Clinical Nomogram Model for Pre-Operative Prediction of Microvascular Invasion of Hepatocellular Carcinoma before Hepatectomy
Abstract
:1. Introduction
2. Patients and Method
2.1. Study Design
2.2. Pre-Operative Patient Variates Profiles
2.3. Statistical Analysis
3. Results
3.1. Demographic of Clinical Profiles of D-Cohort and V-Cohort
3.2. Clinical Laboratory Data and Nutrition-Based Index for HCC Patients
3.3. Calibration Plot Model and Nomogram for Prediction Probability
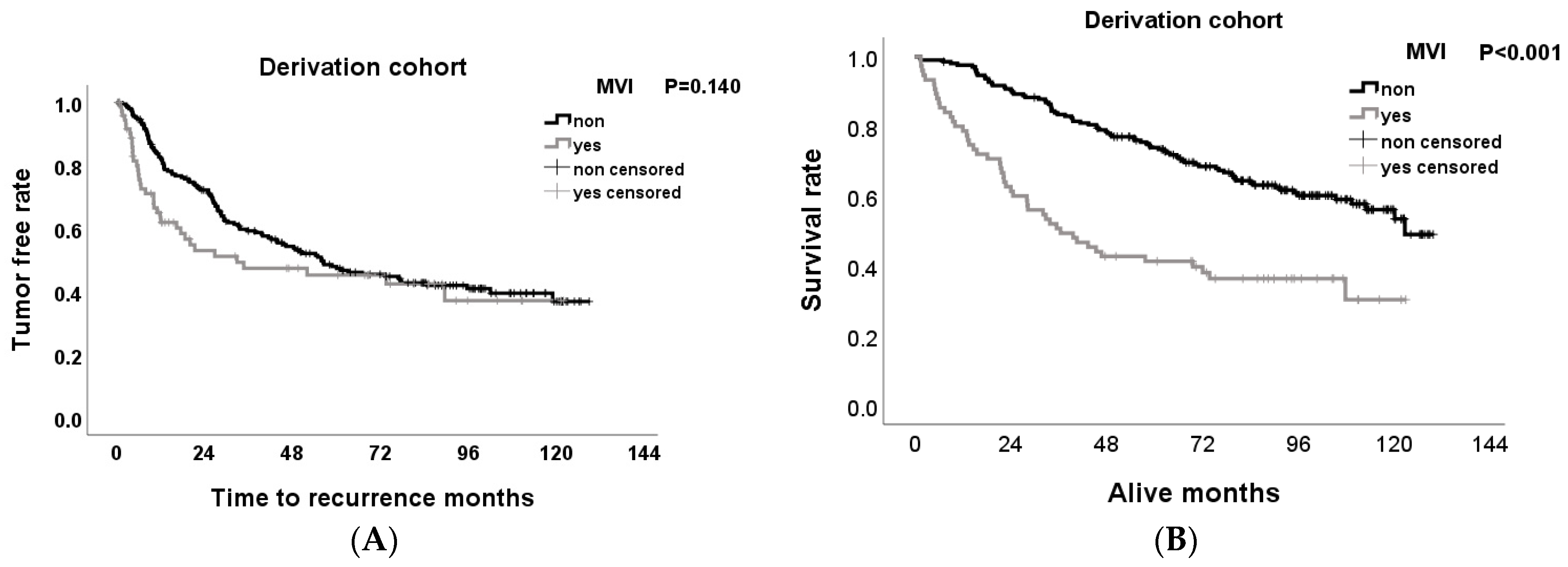
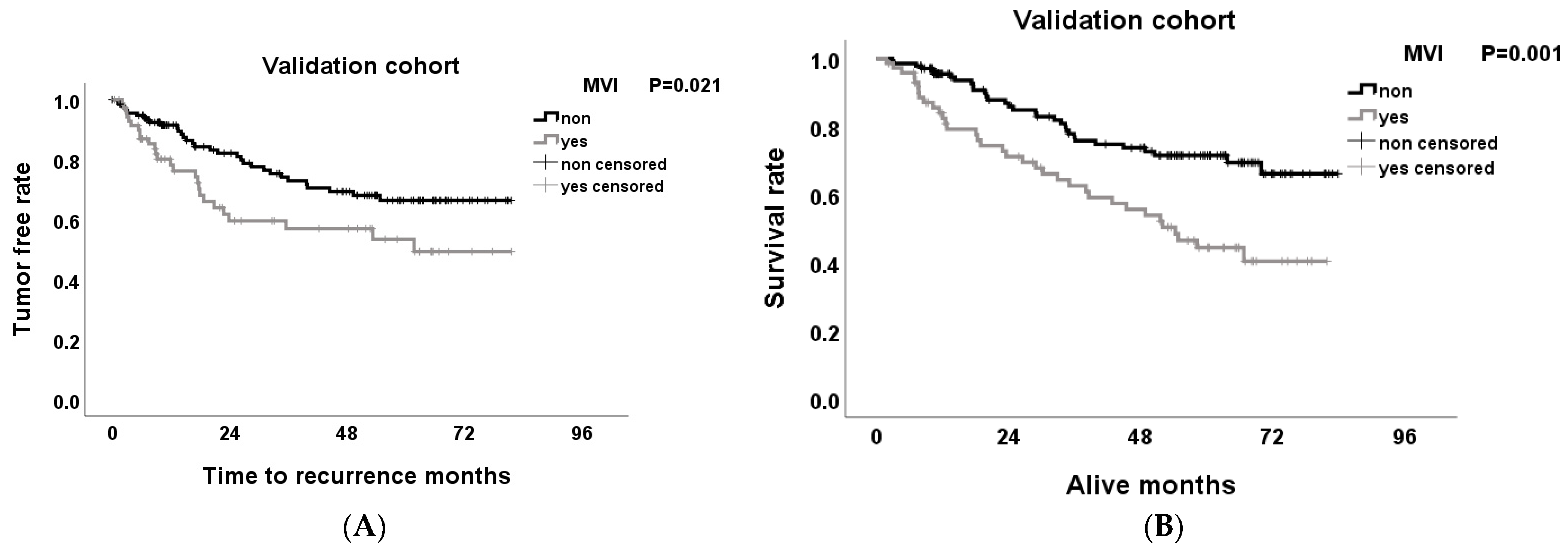
3.4. Effect of MVI on Tumor-Free Survival and Overall Survival Rates after Hepatectomy
3.5. ROC and AUC Score from Multiple Logistic Regression Model in Predicting MVI Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Health Promotion Adminstration Ministry of Health and Welfare T. Cancer Registry Annual Report, 2021, Taiwan. 2021, pp. 2–5. Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=269 (accessed on 12 March 2024).
- Tampaki, M.; Papatheodoridis, G.V.; Cholongitas, E. Intrahepatic recurrence of hepatocellular carcinoma after resection: An update. Clin. J. Gastroenterol. 2021, 14, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Song, S.K.; Kim, B.W.; Park, S.K.; Lee, J.I.; Lim, S.S.; Wang, H.J. Conditional Survival Analysis Demonstrates that Recurrence Risk of Surgically Treated Hepatocellular Carcinoma Evolves with Time. J. Gastrointest. Surg. 2017, 21, 1237–1244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Chung, C. Epidemiology of Hepatocellular Carcinoma in Taiwan. Clin. Pract. 2024, 14, 570–578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kinsey, E.; Lee, H.M. Management of Hepatocellular Carcinoma in 2024: The Multidisciplinary Paradigm in an Evolving Treatment Landscape. Cancers 2024, 16, 666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collaborators GUHD. Burden of liver cancer mortality by county, race, and ethnicity in the USA, 2000-19: A systematic analysis of health disparities. Lancet Public Health 2024, 9, e186–e198. [Google Scholar] [CrossRef]
- Du, M.; Chen, L.; Zhao, J.; Tian, F.; Zeng, H.; Tan, Y.; Sun, H.; Zhou, J.; Ji, Y. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer 2014, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Fan, P.; Chen, S.; Tu, C.; Wan, C. Distinct Recurrence Risk Factors for Intrahepatic Metastasis and Multicenter Occurrence After Surgery in Patients with Hepatocellular Carcinoma. J. Gastrointest. Surg. 2017, 21, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Erstad, D.J.; Tanabe, K.K. Scoring microvascular invasion in hepatocellular carcinoma: Are we meeting the grade? Hepatobiliary Surg. Nutr. 2024, 13, 184–187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Renzulli, M.; Brocchi, S.; Cucchetti, A.; Mazzotti, F.; Mosconi, C.; Sportoletti, C.; Brandi, G.; Pinna, A.D.; Golfieri, R. Can Current Preoperative Imaging Be Used to Detect Microvascular Invasion of Hepatocellular Carcinoma? Radiology 2016, 279, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.I.; Imai, K.; Yusa, T.; Nakao, Y.; Kitano, Y.; Nakagawa, S.; Okabe, H.; Chikamoto, A.; Ishiko, T.; Yashizumi, T.; et al. Microvascular invasion of single small hepatocellular carcinoma ≤3 cm: Predictors and optimal treatments. Ann. Gastroenterol. Surg. 2018, 2, 197–203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poté, N.; Cauchy, F.; Albuquerque, M.; Voitot, H.; Belghiti, J.; Castera, L.; Puy, H.; Bedossa, P.; Paradis, V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J. Hepatol. 2015, 62, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.C.; Shi, S.; Yan, L.J.; Wang, H.C.; Ding, Z.N.; Liu, H.; Pan, Z.Q.; Zhang, X.; Han, C.L.; Tian, B.W.; et al. A model based on adipose and muscle-related indicators evaluated by CT images for predicting microvascular invasion in HCC patients. Biomark. Res. 2023, 11, 87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nimitrungtawee, N.; Inmutto, N.; Amantakul, A.; Jantarangkoon, A. Prediction microvascular invasion of hepatocellular carcinoma based on tumour margin enhancing pattern in multiphase computed tomography images. Pol. J. Radiol. 2023, 88, e238–e243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryu, T.; Takami, Y.; Wada, Y.; Tateishi, M.; Hara, T.; Yoshitomi, M.; Monosaki, M.; Yasumori, K.; Saitsu, H.; Okuda, K. A Clinical Scoring System for Predicting Microvascular Invasion in Patients with Hepatocellular Carcinoma within the Milan Criteria. J. Gastrointest. Surg. 2019, 23, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Breen, I.; Pershad, Y.; Naidu, S.; Knuttinen, M.G.; Alzubaidi, S.; Sheth, R.; Albadawi, H.; Kuo, M.; Oklu, R. Radiogenomics and Radiomics in Liver Cancers. Diagnostics 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Guo, T.; Zhu, B.; Liu, Y. A novel nomogram for predicting microvascular invasion in hepatocellular carcinoma. Ann. Hepatol. 2023, 28, 101136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zuo, X.; Zhang, Y.; Han, G.; Zhang, L.; Ding, W.; Wu, J.; Wang, X. Derivation and validation of machine learning models for preoperative estimation of microvascular invasion risk in hepatocellular carcinoma. Ann. Transl. Med. 2023, 11, 249–262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, W.; Zhang, L.; Xin, Z.; Zhang, H.; You, L.; Bai, L.; Zhou, J.; Ying, H. A Promising Preoperative Prediction Model for Microvascular Invasion in Hepatocellular Carcinoma Based on an Extreme Gradient Boosting Algorithm. Front. Oncol. 2022, 12, 852736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Otto, G.; Heuschen, U.; Hofmann, W.J.; Krumm, G.; Hinz, U.; Herfarth, C. Survival and recurrence after liver transplantation versus liver resection for hepatocellular carcinoma: A retrospective analysis. Ann. Surg. 1998, 227, 424–432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poon, T.P.R.; Fan, S.T.; Wong, J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann. Surg. 2000, 232, 10–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrero, A.; Toubert, C.; Bedoya, J.U.; Assenat, E.; Guiu, B.; Panaro, F.; Bardol, F.; Cassese, G. Management of hepatocellular carcinoma recurrence after liver surgery and thermal ablations: State of the art and future perspectives. Hepatobiliary Surg. Nutr. 2024, 13, 71–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iwatsuki, S.; Dvorchik, I.; Marsh, J.W.; Madariaga, J.R.; Carr, B.; Fung, J.J.; Starzl, T.E. Liver transplantation for hepatocellular carcinoma: A proposal of a prognostic scoring system. J. Am. Coll. Surg. 2000, 191, 389–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Li, T.; Hu, J.; Liu, J. A nomogram to predict microvascular invasion in early hepatocellular carcinoma. J. Cancer Res. Ther. 2021, 17, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.J.; Yin, S.Y.; Sheng, X.; Wang, H.X.; Lau, W.Y.; Dong, H.; Cong, W.M. A Grading System of Microvascular Invasion for Patients with Hepatocellular Carcinoma Undergoing Liver Resection with Curative Intent: A Multicenter Study. J. Hepatocell. Carcinoma 2024, 11, 191–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Y.Z.; He, K.; Huang, R.Q.; Wang, Z.L.; Ye, S.W.; Liu, L.W.; Lou, Q.J.; Hu, Z.M. Preoperative evaluation and prediction of clinical scores for hepatocellular carcinoma microvascular invasion: A single-center retrospective analysis. Ann. Hepatol. 2020, 19, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Arnaoutakis, D.J.; Mavros, M.N.; Shen, F.; Alexandrescu, S.; Firoozmand, A.; Popescu, I.; Weiss, M.; Wolfgang, C.; Choti, M.A.; Pawlik, T.M. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: A multi-institutional analysis. Ann. Surg. Oncol. 2014, 21, 147–154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez-Perálvarez, M.; Luong, T.V.; Andreana, L.; Meyer, T.; Dhillon, A.P.; Burroughs, A.K. A systematic review of microvascular invasion in hepatocellular carcinoma: Diagnostic and prognostic variability. Ann. Surg. Oncol. 2013, 20, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.L.; Cheng, Q.; Huang, Z.Y.; Dong, W. Revisiting Surgical Strategies for Hepatocellular Carcinoma With Microvascular Invasion. Front. Oncol. 2021, 11, 691354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanemura, A.; Mizuno, S.; Hayasaki, A.; Gyoten, K.; Fujii, T.; Iizawa, Y.; Kato, H.; Murata, Y.; Kuriyama, N.; Kishiwada, M.; et al. Onodera’s prognostic nutritional index is a strong prognostic indicator for patients with hepatocellular carcinoma after initial hepatectomy, especially patients with preserved liver function. BMC Surg. 2020, 20, 261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, S.; Tian, M.; Hu, K.; Chen, R.; Zhang, B.; Ren, Z.; Shi, Y.; Yin, X. Albumin-to-Alkaline Phosphatase Ratio is an Independent Prognostic Indicator in Combined Hepatocellular and Cholangiocarcinoma. J. Cancer 2020, 11, 5177–5186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, P.J.; Dhanaraj, S.; Berhane, S.; Bonnett, L.; Ma, Y.T. The prognostic and diagnostic significance of the neutrophil-to-lymphocyte ratio in hepatocellular carcinoma: A prospective controlled study. Br. J. Cancer 2021, 125, 714–716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanno, H.; Goto, Y.; Sasaki, S.; Fukutomi, S.; Hisaka, T.; Fujita, F.; Akagi, Y.; Okuda, K. Geriatric nutritional risk index predicts prognosis in hepatocellular carcinoma after hepatectomy: A propensity score matching analysis. Sci. Rep. 2021, 11, 9038–9044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Dennis, C.; Prince, D.S.; Marsh-Wakefield, F.; Santhakumar, C.; Gamble, J.R.; Strasser, S.I.; McCaughan, G.W. Vessels that encapsulate tumour clusters vascular pattern in hepatocellular carcinoma. JHEP Rep. Innov. Hepatol. 2023, 5, 100792. [Google Scholar] [CrossRef] [PubMed]
- Isik, B.; Gonultas, F.; Sahin, T.; Yilmaz, S. Microvascular Venous Invasion in Hepatocellular Carcinoma: Why Do Recurrences Occur? J. Gastrointest. Cancer 2020, 51, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Li, J.; Wu, D.; Xia, Y.; Wang, Q.; Si, A.; Wang, K.; Wan, X.; Lau, W.Y.; Wu, M.; et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma within the Milan Criteria. JAMA Surg. 2016, 151, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, Y.; Zhu, C.; Hu, X.; Zou, H.; Sun, C. New insights into a microvascular invasion prediction model in hepatocellular carcinoma: A retrospective study from the SEER database and China. Front. Surg. 2023, 9, 1046713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheng, H.; Mao, M.; Huang, K.; Zheng, H.; Liu, W.; Liang, Y. A Clinical Tool to Predict the Microvascular Invasion Risk in Patients with Hepatocellular Carcinoma. Technol. Cancer Res. Treat. 2023, 22, 15330338231182526. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wei, X.; Wang, N.; Pu, X.; Yang, J.; Jiang, L. A new method for predicting the microvascular invasion status of hepatocellular carcinoma through neural network analysis. BMC Surg. 2023, 23, 100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McHugh, P.P.; Gilbert, J.; Vera, S.; Koch, A.; Ranjan, D.; Gedaly, R. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB 2010, 12, 56–61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.C.; Hung, H.C.; Wang, Y.C.; Cheng, C.H.; Wu, T.H.; Lee, C.F.; Wu, T.H.; Lee, C.F.; Chou, H.S.; Chan, K.M.; et al. Risk Score Model for Microvascular Invasion in Hepatocellular Carcinoma: The Role of Tumor Burden and Alpha-Fetoprotein. Cancers 2021, 13, 4403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, B.; Ji, W.D.; Wang, W.C.; Chen, L.; Ma, J.Y.; Tang, E.J.; Lin, M.B.; Zhang, X.F. Circulating tumor cells participate in the formation of microvascular invasion and impact on clinical outcomes in hepatocellular carcinoma. Front. Genet. 2023, 14, 1265866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deng, Y.; Yang, D.; Tan, X.; Xu, H.; Xu, L.; Ren, A.; Liu, P.; Yang, Z. Preoperative evaluation of microvascular invasion in hepatocellular carcinoma with a radiological feature-based nomogram: A bi-centre study. BMC Med. Imaging 2024, 24, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lei, Y.; Feng, B.; Wan, M.; Xu, K.; Cui, J.; Ma, C.; Sun, J.; Yao, C.; Gan, S.; Shi, J.; et al. Predicting microvascular invasion in hepatocellular carcinoma with a CT- and MRI-based multimodal deep learning model. Abdom. Radiol. 2024, 49, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Z.; Zhou, H.; Leng, C.; Hou, B.; Zhou, C.; Hu, X.; Wang, J.; Chen, X. Preoperative circulating tumor cells to predict microvascular invasion and dynamical detection indicate the prognosis of hepatocellular carcinoma. BMC Cancer 2020, 20, 1047. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, Z.W.; Chen, K.; Liu, Y.Z.; Li, N.; Hu, M.G. A predictive and prognostic model for hepatocellular carcinoma with microvascular invasion based TCGA database genomics. BMC Cancer 2021, 21, 1337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Wang, K.; Wei, X.; Li, L.Q.; Sun, H.; Wen, T.; Chai, Z.T.; Chen, Z.H.; Shi, J.; Guo, W.X.; et al. An Eastern Hepatobiliary Surgery Hospital Microvascular Invasion Scoring System in Predicting Prognosis of Patients with Hepatocellular Carcinoma and Microvascular Invasion After R0 Liver Resection: A Large-Scale, Multicenter Study. Oncologist 2019, 24, e1476–e1488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Nakashima, Y.; Nakashima, O.; Tanaka, M.; Okuda, K.; Nakashima, M.; Kojiro, M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol. Res. 2003, 26, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, X.H.; Hu, W.J.; Chen, D.H.; Hu, Z.L.; Li, S.Q. Patterns, Risk Factors, and Outcomes of Recurrence After Hepatectomy for Hepatocellular Carcinoma with and without Microvascular Invasion. J. Hepatocell. Carcinoma 2024, 11, 801–812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, W.D.; Ye, L.N.; Song, Z.S.; Wang, K.P.; Feng, Y.F.; Pan, C.Y. Wide surgical margins improve prognosis for HCC with microvascular invasion. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Govalan, R.; Lauzon, M.; Luu, M.; Ahn, J.C.; Kosari, K.; Todo, T.; Kim, I.K.; Noureddin, M.; Kuo, A.; Walid, A.S.; et al. Comparison of Surgical Resection and Systemic Treatment for Hepatocellular Carcinoma with Vascular Invasion: National Cancer Database Analysis. Liver Cancer 2021, 10, 407–418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Zhuang, G.; Bai, S.; Hu, Z.; Xia, Y.; Lu, C.; Wang, J.; Wang, C.; Liu, L.; Li, F.; et al. The Comparison of Surgical Margins and Type of Hepatic Resection for Hepatocellular Carcinoma With Microvascular Invasion. Oncologist 2023, 28, e1043–e1051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, J.; Liang, H.; Hu, K.; Xiong, Z.; Cao, M.; Zhong, Z.; Yao, Z.; Deng, M. The effects of several postoperative adjuvant therapies for hepatocellular carcinoma patients with microvascular invasion after curative resection: A systematic review and meta-analysis. Cancer Cell Int. 2021, 21, 92–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, W.; Guo, Y.; Zhong, J.; Wang, Q.; Wang, X.; Wei, H.; Li, J.; Xiu, P. The clinical significance of microvascular invasion in the surgical planning and postoperative sequential treatment in hepatocellular carcinoma. Sci. Rep. 2021, 11, 2415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Variables | Total n = 489 n (%) | D-Cohort n = 281 | V-Cohort n = 208 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MVI(−) n = 206 | MVI(+) n = 75 | p | MVI(−) n = 136 | MVI(+) n = 72 | p | ||||||
| n | % | n | % | n | % | n | % | ||||
| Age | 60.8 ± 10.7 | 60.4 ± 10.9 | 60.8 ± 10.9 | 0.788 | 61.3 ± 9.6 | 60.8 ± 12.3 | 0.786 | ||||
| <65 | 307 (62.8) | 135 | 73.4 | 49 | 26.6 | 0.975 | 80 | 65.0 | 43 | 35.0 | 0.900 |
| ≥65 | 182 (37.2) | 71 | 73.2 | 26 | 26.8 | 56 | 65.9 | 29 | 34.1 | ||
| Sex | |||||||||||
| Male | 378 (77.3) | 152 | 70.7 | 63 | 29.3 | 0.074 | 109 | 66.9 | 54 | 33.1 | 0.391 |
| Female | 111 (22.7) | 54 | 81.8 | 12 | 18.2 | 27 | 60.0 | 18 | 40.0 | ||
| BMI | 24.9 ± 3.8 | 25.1 ± 4.0 | 24.7 ± 3.4 | 0.522 | 25.1 ± 3.7 | 24.2 ± 3.8 | 0.077 | ||||
| <24 | 200 (41.5) | 88 | 73.3 | 32 | 26.7 | 0.968 | 50 | 62.5 | 30 | 37.5 | 0.515 |
| ≥24 | 282 (58.5) | 114 | 73.5 | 41 | 26.5 | 85 | 66.9 | 42 | 33.1 | ||
| DM | |||||||||||
| yes | 126 (25.8) | 52 | 74.3 | 18 | 25.7 | 0.831 | 37 | 66.1 | 19 | 33.9 | 0.899 |
| non | 363 (74.2) | 154 | 73.0 | 57 | 27.0 | 99 | 65.1 | 53 | 34.9 | ||
| HbA1c (%) | 7.1 ± 1.6 | 7.6 ± 2.0 | 6.4 ± 1.5 | 0.048 | 7.1 ± 1.4 | 6.9 ± 1.3 | 0.581 | ||||
| <6.5 | 39 (41.1) | 9 | 52.9 | 8 | 47.1 | 0.085 | 15 | 68.2 | 7 | 31.8 | 0.952 |
| ≥6.5 | 56 (58.9) | 21 | 77.8 | 6 | 22.2 | 20 | 69.0 | 9 | 31.0 | ||
| Alcohol | |||||||||||
| non | 317 (66.3) | 129 | 75.0 | 43 | 25.0 | 0.471 | 98 | 67.6 | 47 | 32.4 | 0.339 |
| 1 + 2 | 161 (33.7) | 71 | 71.0 | 29 | 29.0 | 37 | 60.7 | 24 | 39.3 | ||
| ECOG | |||||||||||
| 0 + 1 | 482 (99.2) | 203 | 73.8 | 72 | 26.2 | 1.000 | 136 | 65.7 | 71 | 34.3 | 0.168 |
| 2 + 3 + 4 | 4 (0.8) | 2 | 66.7 | 1 | 33.3 | 0 | 0 | 1 | 100 | ||
| BCLC | |||||||||||
| 0 + 1 | 281 (57.5) | 133 | 81.6 | 30 | 18.4 | <0.001 | 90 | 76.3 | 28 | 23.7 | <0.001 |
| 2 + 3 | 208 (42.5) | 73 | 61.9 | 45 | 38.1 | 46 | 51.1 | 44 | 48.9 | ||
| Child–Pugh | |||||||||||
| A | 471 (98.3) | 194 | 72.7 | 73 | 27.3 | 0.668 | 134 | 65.7 | 70 | 34.3 | 1.000 |
| B | 8 (1.7) | 4 | 66.7 | 2 | 33.3 | 1 | 50.0 | 1 | 50.0 | ||
| Hepatitis | |||||||||||
| non | 93 (19.0) | 38 | 71.7 | 15 | 28.3 | 0.768 | 30 | 75.0 | 10 | 25.0 | 0.155 |
| B/C | 396 (81.0) | 168 | 73.7 | 60 | 26.3 | 106 | 63.1 | 62 | 36.9 | ||
| T location * | |||||||||||
| seg. 1 | 7 (1.4) | 4 | 100 | 0 | 0 | 0.462 | 3 | 100 | 0 | 0 | 0.323 |
| left (seg. 2–3) | 94 (19.3) | 40 | 69.0 | 18 | 31.0 | 20 | 55.6 | 16 | 44.4 | ||
| right (seg. 4–8) | 380 (77.9) | 159 | 74.3 | 55 | 25.7 | 111 | 66.9 | 55 | 33.1 | ||
| seg. 2 + 3 | 7 (1.4) | 3 | 60.0 | 2 | 40.0 | 1 | 50.0 | 1 | 50.0 | ||
| T extension | |||||||||||
| non | 451 (92.2) | 194 | 76.1 | 61 | 23.9 | 0.001 | 132 | 67.3 | 64 | 32.7 | 0.026 |
| yes | 38 (7.8) | 12 | 46.2 | 14 | 53.8 | 4 | 33.3 | 8 | 66.7 | ||
| T size (mm) | 52.5 ± 36.4 | 45.5 ± 29.5 | 70.1 ± 38.7 | <0.001 | 45.8 ± 32.9 | 67.2 ± 46.8 | 0.001 | ||||
| <50 mm | 292 (59.7) | 139 | 83.7 | 27 | 16.3 | <0.001 | 92 | 73.0 | 34 | 27.0 | 0.004 |
| ≥50 mm | 197 (40.3) | 67 | 58.3 | 48 | 41.7 | 44 | 53.7 | 38 | 46.3 | ||
| T number | 1.06 ± 0.41 | 1.06 ± 0.38 | 1.21 ± 0.60 | 0.039 | 1.00 ± 0.33 | 1.03 ± 0.34 | 0.471 | ||||
| 1 | 421 (90.1) | 179 | 76.8 | 54 | 23.2 | 0.002 | 124 | 66.0 | 64 | 34.0 | 0.322 |
| ≥2 | 46 (9.9) | 19 | 52.8 | 17 | 47.2 | 5 | 50.0 | 5 | 50.0 | ||
| Satellite nodule | |||||||||||
| no | 415 (84.9) | 189 | 81.8 | 42 | 18.2 | <0.001 | 130 | 70.7 | 54 | 29.3 | <0.001 |
| yes | 74 (15.1) | 17 | 34.0 | 33 | 66.0 | 6 | 25.0 | 18 | 75.0 | ||
| Pre-op Tx ** | |||||||||||
| no | 434 (88.8) | 184 | 74.8 | 62 | 25.2 | 0.135 | 124 | 66.0 | 64 | 34.0 | 0.594 |
| yes | 55 (11.2) | 22 | 62.9 | 13 | 37.1 | 12 | 60.0 | 8 | 40.0 | ||
| Variables | Total n = 489 n (%) | D-Cohort n = 281 | V-Cohort n = 208 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Micro(−) n = 206 | Micro(+) n = 75 | p | Micro(−) n = 136 | Micro(+) n = 72 | p | ||||||
| n | % | n | % | n | % | n | % | ||||
| INR | 1.24 ± 1.82 | 1.12 ± 0.83 | 1.54 ± 3.38 | 0.283 | 1.20 ± 1.33 | 1.34 ± 2.31 | 0.598 | ||||
| <1.24 | 467 (95.5) | 198 | 73.3 | 72 | 26.7 | 0.999 | 131 | 66.5 | 66 | 35.5 | 0.195 |
| ≥1.24 | 22 (4.5) | 8 | 72.7 | 3 | 27.3 | 5 | 45.5 | 6 | 54.5 | ||
| ICG (%) | 12.48 ± 9.23 | 12.78 ± 8.86 | 11.88 ± 6.48 | 0.422 | 12.54 ± 9.00 | 12.16 ± 12.59 | 0.802 | ||||
| <20 | 416 (87.0) | 174 | 72.5 | 66 | 27.5 | 0.505 | 111 | 63.1 | 65 | 36.9 | 0.320 |
| ≥20 | 62 (13.0) | 28 | 77.8 | 8 | 22.2 | 19 | 73.1 | 7 | 26.9 | ||
| AFP (×102 ng/mL) | 89.95 ± 446.61 | 31.66 ± 220.06 | 166.19 ± 449.3 | 0.017 | 44.36 ± 358.09 | 256.53 ± 841.3 | 0.045 | ||||
| <200 IU | 355 (71.0) | 150 | 79.8 | 38 | 20.2 | <0.001 | 103 | 70.1 | 44 | 29.9 | 0.004 |
| ≥200IU | 137 (29.0) | 49 | 59.0 | 34 | 41.0 | 26 | 48.1 | 28 | 51.9 | ||
| WBC (×103/µL) | 6.58 ± 2.51 | 6.40 ± 2.67 | 6.87 ± 2.56 | 0.184 | 6.51 ± 2.15 | 6.96 ± 2.58 | 0.182 | ||||
| <5.93 | 213 (44.1) | 102 | 78.5 | 28 | 21.5 | 0.087 | 61 | 73.5 | 22 | 26.5 | 0.048 |
| ≥5.93 | 270 (55.9) | 102 | 69.4 | 45 | 30.6 | 74 | 60.2 | 49 | 39.8 | ||
| Neutro (×103/µL) | 4.59 ± 2.63 | 4.20 ± 2.71 | 4.94 ± 2.51 | 0.160 | 4.77 ± 2.36 | 4.99 ± 2.93 | 0.714 | ||||
| <7.65 | 176 (88.4) | 80 | 73.4 | 29 | 26.6 | 0.007 | 45 | 67.2 | 22 | 32.8 | 0.186 |
| ≥7.65 | 23 (11.6) | 4 | 33.3 | 8 | 66.7 | 5 | 45.5 | 6 | 54.5 | ||
| Lympho (×103/µL) | 1.71 ± 0.96 | 1.75 ± 1.12 | 1.75 ± 0.96 | 0.980 | 1.64 ± 0.69 | 1.65 ± 0.88 | 0.949 | ||||
| <2.38 | 164 (83.2) | 71 | 71.0 | 29 | 29.0 | 0.331 | 44 | 68.8 | 20 | 31.3 | 0.200 |
| ≥2.38 | 33 (16.8) | 12 | 60.0 | 8 | 40.0 | 6 | 46.2 | 7 | 53.8 | ||
| Platelet (×103/µL) | 194.02 ± 77.5 | 180.33 ± 67.4 | 205.93 ± 71.2 | 0.006 | 191.26 ± 66.9 | 226.42 ± 112.4 | 0.017 | ||||
| <178.50 | 218 (45.0) | 109 | 82.6 | 23 | 17.4 | 0.001 | 64 | 74.4 | 22 | 25.6 | 0.026 |
| ≥178.50 | 266 (55.0) | 95 | 65.5 | 50 | 34.5 | 72 | 59.5 | 49 | 40.5 | ||
| Bil, (mg/dL) | 0.92 ± 0.37 | 0.95 ± 0.37 | 0.91 ± 0.39 | 0.437 | 0.87 ± 0.37 | 0.93 ± 0.35 | 0.275 | ||||
| <1.2 | 379 (79.0) | 153 | 70.2 | 65 | 29.8 | 0.019 | 108 | 67.1 | 53 | 32.9 | 0.247 |
| ≥1.2 | 101 (21.0) | 48 | 85.7 | 8 | 14.3 | 26 | 57.8 | 19 | 42.2 | ||
| GOT/GPT (µL) | 1.35 ± 1.34 | 1.20 ± 0.76 | 1.51 ± 1.20 | 0.040 | 1.35 ± 1.81 | 1.60 ± 1.65 | 0.342 | ||||
| <1.35 | 338 (70.1) | 152 | 78.8 | 41 | 21.2 | 0.002 | 102 | 70.3 | 43 | 29.7 | 0.023 |
| ≥1.35 | 144 (29.9) | 49 | 60.5 | 32 | 39.5 | 34 | 54.0 | 29 | 46.0 | ||
| Alb. (g/dL) | 4.14 ± 0.36 | 4.15 ± 0.40 | 4.12 ± 0.36 | 0.516 | 4.17 ± 0.29 | 4.09 ± 0.37 | 0.163 | ||||
| A < 4.15 | 213 (45.1) | 88 | 71.0 | 36 | 29.0 | 0.201 | 53 | 56.9 | 36 | 40.4 | 0.136 |
| ≥4.15 | 259 (54.9) | 112 | 77.8 | 32 | 22.2 | 80 | 69.6 | 35 | 30.4 | ||
| Alk-P (µL) | 338.68 ± 277.1 | 321.03 ± 201.4 | 486.35 ± 605.5 | 0.069 | 305.85 ± 119.0 | 317.35 ± 142.7 | 0.594 | ||||
| A < 384.50 | 289 (42.6) | 120 | 81.1 | 28 | 18.9 | 0.003 | 80 | 66.1 | 41 | 33.9 | 0.364 |
| ≥384.50 | 84 (57.4) | 31 | 60.8 | 20 | 39.2 | 19 | 57.6 | 14 | 42.4 | ||
| PNI | 41.42 ± 3.59 | 41.52 ± 3.96 | 41.17 ± 3.58 | 0.517 | 41.66 ± 2.88 | 40.95 ± 3.70 | 0.162 | ||||
| <41.00 | 165 (35.0) | 71 | 71.7 | 28 | 28.3 | 0.402 | 38 | 57.6 | 28 | 42.4 | 0.114 |
| ≥41.00 | 307 (65.0) | 129 | 76.3 | 40 | 23.7 | 95 | 68.8 | 43 | 31.2 | ||
| AAR | 0.16 ± 0.70 | 0.17 ± 0.08 | 0.13 ± 0.07 | 0.014 | 0.16 ± 0.06 | 0.15 ± 0.06 | 0.819 | ||||
| <0.09 | 53 (15.3) | 20 | 60.6 | 13 | 39.4 | 0.015 | 10 | 50.0 | 10 | 50.0 | 0.147 |
| ≥0.09 | 294 (84.7) | 130 | 80.2 | 32 | 19.8 | 88 | 66.7 | 44 | 33.3 | ||
| ALBI | −2.67 ± 0.62 | −2.70 ± 0.54 | −2.49 ± 0.95 | 0.087 | −2.74 ± 0.52 | −2.66 ± 0.54 | 0.310 | ||||
| <−2.86 | 181 (37.7) | 81 | 77.9 | 23 | 22.1 | 0.185 | 56 | 72.7 | 21 | 27.3 | 0.074 |
| ≥−2.86 | 299 (62.3) | 120 | 70.6 | 50 | 29.4 | 78 | 60.5 | 51 | 39.5 | ||
| GNRI | 106.8 ± 14.2 | 107.90 ± 12.8 | 101.88 ± 21.6 | 0.026 | 108.33 ± 11.1 | 105.93 ± 12.5 | 0.157 | ||||
| <98.87 | 77 (15.9) | 33 | 63.5 | 19 | 36.5 | 0.076 | 7 | 28.0 | 18 | 72.0 | <0.001 |
| ≥98.87 | 407 (84.1) | 170 | 75.6 | 55 | 24.4 | 128 | 70.3 | 54 | 29.7 | ||
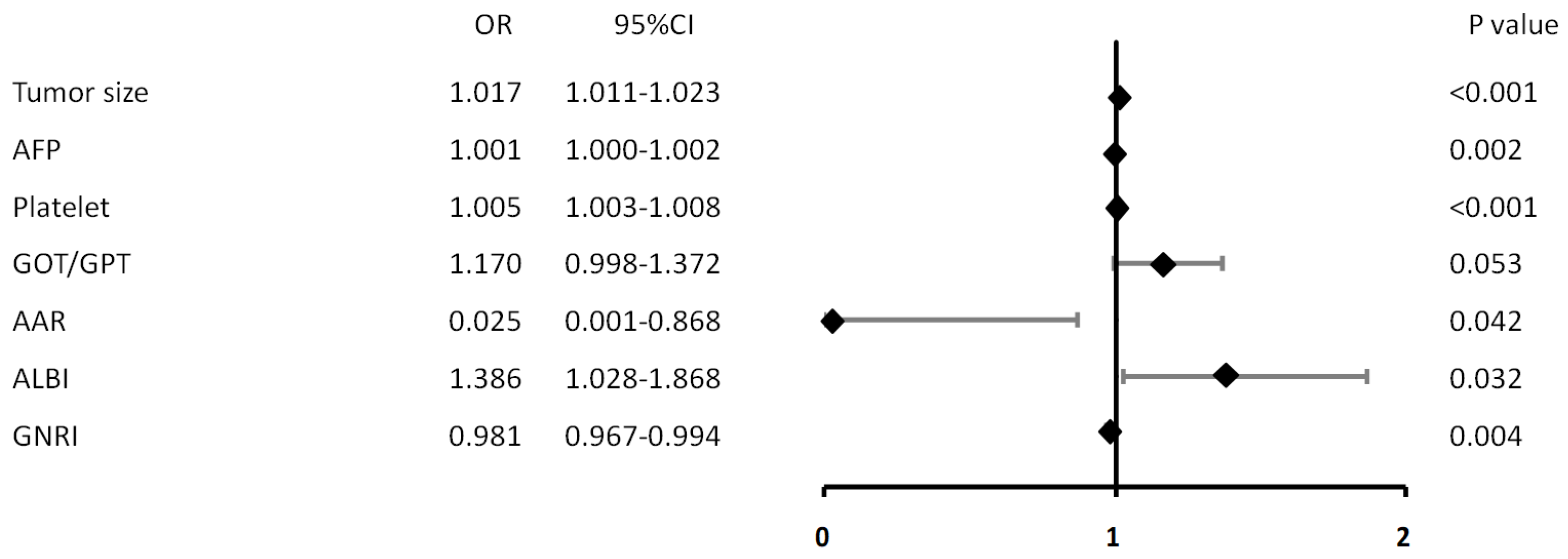
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| BCLC | ||||
| 0 + 1(ref.) | 1 | 1 | ||
| 2 + 3 | 2.876 (1.930, 4.284) | <0.001 | 1.119 (0.510, 2.458) | 0.779 |
| Tumor extension | ||||
| non (ref.) | 1 | 1 | ||
| yes | 3.586 (1.824, 7.051) | <0.001 | 1.771 (0.575, 5.453) | 0.319 |
| Tumor size (mm) | 1.017 (1.011, 1.023) | <0.001 | 1.006 (0.995, 1.017) | 0.306 |
| Tumor number | 1.695 (1.062, 2.705) | 0.027 | 1.236 (0.616, 2.482) | 0.551 |
| Satellite nodule | ||||
| non (ref.) | 1 | 1 | ||
| yes | 7.368 (4.283, 12.677) | <0.001 | 5.660 (2.553, 12.547) | <0.001 |
| AFP (×102 ng/mL) | 1.001 (1.000, 1.002) | 0.002 | 1.000 (1.000, 1.001) | 0.200 |
| Platelet (×103/µL) | 1.005 (1.003, 1.008) | <0.001 | 1.004 (1.000, 1.008) | 0.031 |
| Alk.P (µL) | 1.001 (1.000, 1.002) | 0.033 | 1.000 (0.999, 1.001) | 0.810 |
| AAR | 0.025 (0.001, 0.868) | 0.042 | 2.674 (0.016, 450.862) | 0.707 |
| ALBI | 1.386 (1.028, 1.868) | 0.032 | 0.915 (0.303, 2.766) | 0.875 |
| GNRI | 0.981 (0.967, 0.994) | 0.004 | 0.997 (0.962, 1.034) | 0.878 |
| Pre-Operative Risk Variable | Threshold | Sensitivity | Specificity | Youden Index | Accuracy | Precision | AUC |
|---|---|---|---|---|---|---|---|
| Tumor size (mm) | 42.50 | 66.7% | 62.0% | 0.287 | 64.4% | 63.7% | 0.684 |
| AFP(×102 ng/mL) | 30.24 | 66.0% | 61.6% | 0.276 | 63.8% | 63.2% | 0.631 |
| Platelet(×103/µL) | 178.50 | 68.8% | 50.9% | 0.196 | 59.9% | 58.4% | 0.627 |
| GOT/GPT | 1.33 | 43.4% | 75.1% | 0.185 | 59.3% | 63.5% | 0.550 |
| AAR | 0.09 | 23.2% | 88.3% | 0.115 | 55.8% | 66.5% | 0.564 |
| ALBI | −2.86 | 70.3% | 40.9% | 0.112 | 55.6% | 54.3% | 0.521 |
| GNRI | 98.87 | 25.3% | 88.2% | 0.135 | 56.8% | 68.2% | 0.521 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-L.; Chen, Y.-S.; Hsieh, K.-C.; Lee, H.-M.; Chen, C.-Y.; Chen, J.-H.; Hung, C.-M.; Hsu, C.-T.; Huang, Y.-L.; Ker, C.-G. Clinical Nomogram Model for Pre-Operative Prediction of Microvascular Invasion of Hepatocellular Carcinoma before Hepatectomy. Medicina 2024, 60, 1410. https://doi.org/10.3390/medicina60091410
Chen J-L, Chen Y-S, Hsieh K-C, Lee H-M, Chen C-Y, Chen J-H, Hung C-M, Hsu C-T, Huang Y-L, Ker C-G. Clinical Nomogram Model for Pre-Operative Prediction of Microvascular Invasion of Hepatocellular Carcinoma before Hepatectomy. Medicina. 2024; 60(9):1410. https://doi.org/10.3390/medicina60091410
Chicago/Turabian StyleChen, Jen-Lung, Yaw-Sen Chen, Kun-Chou Hsieh, Hui-Ming Lee, Chung-Yen Chen, Jian-Han Chen, Chao-Ming Hung, Chao-Tien Hsu, Ya-Ling Huang, and Chen-Guo Ker. 2024. "Clinical Nomogram Model for Pre-Operative Prediction of Microvascular Invasion of Hepatocellular Carcinoma before Hepatectomy" Medicina 60, no. 9: 1410. https://doi.org/10.3390/medicina60091410





