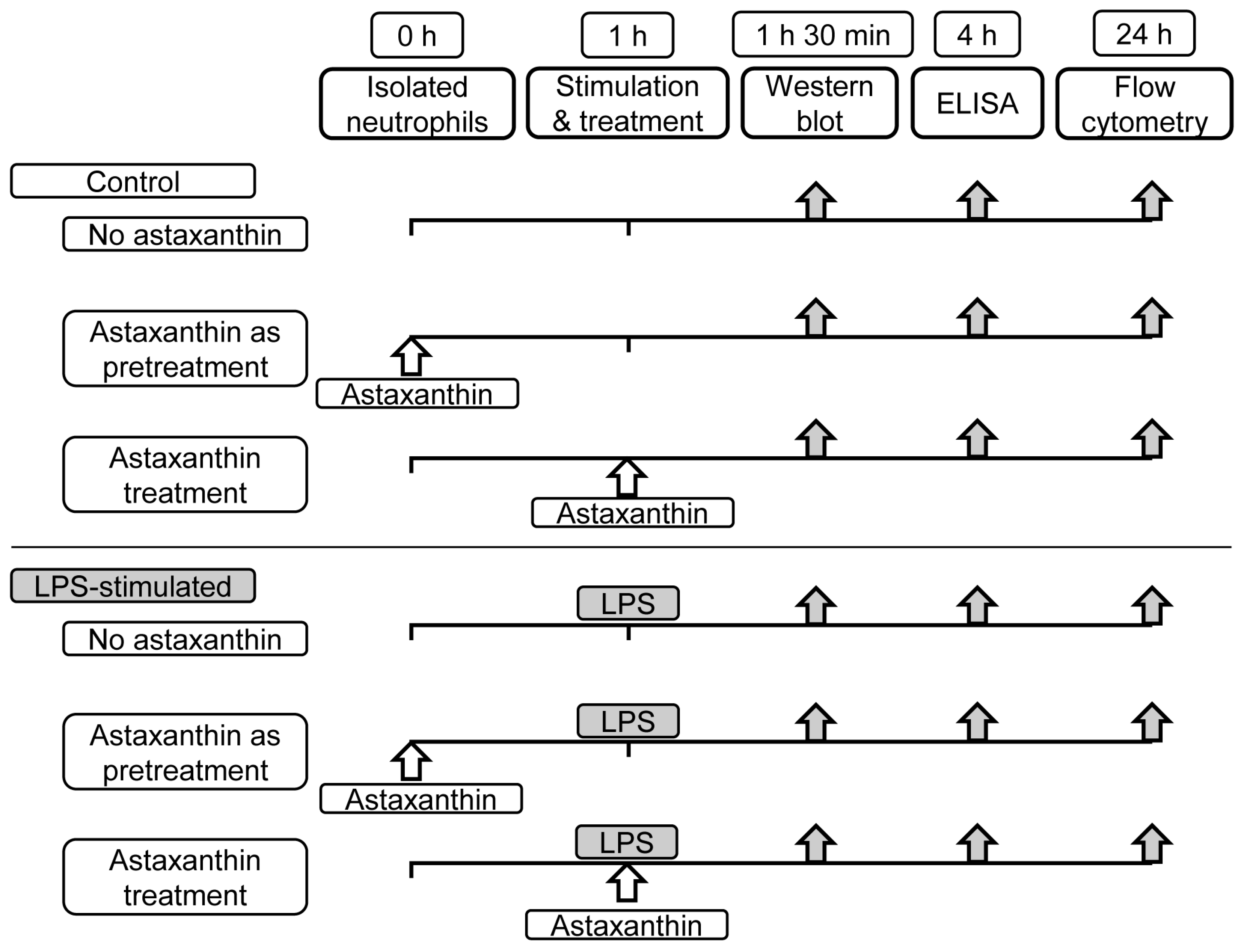
2. Materials and Methods
2.1. Neutrophils Isolation
2.2. Cytokine Quantification
2.3. Western Blot Analysis
2.4. Apoptosis Assessment
2.5. Statistical Analysis
3. Results
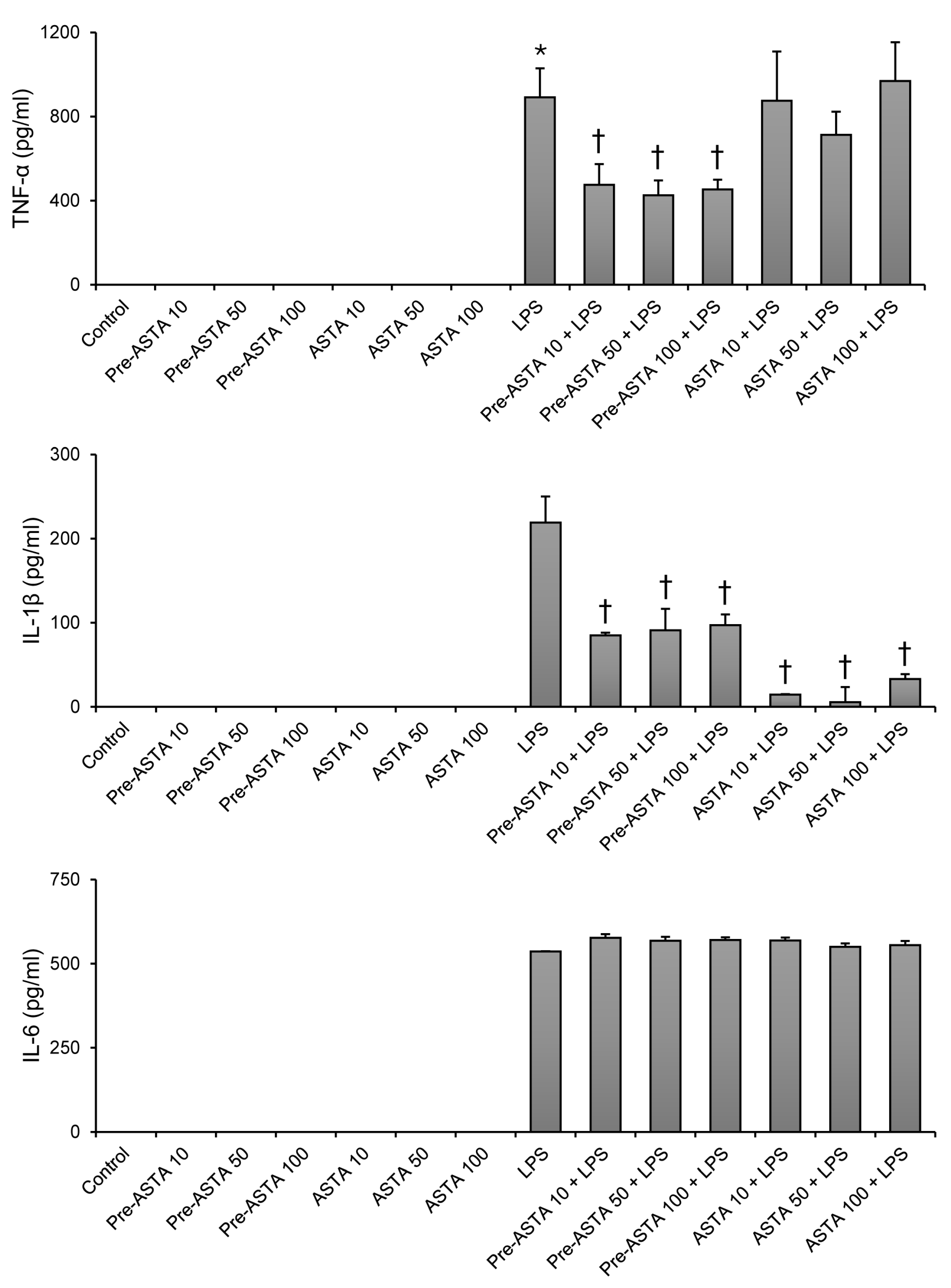
3.1. Impact of Astaxanthin on Cytokine Production in LPS-Stimulated Neutrophils
3.2. Effects of Astaxanthin on MAPKs and NF-κB Signaling in LPS-Stimulated Neutrophils
3.3. Role of Astaxanthin in Modulating Neutrophil Apoptosis Delayed by LPS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caraballo, C.; Jaimes, F. Focus: Death: Organ Dysfunction in Sepsis: An Ominous Trajectory From Infection To Death. Yale J. Biol. Med. 2019, 92, 629. [Google Scholar]
- Ferrer, R.; Martin-Loeches, I.; Phillips, G.; Osborn, T.M.; Townsend, S.; Dellinger, R.P.; Artigas, A.; Schorr, C.; Levy, M.M. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: Results from a guideline-based performance improvement program. Crit. Care Med. 2014, 42, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Dantes, R.; Epstein, L.; Murphy, D.J.; Seymour, C.W.; Iwashyna, T.J.; Kadri, S.S.; Angus, D.C.; Danner, R.L.; Fiore, A.E. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. J. Am. Med. Assoc. 2017, 318, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- van Zanten, A.R.; Brinkman, S.; Arbous, M.S.; Abu-Hanna, A.; Levy, M.M.; de Keizer, N.F. Guideline bundles adherence and mortality in severe sepsis and septic shock. Crit. Care Med. 2014, 42, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.M.; Allen, L.-A.H. Regulation of human neutrophil apoptosis and lifespan in health and disease. J. Cell Death 2014, 7, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Umare, V.; Pradhan, V.; Nadkar, M.; Rajadhyaksha, A.; Patwardhan, M.; Ghosh, K.K.; Nadkarni, A.H. Effect of proinflammatory cytokines (IL-6, TNF-α, and IL-1β) on clinical manifestations in Indian SLE patients. Mediat. Inflamm. 2014, 2014, 385297. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.B.; Park, C.H.; Kim, J.; Tin, T.D.; Kwak, S.H. Protective role of curcumin against lipopolysaccharide-induced inflammation and apoptosis in human neutrophil. Anesth. Pain. Med. 2020, 15, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ju, W.; Tin, T.D.; Kim, J.; Lee, J.S.; Park, C.H.; Kwak, S.H. Effect of BMS-470539 on lipopolysaccharide-induced neutrophil activation. Korean J. Anesth. Anesthesiol. 2020, 73, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Kim, J.; Bae, H.B.; Tin, T.D.; Ju, W.; Kwak, S.H. The Effects of Flecainide Acetate on Inflammatory-Immune Response in Lipopolysaccharide-Stimulated Neutrophils and on Mortality in Septic Rats. Acute Crit. Care 2018, 33, 34–41. [Google Scholar] [CrossRef]
- Margalith, P. Production of ketocarotenoids by microalgae. Appl. Microbiol. Biotechnol. 1999, 51, 431–438. [Google Scholar] [CrossRef]
- Choi, S.; Koo, S. Efficient syntheses of the keto-carotenoids canthaxanthin, astaxanthin, and astacene. J. Org. Chem. 2005, 70, 3328–3331. [Google Scholar] [CrossRef]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases. Int. J. Mol. Med. 2021, 47, 37–48. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58D–68D. [Google Scholar] [CrossRef]
- Li, M.-Y.; Sun, L.; Niu, X.-T.; Chen, X.-M.; Tian, J.-X.; Kong, Y.-D.; Wang, G.-Q. Astaxanthin protects lipopolysaccharide-induced inflammatory response in Channa argus through inhibiting NF-κB and MAPKs signaling pathways. Fish. Shellfish. Immunol. 2019, 86, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xu, N.; Shi, Y.; Zhou, B.; Sun, D.; Ma, B.; Xu, Z.; Yang, J.; Li, C. Astaxanthin Protects Dendritic Cells from Lipopolysaccharide-Induced Immune Dysfunction. Mar. Drugs 2021, 19, 346. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Gao, M.; Xiao, Z.; Zhang, J.; Li, X.; Wang, A. Protective effect of astaxanthin against multiple organ injury in a rat model of sepsis. J. Surg. Res. 2015, 195, 559–567. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Diksha; Kumari, A.; Panwar, A. Astaxanthin: A super antioxidant from microalgae and its therapeutic potential. J. Basic. Microbiol. 2022, 62, 1064–1082. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Makita, H.; Ohnishi, M.; Mori, H.; Satoh, K.; Hara, A. Chemoprevention of rat oral carcinogenesis by naturally occurring xanthophylls, astaxanthin and canthaxanthin. Cancer Res. 1995, 55, 4059–4064. [Google Scholar] [PubMed]
- Chew, B.P.; Park, J.S.; Wong, M.W.; Wong, T.S. A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer. Res. 1999, 19, 1849–1853. [Google Scholar]
- Wang, X.; Willen, R.; Wadstrom, T. Astaxanthin-rich algal meal and vitamin C inhibit Helicobacter pylori infection in BALB/cA mice. Antimicrob. Agents Chemother. 2000, 44, 2452–2457. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of astaxanthin in obese mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2007, 71, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.; Kowshik, J.; Kishore, T.K.; Baba, A.B.; Nagini, S. Astaxanthin inhibits NF-kappaB and Wnt/beta-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. Biochim. Biophys. Acta 2013, 1830, 4433–4444. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Chen, Y.; Xie, X.; Yao, D.; Ding, C.; Chen, M. Astaxanthin prevents against lipopolysaccharide-induced acute lung injury and sepsis via inhibiting activation of MAPK/NF-kappaB. Am. J. Transl. Res. 2019, 11, 1884–1894. [Google Scholar]
- Macedo, R.C.; Bolin, A.P.; Marin, D.P.; Otton, R. Astaxanthin addition improves human neutrophils function: In vitro study. Eur. J. Nutr. 2010, 49, 447–457. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Son, S.K.; Cho, E.; Yoo, S.; Jang, E.-A.; Kwak, S.H. Protective Role of Astaxanthin in Regulating Lipopolysaccharide-Induced Inflammation and Apoptosis in Human Neutrophils. Curr. Issues Mol. Biol. 2024, 46, 8567-8575. https://doi.org/10.3390/cimb46080504
Lee S, Son SK, Cho E, Yoo S, Jang E-A, Kwak SH. Protective Role of Astaxanthin in Regulating Lipopolysaccharide-Induced Inflammation and Apoptosis in Human Neutrophils. Current Issues in Molecular Biology. 2024; 46(8):8567-8575. https://doi.org/10.3390/cimb46080504
Chicago/Turabian StyleLee, Seongheon, Sung Kuk Son, Eunye Cho, Sungah Yoo, Eun-A Jang, and Sang Hyun Kwak. 2024. "Protective Role of Astaxanthin in Regulating Lipopolysaccharide-Induced Inflammation and Apoptosis in Human Neutrophils" Current Issues in Molecular Biology 46, no. 8: 8567-8575. https://doi.org/10.3390/cimb46080504





