Multiorgan Toxicity from Dual Checkpoint Inhibitor Therapy, Resulting in a Complete Response—A Case Report
Abstract
:1. Introduction
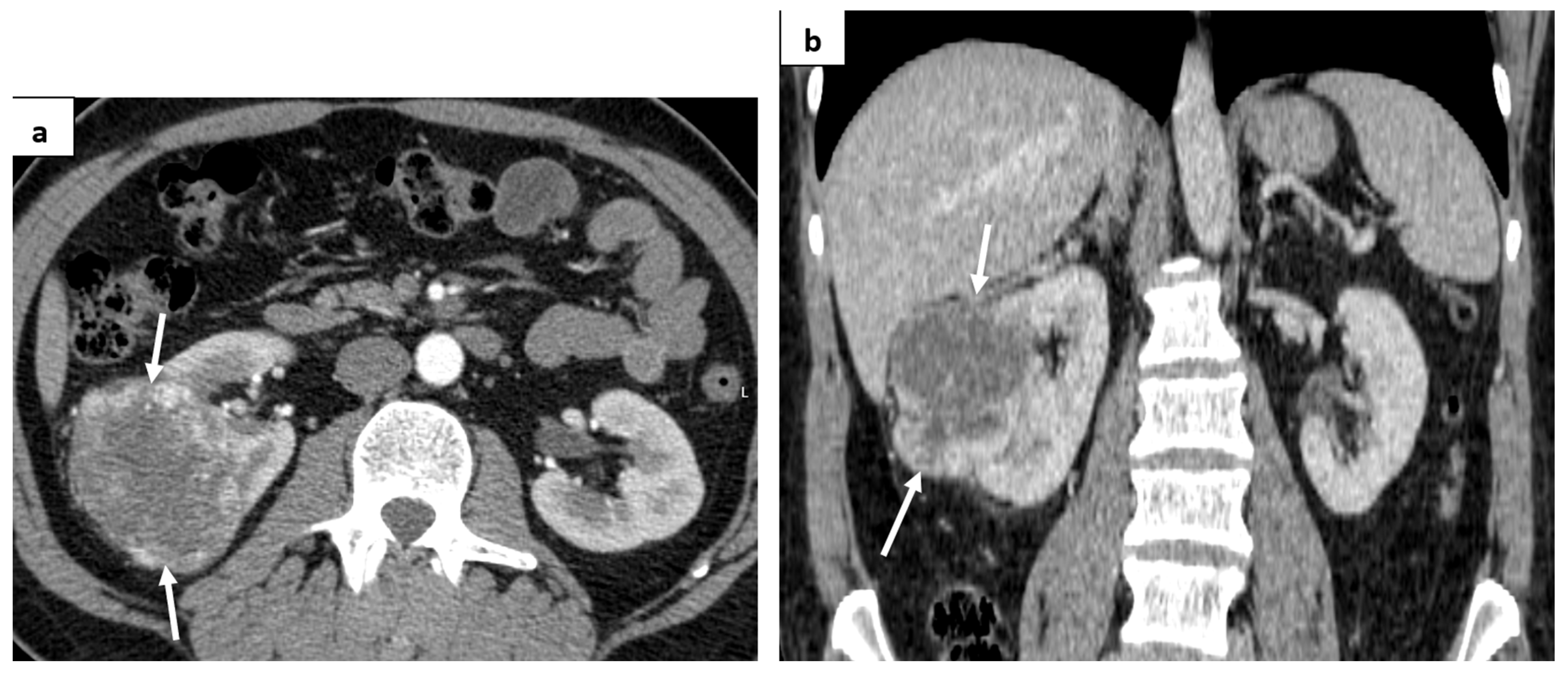
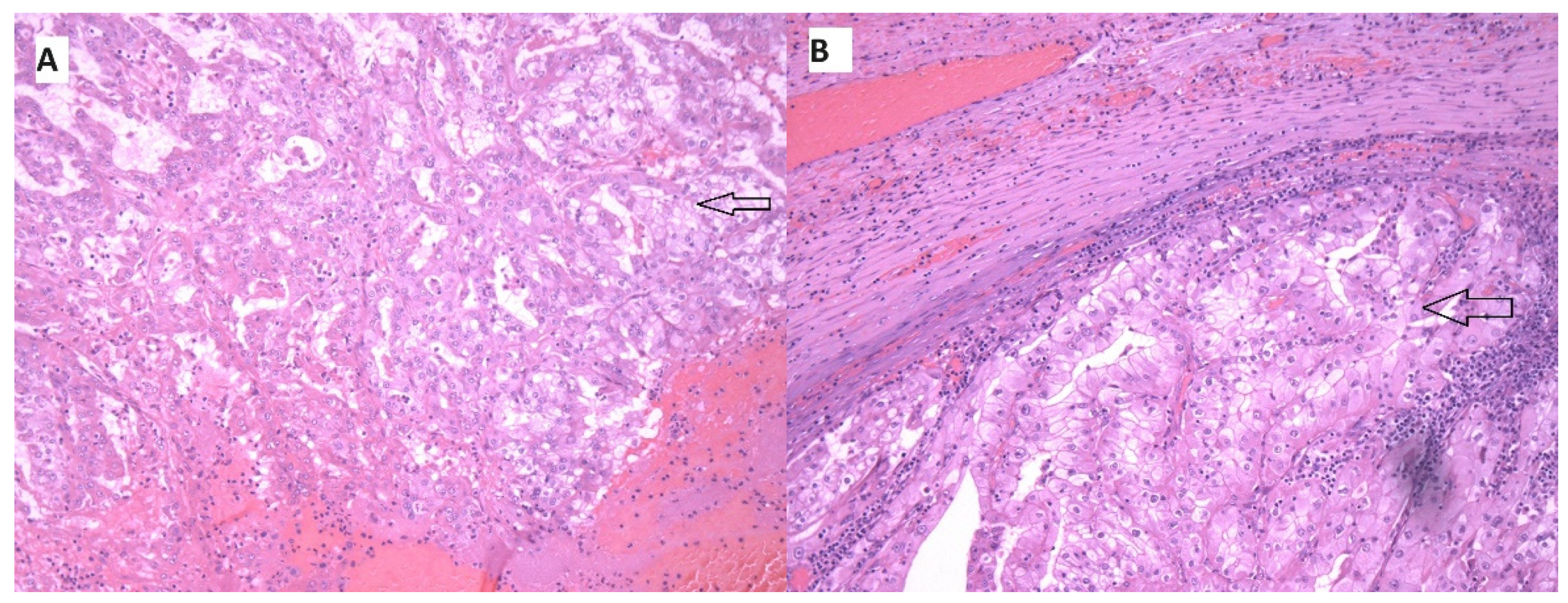
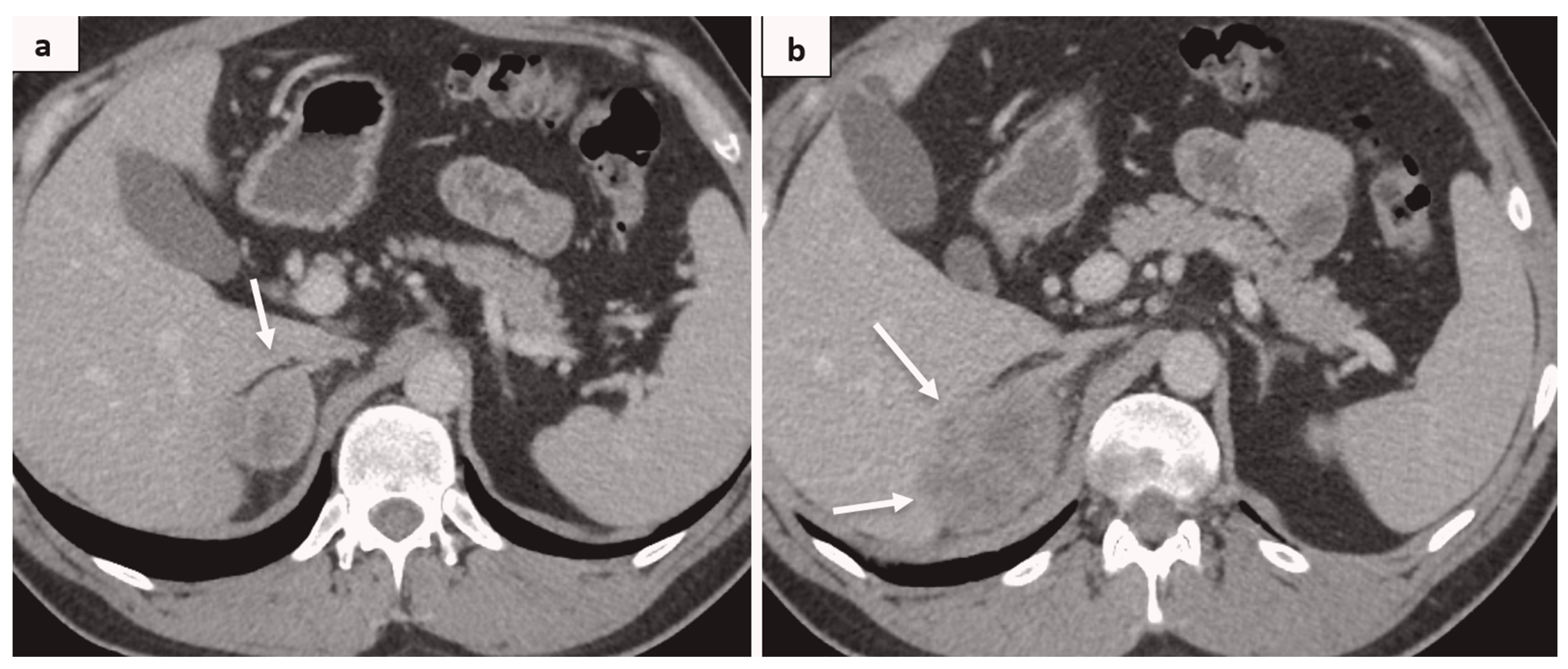
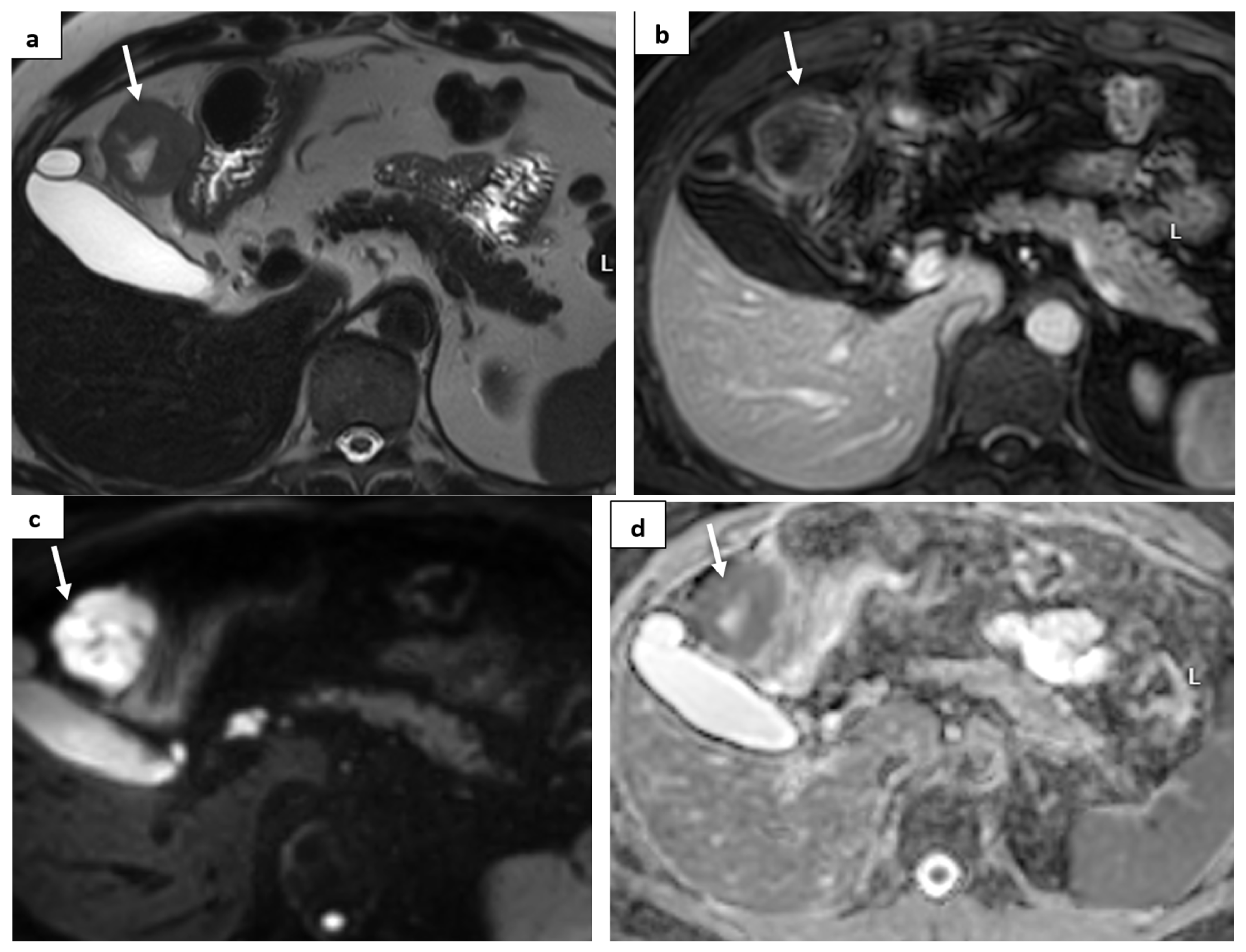
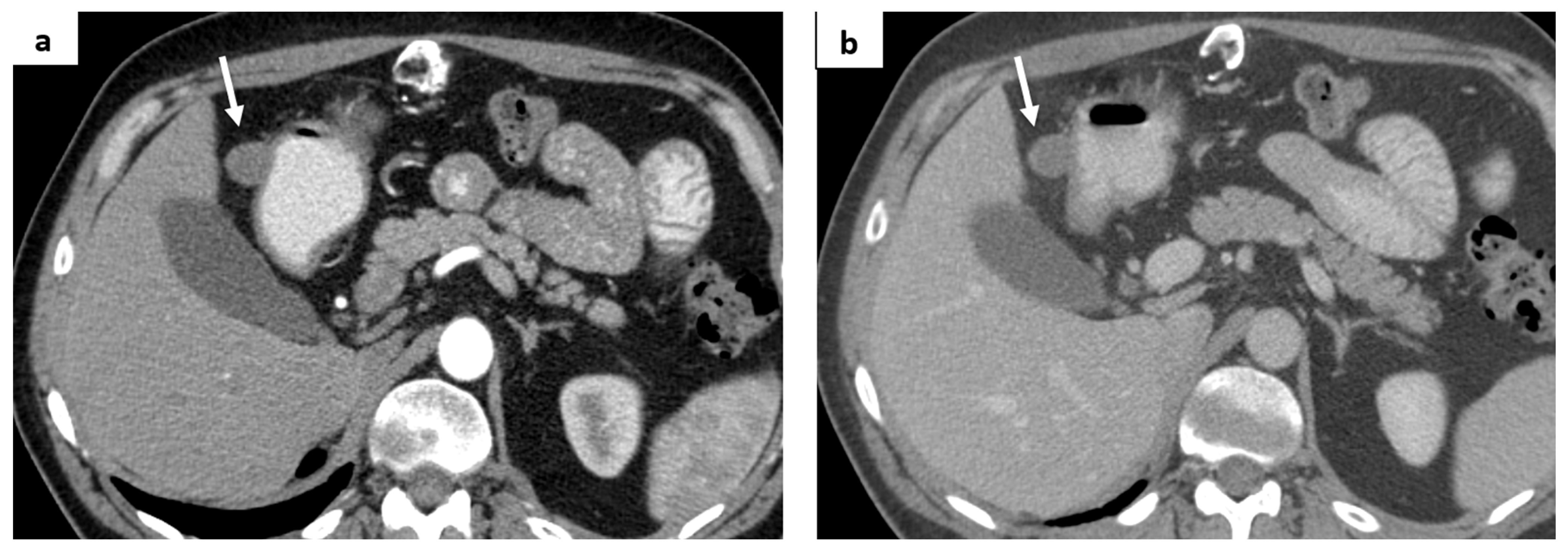
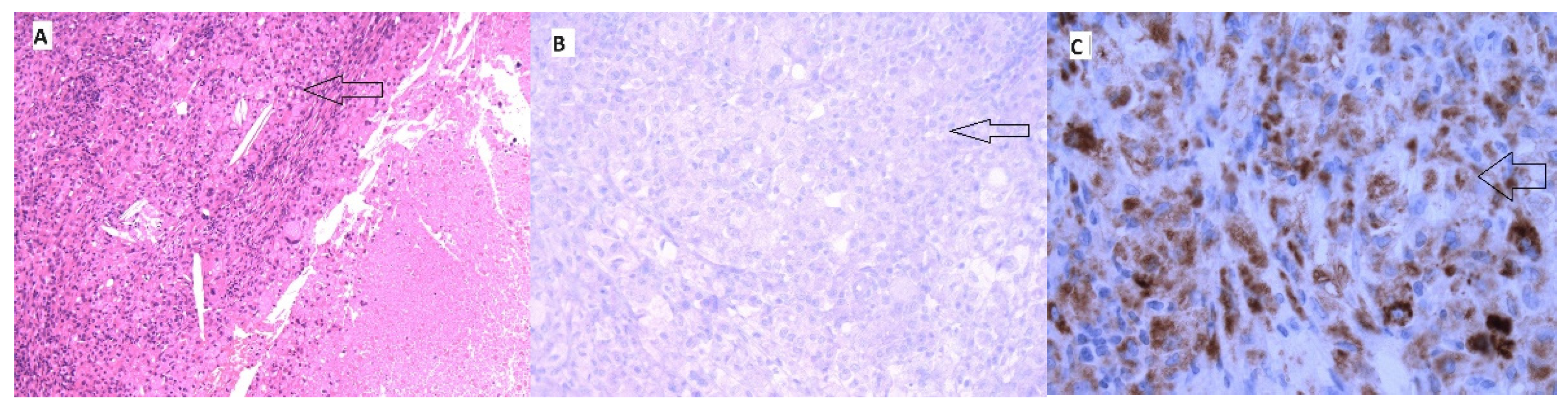
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Maher, V.E.; Fernandes, L.L.; Weinstock, C.; Tang, S.; Agarwal, S.; Brave, M.; Ning, Y.-M.; Singh, H.; Suzman, D.; Xu, J.; et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J. Clin. Oncol. 2019, 37, 2730–2737. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Lee, H.J.; Farmer, J.R.; Reynolds, K.L. Mechanisms driving immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Curr. Cardiol. Rep. 2021, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wu, L.; Han, L.; Zheng, X.; Tong, R.; Li, L.; Bai, L.; Bian, Y. Immune-related adverse events of immune checkpoint inhibitors: A review. Front. Immunol. 2023, 14, 1167975. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. Management of immunotherapy-related toxicities, version 1. J. Natl. Compr. Cancer Netw. 2019, 17, 255–289. [Google Scholar] [CrossRef]
- Kröner, P.T.; Mody, K.; Farraye, F.A. Immune checkpoint inhibitor-related luminal GI adverse events. Gastrointest. Endosc. 2019, 90, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Bellaguarda, E.; Hanauer, S. Checkpoint Inhibitor-Induced Colitis. Am. J. Gastroenterol. 2020, 115, 202. [Google Scholar] [CrossRef]
- Pizuorno Machado, A.; Shatila, M.; Liu, C.; Lu, Y.; Altan, M.; Glitza Oliva, I.C.; Zhao, D.; Zhang, H.C.; Thomas, A.; Wang, Y. Characteristics, treatment, and outcome of patients with bowel perforation after immune checkpoint inhibitor exposure. J. Cancer Res. Clin. Oncol. 2023, 149, 5989–5998. [Google Scholar] [CrossRef]
- Li, H.; Fu, Z.Y.; Arslan, M.E.; Lee, H.; Cho, D. Differential diagnosis and management of immune checkpoint inhibitor-induced colitis: A comprehensive review. World J. Exp. Med. 2021, 11, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Hashash, J.G.; Francis, F.F.; Farraye, F.A. Diagnosis and management of immune checkpoint inhibitor colitis. Gastroenterol. Hepatol. 2021, 17, 358–366. [Google Scholar]
- Wang, Y.; Abu-Sbeih, H.; Mao, E.; Ali, N.; Ali, F.S.; Qiao, W.; Lum, P.; Raju, G.; Shuttlesworth, G.; Stroehlein, J.; et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: Retrospective review at MD Anderson. J. Immunother. Cancer 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368. [Google Scholar] [CrossRef]
- Grover, S.; Dougan, M.; Tyan, K.; Giobbie-Hurder, A.; Blum, S.M.; Ishizuka, J.; Qazi, T.; Elias, R.; Vora, K.B.; Ruan, A.B.; et al. Vitamin D intake is associated with decreased risk of immune checkpoint inhibitor-induced colitis. Cancer 2020, 126, 3758. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; Van den Eertwegh, A.J.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Rahma, O.E.; Hashemi, N.; Lim, R.M. Gastrointestinal and Hepatic Toxicities of Checkpoint Inhibitors: Algorithms for Management. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 13. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Elhalawani, H.; Fouad, M. Risk of gastrointestinal complications in cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Immunotherapy 2015, 7, 1213–1227. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Vozy, A.; De Martin, E.; Johnson, D.B.; Lebrun-Vignes, B.; Moslehi, J.J.; Salem, J.E. Increased reporting of fatal hepatitis associated with immune checkpoint inhibitors. Eur. J. Cancer. 2019, 123, 112–115. [Google Scholar] [CrossRef]
- Farshidpour, M.; Hutson, W. Immune Checkpoint Inhibitors Induced Hepatotoxicity: Gastroenterologists’ Perspectives. Middle East. J. Dig. Dis. 2022, 14, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Miao, K.; Zhang, L. Incidence rate and treatment strategy of immune checkpoint inhibitor mediated hepatotoxicity: A systematic review. Cancer Pathog. Ther. 2023, 1, 46–55. [Google Scholar] [CrossRef]
- Remash, D.; Prince, D.S.; McKenzie, C.; Strasser, S.I.; Kao, S.; Liu, K. Immune checkpoint inhibitor-related hepatotoxicity: A review. World J. Gastroenterol. 2021, 27, 5376–5539. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, T.; Wu, G.Y.; Vaziri, H. Immunotherapy-induced Hepatotoxicity: A Review. J. Clin. Transl. Hepatol. 2022, 10, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Hercun, J.; Vincent, C.; Bilodeau, M.; Lapierre, P. Immune-Mediated Hepatitis During Immune Checkpoint Inhibitor cancer Immunotherapy: Lessons from Autoimmune Hepatitis and Liver Immunology. Front. Immunol. 2022, 13, 907591. [Google Scholar] [CrossRef] [PubMed]
- Seethapathy, H.; Zhao, S.; Chute, D.F.; Zubiri, L.; Oppong, Y.; Strohbehn, I.; Cortazar, F.B.; Leaf, D.E.; Mooradian, M.J.; Villani, A.-C.; et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin. J. Am. Soc. Nephrol. 2019, 14, 1692–1700. [Google Scholar] [CrossRef]
- Moturi, K.; Sharma, H.; Hashemi-Sadraei, N. Nephrotoxicity in the Age of Immune Checkpoint Inhibitors: Mechanisms, Diagnosis, and Management. Int. J. Mol. Sci. 2023, 25, 414. [Google Scholar] [CrossRef]
- Waeckerle-Men, Y.; Starke, A.; Wüthrich, R.P. PD-L1 partially protects renal tubular epithelial cells from the attack of CD8+cytotoxic T cells. Nephrol. Dial. Transplant. 2007, 22, 1527–1536. [Google Scholar] [CrossRef]
- Gupta, S.; Short, S.A.P.; Sise, M.E.; Prosek, J.M.; Madhavan, S.M.; Soler, M.J. Acute kidney injury in patients treated with immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e003467. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Kibbelaar, Z.A.; Glezerman, I.G.; Abudayyeh, A.; Mamlouk, O.; Motwani, S.S. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: A multicenter study. J. Am. Soc. Nephrol. 2020, 31, 435–446. [Google Scholar] [CrossRef]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astašauskaitė, S.; Kupčinskaitė-Noreikienė, R.; Zaborienė, I.; Vaičiūnienė, R.; Vanagas, T.; Pranys, D.; Poškienė, L.; Juozaitytė, E. Multiorgan Toxicity from Dual Checkpoint Inhibitor Therapy, Resulting in a Complete Response—A Case Report. Medicina 2024, 60, 1129. https://doi.org/10.3390/medicina60071129
Astašauskaitė S, Kupčinskaitė-Noreikienė R, Zaborienė I, Vaičiūnienė R, Vanagas T, Pranys D, Poškienė L, Juozaitytė E. Multiorgan Toxicity from Dual Checkpoint Inhibitor Therapy, Resulting in a Complete Response—A Case Report. Medicina. 2024; 60(7):1129. https://doi.org/10.3390/medicina60071129
Chicago/Turabian StyleAstašauskaitė, Skaistė, Rita Kupčinskaitė-Noreikienė, Inga Zaborienė, Rūta Vaičiūnienė, Tomas Vanagas, Darius Pranys, Lina Poškienė, and Elona Juozaitytė. 2024. "Multiorgan Toxicity from Dual Checkpoint Inhibitor Therapy, Resulting in a Complete Response—A Case Report" Medicina 60, no. 7: 1129. https://doi.org/10.3390/medicina60071129




